Acute and Chronic Exercise Effects on Human Memory: What We Know and Where to Go from Here
1. Memory Systems
2. Effects of Acute Exercise on Memory
3. Effects of Chronic Exercise on Memory
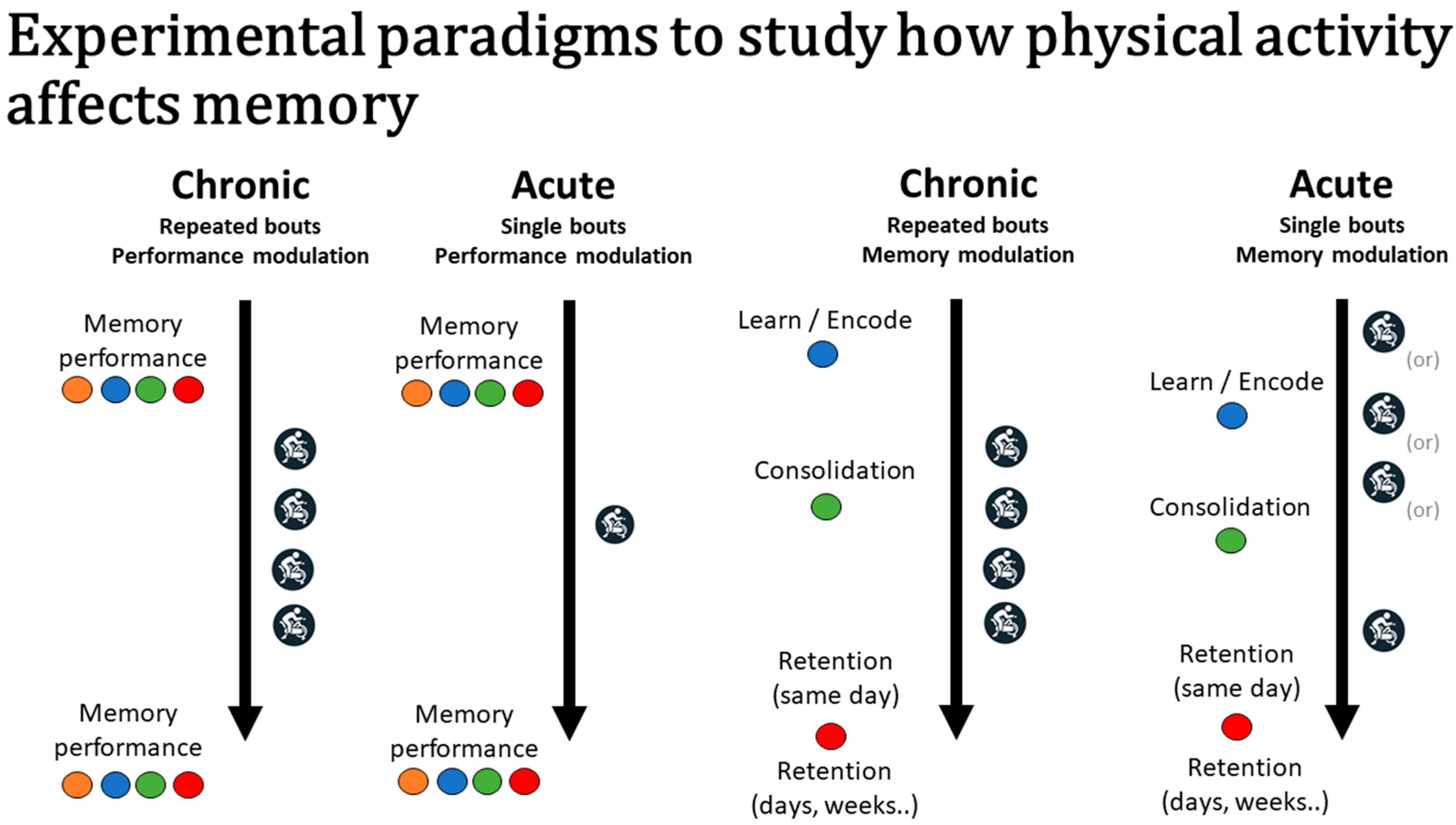
4. Selective Use of Study Design to Target Memory Phases and Exercise Mechanisms
5. Controversies and Inconsistencies: Discussion of Moderators
6. Suggestions for Future Research
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simons, J.S.; Spiers, H.J. Prefrontal and medial temporal lobe interactions in long-term memory. Nat. Rev. Neurosci. 2003, 4, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Squire, L.R. Memory systems of the brain: A brief history and current perspective. Neurobiol. Learn. Mem. 2004, 82, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Dudai, Y.; Karni, A.; Born, J. The Consolidation and Transformation of Memory. Neuron 2015, 88, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yonelinas, A.P.; Ranganath, C.; Ekstrom, A.D.; Wiltgen, B.J. A contextual binding theory of episodic memory: Systems consolidation reconsidered. Nat. Rev. Neurosci. 2019, 20, 364–375. [Google Scholar] [CrossRef]
- Poo, M.M.; Pignatelli, M.; Ryan, T.J.; Tonegawa, S.; Bonhoeffer, T.; Martin, K.C.; Rudenko, A.; Tsai, L.H.; Tsien, R.W.; Fishell, G.; et al. What is memory? The present state of the engram. BMC Biol. 2016, 14, 40. [Google Scholar] [CrossRef] [Green Version]
- Alberini, C.M. The role of reconsolidation and the dynamic process of long-term memory formation and storage. Front. Behav. Neurosci. 2011, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Baddeley, A.; Scott, D. Short term forgetting in the absence of proactive interference. Q. J. Exp. Psychol. 1971, 23, 275–283. [Google Scholar] [CrossRef]
- Nadel, L.; Hardt, O. Update on memory systems and processes. Neuropsychopharmacology 2011, 36, 251–273. [Google Scholar] [CrossRef] [Green Version]
- Tomporowski, P.D.; Qazi, A.S. Cognitive-Motor Dual Task Interference Effects on Declarative Memory: A Theory-Based Review. Front. Psychol. 2020, 11, 1015. [Google Scholar] [CrossRef]
- McMorris, T. History of Research into the Acute Exercise-Cognition Interaction: A cognitive Psychology Approach. In Exercise-Cognition Interaction: Neuroscience Perspectives; McMorris, T., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2016; pp. 1–28. [Google Scholar]
- Tomporowski, P.; Ellis, N.R. Effects of exercise on cognitive processes: A review. Psychol. Bull. 1986, 99, 338346. [Google Scholar] [CrossRef]
- Etnier, J.L.; Salazar, W.; Landers, D.M.; Petruzzello, S.J.; Han, M.; Nowell, P. The influence of physical fitness and exercise upon cognitive functioning: A meta-analysis. J. Sport Exerc. Psychol. 1997, 19, 249–277. [Google Scholar] [CrossRef] [Green Version]
- Tomporowski, P.D. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003, 112, 297–324. [Google Scholar] [CrossRef]
- Chang, Y.K.; Labban, J.D.; Gapin, J.I.; Etnier, J.L. The effects of acute exercise on cognitive performance: A meta-analysis. Brain Res. 2012, 1453, 87–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roig, M.; Nordbrandt, S.; Geertsen, S.S.; Nielsen, J.B. The effects of cardiovascular exercise on human memory: A review with meta-analysis. Neurosci. Biobehav. Rev. 2013, 37, 1645–1666. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Blough, J.; Crawford, L.; Ryu, S.; Zou, L.; Li, H. The temporal effects of acute exercise on episodic memory function: Systematic review with meta-analysis. Brain Sci. 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomstrand, P.; Engvall, J. Effects of a single exercise workout on memory and learning functions in young adults—A systematic review. Transl. Sports Med. 2020, 4, 115–127. [Google Scholar] [CrossRef]
- Etnier, J.L.; Sprick, P.M.; Labban, J.D.; Shih, C.H.; Glass, S.M.; Vance, J.C. Effects of an aerobic fitness test on short- and long-term memory in elementary-aged children. J. Sports Sci. 2020, 38, 2264–2272. [Google Scholar] [CrossRef]
- Etnier, J.; Labban, J.D.; Piepmeier, A.; Davis, M.E.; Henning, D.A. Effects of an acute bout of exercise on memory in 6th grade children. Pediatr. Exerc. Sci. 2014, 26, 250–258. [Google Scholar] [CrossRef] [Green Version]
- Labban, J.D.; Etnier, J.L. The Effect of Acute Exercise on Encoding and Consolidation of Long-Term Memory. J. Sport Exerc. Psychol. 2018, 40, 336–342. [Google Scholar] [CrossRef]
- Labban, J.D.; Etnier, J.L. Effects of acute exercise on long-term memory. Res. Q. Exerc. Sport 2011, 82, 712–721. [Google Scholar] [CrossRef] [Green Version]
- Slutsky-Ganesh, A.B.; Etnier, J.L.; Labban, J.D. Acute exercise, memory, and neural activation in young adults. Int. J. Psychophysiol 2020, 158, 299–309. [Google Scholar] [CrossRef]
- Etnier, J.L.; Vance, J.C.; Ueno, A. Effects of Acute Exercise on Memory Performance in Middle-Aged and Older Adults. J. Aging Phys. Act. 2021, 1, 1–8. [Google Scholar]
- Roig, M.; Thomas, R.; Mang, C.S.; Snow, N.J.; Ostadan, F.; Boyd, L.A.; Lundbye-Jensen, J. Time-Dependent Effects of Cardiovascular Exercise on Memory. Exerc. Sport Sci. Rev. 2016, 44, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Etnier, J.L.; Shih, C.; Piepmeier, A.T. The History of Research on Chronic Physical Activity and Cognitive Performance. In Exercise-Cognition Interaction: Neuroscience Perspectives; McMorris, T., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2016; pp. 29–42. [Google Scholar]
- Rathore, A.; Lom, B. The effects of chronic and acute physical activity on working memory performance in healthy participants: A systematic review with meta-analysis of randomized controlled trials. Syst. Rev. 2017, 6, 124. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Zou, L.; Li, H. The Endocannabinoid System as a Potential Mechanism through which Exercise Influences Episodic Memory Function. Brain Sci. 2019, 9, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, B.M.; Bringard, A.; Logrieco, M.G.; Lauer, E.; Imobersteg, N.; Thomas, A.; Ferretti, G.; Schwartz, S.; Igloi, K. A single session of moderate intensity exercise influences memory, endocannabinoids and brain derived neurotrophic factor levels in men. Sci. Rep. 2021, 11, 14371. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Day, S.; Hendry, R.; Hoffman, S.; Love, A.; Marable, S.; McKee, E.; Stec, S.; Watson, H.; Gilliland, B. The Effects of Acute Exercise on Short- and Long-Term Memory: Considerations for the Timing of Exercise and Phases of Memory. Eur. J. Psychol. 2021, 17, 85–103. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Ponce, P.; Frith, E. Hypothesized mechanisms through which acute exercise influences episodic memory. Physiol. Int. 2018, 105, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D.; Edwards, M.K.; Frith, E. Potential avenues for exercise to activate episodic memory-related pathways: A narrative review. Eur. J. Neurosci. 2017, 46, 2067–2077. [Google Scholar] [CrossRef]
- Moore, D.; Loprinzi, P.D. Exercise influences episodic memory via changes in hippocampal neurocircuitry and long-term potentiation. Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef]
- Pontifex, M.B.; McGowan, A.L.; Chandler, M.C.; Gwizdala, K.L.; Parks, A.C.; Fenn, K.; Kamijo, K. A primer on investigating the after effects of acute bouts of physical activity on cognition. Psychol. Sport Exerc. 2019, 40, 1–22. [Google Scholar] [CrossRef]
- Voss, M.W.; Vivar, C.; Kramer, A.F.; van Praag, H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn. Sci. 2013, 17, 525–544. [Google Scholar] [CrossRef] [Green Version]
- El-Sayes, J.; Harasym, D.; Turco, C.V.; Locke, M.B.; Nelson, A.J. Exercise-Induced Neuroplasticity: A Mechanistic Model and Prospects for Promoting Plasticity. Neuroscientist 2019, 25, 65–85. [Google Scholar] [CrossRef] [PubMed]
- McMorris, T. Developing the catecholamines hypothesis for the acute exercise-cognition interaction in humans: Lessons from animal studies. Physiol. Behav. 2016, 165, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D.; Moore, D.; Loenneke, J.P. Does Aerobic and Resistance Exercise Influence Episodic Memory through Unique Mechanisms? Brain Sci. 2020, 10, 913. [Google Scholar] [CrossRef] [PubMed]
- Basso, J.C.; Suzuki, W.A. The Effects of Acute Exercise on Mood, Cognition, Neurophysiology, and Neurochemical Pathways: A Review. Brain Plast. 2017, 2, 127–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Yu, Q.; Liu, S.; Loprinzi, P.D. Exercise on Visuo-Spatial Memory: Direct Effects and Underlying Mechanisms. Am. J. Health Behav. 2020, 44, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Hotting, K.; Schickert, N.; Kaiser, J.; Roder, B.; Schmidt-Kassow, M. The effects of acute physical exercise on memory, peripheral BDNF, and cortisol in young adults. Neural Plast. 2016, 2016, 6860573. [Google Scholar] [CrossRef] [Green Version]
- Etnier, J.L.; Wideman, L.; Labban, J.D.; Piepmeier, A.T.; Pendleton, D.M.; Dvorak, K.K.; Becofsky, K. The Effects of Acute Exercise on Memory and Brain-Derived Neurotrophic Factor (BDNF). J. Sport Exerc. Psychol. 2016, 38, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Kassow, M.; Zink, N.; Mock, J.; Thiel, C.; Vogt, L.; Abel, C.; Kaiser, J. Treadmill walking during vocabulary encoding improves verbal long-term memory. Behav. Brain Funct. BBF 2014, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Piepmeier, A.T.; Etnier, J.L.; Wideman, L.; Berry, N.T.; Kincaid, Z.; Weaver, M.A. A preliminary investigation of acute exercise intensity on memory and BDNF isoform concentrations. Eur. J. Sport Sci. 2019, 8, 1–12. [Google Scholar] [CrossRef]
- Horowitz, A.M.; Fan, X.; Bieri, G.; Smith, L.K.; Sanchez-Diaz, C.I.; Schroer, A.B.; Gontier, G.; Casaletto, K.B.; Kramer, J.H.; Williams, K.E.; et al. Blood factors transfer beneficial effects of exercise on neurogenesis and cognition to the aged brain. Science 2020, 369, 167–173. [Google Scholar] [CrossRef]
- Suwabe, K.; Byun, K.; Hyodo, K.; Reagh, Z.M.; Roberts, J.M.; Matsushita, A.; Saotome, K.; Ochi, G.; Fukuie, T.; Suzuki, K.; et al. Rapid stimulation of human dentate gyrus function with acute mild exercise. Proc. Natl. Acad. Sci. USA 2018, 115, 10487–10492. [Google Scholar] [CrossRef] [Green Version]
- Voss, M.W.; Weng, T.B.; Narayana-Kumanan, K.; Cole, R.C.; Wharff, C.; Reist, L.; DuBose, L.; Schmidt, P.G.; Sigurdsson, G.; Mills, J.A.; et al. Acute Exercise Effects Predict Training Change in Cognition and Connectivity. Med. Sci. Sports Exerc. 2020, 52, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Norman, J.E.; Rutkowsky, J.; Bodine, S.; Rutledge, J.C. The Potential Mechanisms of Exercise-induced Cognitive Protection: A Literature Review. Curr. Pharm. Des. 2018, 24, 1827–1831. [Google Scholar] [CrossRef]
- Kramer, A.F.; Erickson, K.I. Capitalizing on cortical plasticity: Influence of physical activity on cognition and brain function. Trends Cogn. Sci. 2007, 11, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Marmeleira, J. An examination of the mechanisms underlying the effects of physical activity on brain and cognition. Eur. J. Aging Phys. Act. 2013, 10, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Voss, M.W.; Nagamatsu, L.S.; Liu-Ambrose, T.; Kramer, A.F. Exercise, brain, and cognition across the life span. J. Appl. Physiol. 2011, 111, 1505–1513. [Google Scholar] [CrossRef] [Green Version]
- Herold, F.; Torpel, A.; Hamacher, D.; Budde, H.; Zou, L.; Strobach, T.; Müller, N.G.; Gronwald, T. Causes and consequences of interindividual response variability: A call to apply a more rigorous research design in acute exercise-cognition studies. Front. Physiol. 2021, 12, 682891. [Google Scholar] [CrossRef] [PubMed]
- Hogan, C.L.; Mata, J.; Carstensen, L.L. Exercise holds immediate benefits for affect and cognition in younger and older adults. Psychol. Aging 2013, 28, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Asperholm, M.; Nagar, S.; Dekhtyar, S.; Herlitz, A. The magnitude of sex differences in verbal episodic memory increases with social progress: Data from 54 countries across 40 years. PLoS ONE 2019, 14, e0214945. [Google Scholar] [CrossRef]
- Johnson, L.; Loprinzi, P.D. The effects of acute exercise on episodic memory function among young University students: Moderation considerations by biological sex. Health Promot. Perspect. 2019, 9, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D.; Frith, E. The Role of Sex in Memory Function: Considerations and Recommendations in the Context of Exercise. J. Clin. Med. 2018, 7, 132. [Google Scholar] [CrossRef] [Green Version]
- Most, S.B.; Kennedy, B.L.; Petras, E.A. Evidence for improved memory from 5 minutes of immediate, post-encoding exercise among women. Cogn. Res. Princ. Implic. 2017, 2, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [Green Version]
- Hopkins, M.E.; Davis, F.C.; Vantieghem, M.R.; Whalen, P.J.; Bucci, D.J. Differential effects of acute and regular physical exercise on cognition and affect. Neuroscience 2012, 215, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crush, E.A.; Loprinzi, P.D. Dose-Response Effects of Exercise Duration and Recovery on Cognitive Functioning. Percept. Mot. Skills. 2017, 124, 1164–1193. [Google Scholar] [CrossRef] [PubMed]
- Sibley, B.A.; Beilock, S.L. Exercise and working memory: An individual differences investigation. J. Sport Exerc. Psychol. 2007, 29, 783–791. [Google Scholar] [CrossRef]
- Loprinzi, P.D.; Frith, E. A brief primer on the mediational role of BDNF in the exercise-memory link. Clin. Physiol. Funct. Imaging 2019, 39, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Loprinzi, P.D. Does brain-derived neurotrophic factor mediate the effects of exercise on memory? Physician Sportsmed. 2019, 47, 395–405. [Google Scholar] [CrossRef]
- De Las Heras, B.; Rodrigues, L.; Cristini, J.; Weiss, M.; Prats-Puig, A.; Roig, M. Does the Brain-Derived Neurotrophic Factor Val66Met Polymorphism Modulate the Effects of Physical Activity and Exercise on Cognition? Neuroscientist 2020, 1073858420975712. [Google Scholar] [CrossRef]
- Gejl, A.K.; Enevold, C.; Bugge, A.; Andersen, M.S.; Nielsen, C.H.; Andersen, L.B. Associations between serum and plasma brain-derived neurotrophic factor and influence of storage time and centrifugation strategy. Sci. Rep. 2019, 9, 9655. [Google Scholar] [CrossRef] [Green Version]
- Piepmeier, A.; Etnier, J. Brain-derived neurotrophic factor (BDNF) as a potential mechanism of the effects of acute exercise on cognitive performanace. J. Sport Health Sci. 2015, 4, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D. Intensity-specific effects of acute exercise on human memory function: Considerations for the timing of exercise and the type of memory. Health Promot. Perspect. 2018, 8, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Marchant, D.; Hampson, S.; Finnigan, L.; Marrin, K.; Thorley, C. The Effects of Acute Moderate and High Intensity Exercise on Memory. Front. Psychol. 2020, 11, 1716. [Google Scholar] [CrossRef]
- Pyke, W.; Ifram, F.; Coventry, L.; Sung, Y.; Champion, I.; Javadi, A.H. The effects of different protocols of physical exercise and rest on long-term memory. Neurobiol. Learn. Mem. 2020, 167, 107128. [Google Scholar] [CrossRef] [PubMed]
- Sng, E.; Frith, E.; Loprinzi, P.D. Temporal Effects of Acute Walking Exercise on Learning and Memory Function. Am. J. Health Promot. AJHP 2018, 32, 1518–1525. [Google Scholar] [CrossRef]
- Frith, E.; Sng, E.; Loprinzi, P.D. Randomized controlled trial evaluating the temporal effects of high-intensity exercise on learning, short-term and long-term memory, and prospective memory. Eur. J. Neurosci. 2017, 46, 2557–2564. [Google Scholar] [CrossRef] [PubMed]
- Haynes, J.T.; Frith, E.; Sng, E.; Loprinzi, P.D. Experimental Effects of Acute Exercise on Episodic Memory Function: Considerations for the Timing of Exercise. Psychol. Rep. 2019, 122, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Van Dongen, E.V.; Kersten, I.H.; Wagner, I.C.; Morris, R.G.; Fernandez, G. Physical Exercise Performed Four Hours after Learning Improves Memory Retention and Increases Hippocampal Pattern Similarity during Retrieval. Curr. Biol. 2016, 26, 1722–1727. [Google Scholar] [CrossRef] [Green Version]
- McNerney, M.W.; Radvansky, G.A. Mind racing: The influence of exercise on long-term memory consolidation. Memory 2015, 23, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Cantrelle, J.; Burnett, G.; Loprinzi, P.D. Acute exercise on memory function: Open vs. closed skilled exercise. Health Promot. Perspect. 2020, 10, 123–128. [Google Scholar] [CrossRef] [Green Version]
- O'Brien, J.; Ottoboni, G.; Tessari, A.; Setti, A. One bout of open skill exercise improves cross-modal perception and immediate memory in healthy older adults who habitually exercise. PLoS ONE 2017, 12, e0178739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, R.D.; Romine, M.W.; O’Connor, P.J.; Tomporowski, P.D. The influence of exercise-induced fatigue on cognitive function. J. Sports Sci. 2012, 30, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Kang, M.; Blough, J.; Loprinzi, P.D. Experimental effects of acute exercise on cognitive-based short-term memory improvement: A meta-analysis of repeated measures studies. J. Sci. Sport Exerc. 2021, 55, 331. [Google Scholar] [CrossRef]
- Moore, D.C.; Ryu, S.; Loprinzi, P.D. Experimental effects of acute exercise on forgetting. Physiol. Int. 2020. [Google Scholar] [CrossRef] [PubMed]
- Eich, T.S.; Metcalfe, J. Effects of the stress of marathon running on implicit and explicit memory. Psychon. Bull. Rev. 2009, 16, 475–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loprinzi, P.D.; Edwards, M.K. Exercise and Implicit Memory: A Brief Systematic Review. Psychol. Rep. 2018, 121, 1072–1085. [Google Scholar] [CrossRef]
- Keyan, D.; Bryant, R.A. Acute physical exercise in humans enhances reconsolidation of emotional memories. Psychoneuroendocrinology 2017, 86, 144–151. [Google Scholar] [CrossRef]
- Wade, B.; Loprinzi, P.D. The Experimental Effects of Acute Exercise on Long-Term Emotional Memory. J. Clin. Med. 2018, 7, 486. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D.; Frith, E.; Edwards, M.K. Exercise and emotional memory: A systematic review. J. Cogn. Enhanc. 2019, 3, 94–103. [Google Scholar] [CrossRef]
- Green, D.; Loprinzi, P.D. Experimental Effects of Acute Exercise on Prospective Memory and False Memory. Psychol. Rep. 2018, 122, 1313–1326. [Google Scholar] [CrossRef]
- Siddiqui, A.; Loprinzi, P.D. Experimental Investigation of the Time Course Effects of Acute Exercise on False Episodic Memory. J. Clin. Med. 2018, 7, 157. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D.; Loenneke, J.P.; Storm, B.C. Effects of acute aerobic and resistance exercise on episodic memory function. Q. J. Exp. Psychol. 2021, 74, 1264–1283. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Gronwald, T.; Scholkmann, F.; Zohdi, H.; Wyser, D.; Müller, N.G.; Hamacher, D. New Directions in Exercise Prescription: Is There a Role for Brain-Derived Parameters Obtained by Functional Near-Infrared Spectroscopy? Brain Sci. 2020, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Jamnick, N.A.; Pettitt, R.W.; Granata, C.; Pyne, D.B.; Bishop, D.J. An Examination and Critique of Current Methods to Determine Exercise Intensity. Sports Med. 2020, 50, 1729–1756. [Google Scholar] [CrossRef]
- Blough, J.; Loprinzi, P.D. Experimental manipulation of psychological control scenarios: Implications for exercise and memory research. Psych 2019, 1, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Rasch, B.; Born, J. About sleep’s role in memory. Physiol. Rev. 2013, 93, 681–766. [Google Scholar] [CrossRef]
- Mograss, M.; Crosetta, M.; Abi-Jaoude, J.; Frolova, E.; Robertson, E.M.; Pepin, V.; Dang-Vu, T.T. Exercising before a nap benefits memory better than napping or exercising alone. Sleep 2020, 43, zsaa062. [Google Scholar] [CrossRef]
- Austin, M.; Loprinzi, P.D. Acute exercise and mindfulness meditation on learning and memory: Randomized controlled intervention. Health Promot. Perspect. 2019, 9, 314–318. [Google Scholar] [CrossRef]
- Prichard, E.; Propper, R.E.; Christman, S.D. Degree of Handedness, but not Direction, is a Systematic Predictor of Cognitive Performance. Front. Psychol. 2013, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D.; Frith, E. Interhemispheric Activation and Memory Function: Considerations and Recommendations in the Context of Cardiovascular Exercise Research. Psychol. Rep. 2019, 122, 2396–2405. [Google Scholar] [CrossRef]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.J.; Blumenthal, J.A.; Hoffman, B.M.; Cooper, H. Aerobic exercise and neurocognitive performance: A meta-analytic review of randomized controlled trials. Psychosom. Med. 2010, 72, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Buchman, A.S.; Boyle, P.A.; Yu, L.; Shah, R.C.; Wilson, R.S.; Bennett, D.A. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology 2012, 78, 1323–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etnier, J.; Nowell, P.M.; Landers, D.M.; Sibley, B.A. A meta-regression to examine the relationship between aerobic fitness and cognitive performance. Brain Res. Rev. 2006, 52, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Morris, J.K.; Vidoni, E.D.; Johnson, D.K.; Van Sciver, A.; Mahnken, J.D.; Honea, R.A.; Wilkins, H.M.; Brooks, W.M.; Billinger, S.A.; Swerdlow, R.H.; et al. Aerobic exercise for Alzheimer's disease: A randomized controlled pilot trial. PLoS ONE 2017, 12, e0170547. [Google Scholar] [CrossRef]
- Maass, A.; Duzel, S.; Goerke, M.; Becke, A.; Sobieray, U.; Neumann, K.; Lövdén, M.; Lindenberger, U.; Bäckman, L.; Braun-Dullaeus, R.; et al. Vascular hippocampal plasticity after aerobic exercise in older adults. Mol. Psychiatry 2015, 20, 585–593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, R.C.; Hazeltine, E.; Weng, T.B.; Wharff, C.; DuBose, L.E.; Schmid, P.; Sigurdsson, G.; Magnotta, V.A.; Pierce, G.L.; Voss, M.W. Cardiorespiratory fitness and hippocampal volume predict faster episodic associative learning in older adults. Hippocampus 2020, 30, 143–155. [Google Scholar] [CrossRef]
- Voss, M.W.; Weng, T.B.; Burzynska, A.Z.; Wong, C.N.; Cooke, G.E.; Clark, R.; Fanning, J.; Awick, E.; Gothe, N.P.; Olson, E.A.; et al. Fitness, but not physical activity, is related to functional integrity of brain networks associated with aging. NeuroImage 2016, 131, 113–125. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, G.; Batterham, A.M. True and false interindividual differences in the physiological response to an intervention. Exp. Physiol. 2015, 100, 577–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, G.; Williamson, P.; Batterham, A.M. Exercise training response heterogeneity: Statistical insights. Diabetologia 2018, 61, 496–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, G.; Williamson, P.; Batterham, A.M. Issues in the determination of ‘responders’ and ‘non-responders’ in physiological research. Exp. Physiol. 2019, 104, 1215–1225. [Google Scholar] [CrossRef] [PubMed]
- Dankel, S.J.; Loenneke, J.P. A Method to Stop Analyzing Random Error and Start Analyzing Differential Responders to Exercise. Sports Med. 2020, 50, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Crawford, L.K.; Caplan, J.B.; Loprinzi, P.D. The Impact of Acute Exercise Timing on Memory Interference. Percept. Mot. Ski. 2021, 128, 1215–1234. [Google Scholar] [CrossRef]
- Crawford, L.; Loprinzi, P.D. Effects of intensity-specific acute exercise on paired-associative memory and memory interference. Psych 2019, 1, 290–305. [Google Scholar] [CrossRef] [Green Version]
- Crawford, L.K.; Li, H.; Zou, L.; Wei, G.X.; Loprinzi, P.D. Hypothesized Mechanisms Through Which Exercise May Attenuate Memory Interference. Medicina 2020, 56, 129. [Google Scholar] [CrossRef] [Green Version]
- Loprinzi, P.D.; Frith, E.; Crawford, L. Acute exercise and retroactive memory interference. Am. J. Health Promot. 2019, 34, 25–31. [Google Scholar] [CrossRef]
- Chen, J.; Roig, M.; Wright, D.L. Exercise reduces competition between procedural and declarative memory systems. eNeuro 2020, 22, 0070. [Google Scholar]
- Beck, M.M.; Grandjean, M.U.; Hartmand, S.; Spedden, M.E.; Christiansen, L.; Roig, M.; Lundbye-Jensen, J. Acute Exercise Protects Newly Formed Motor Memories Against rTMS-induced Interference Targeting Primary Motor Cortex. Neuroscience 2020, 436, 110–121. [Google Scholar] [CrossRef]
- Jo, J.S.; Chen, J.; Riechman, S.; Roig, M.; Wright, D.L. The protective effects of acute cardiovascular exercise on the interference of procedural memory. Psychol. Res. 2019, 83, 1543–1555. [Google Scholar] [CrossRef]
- Voss, M.W.; Soto, C.; Yoo, S.; Sodoma, M.; Vivar, C.; van Praag, H. Exercise and Hippocampal Memory Systems. Trends Cogn. Sci. 2019, 23, 318–333. [Google Scholar] [CrossRef]
- Funahashi, S.; Andreau, J.M. Prefrontal cortex and neural mechanisms of executive function. J. Physiol. Paris 2013, 107, 471–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pontifex, M.B.; Hillman, C.H.; Fernhall, B.; Thompson, K.M.; Valentini, T.A. The effect of acute aerobic and resistance exercise on working memory. Med. Sci. Sports Exerc. 2009, 41, 927–934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loprinzi, P.D.; Chism, M.; Marable, S. Does Engaging in Acute Exercise Prior to Memory Encoding and During Memory Consolidation have an Additive Effect on Long-Term Memory Function? J. Sci. Sport Exerc. 2019, 2, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Berchtold, N.C.; Chinn, G.; Chou, M.; Kesslak, J.P.; Cotman., C.W. Exercise primes a molecular memory for brain-derived neurotrophic factor protein induction in the rat hippocampus. Neuroscience 2005, 133, 853–861. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loprinzi, P.D.; Roig, M.; Etnier, J.L.; Tomporowski, P.D.; Voss, M. Acute and Chronic Exercise Effects on Human Memory: What We Know and Where to Go from Here. J. Clin. Med. 2021, 10, 4812. https://doi.org/10.3390/jcm10214812
Loprinzi PD, Roig M, Etnier JL, Tomporowski PD, Voss M. Acute and Chronic Exercise Effects on Human Memory: What We Know and Where to Go from Here. Journal of Clinical Medicine. 2021; 10(21):4812. https://doi.org/10.3390/jcm10214812
Chicago/Turabian StyleLoprinzi, Paul D., Marc Roig, Jennifer L. Etnier, Phillip D. Tomporowski, and Michelle Voss. 2021. "Acute and Chronic Exercise Effects on Human Memory: What We Know and Where to Go from Here" Journal of Clinical Medicine 10, no. 21: 4812. https://doi.org/10.3390/jcm10214812
APA StyleLoprinzi, P. D., Roig, M., Etnier, J. L., Tomporowski, P. D., & Voss, M. (2021). Acute and Chronic Exercise Effects on Human Memory: What We Know and Where to Go from Here. Journal of Clinical Medicine, 10(21), 4812. https://doi.org/10.3390/jcm10214812




