Femoral µCT Analysis, Mechanical Testing and Immunolocalization of Bone Proteins in β-Hydroxy β-Methylbutyrate (HMB) Supplemented Spiny Mouse in a Model of Pregnancy and Lactation-Associated Osteoporosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. MECHANICAL Testing
2.3. Immunohistochemical Staining
2.4. Micro Computed Tomography Analysis
2.5. Statistical Analysis
3. Results
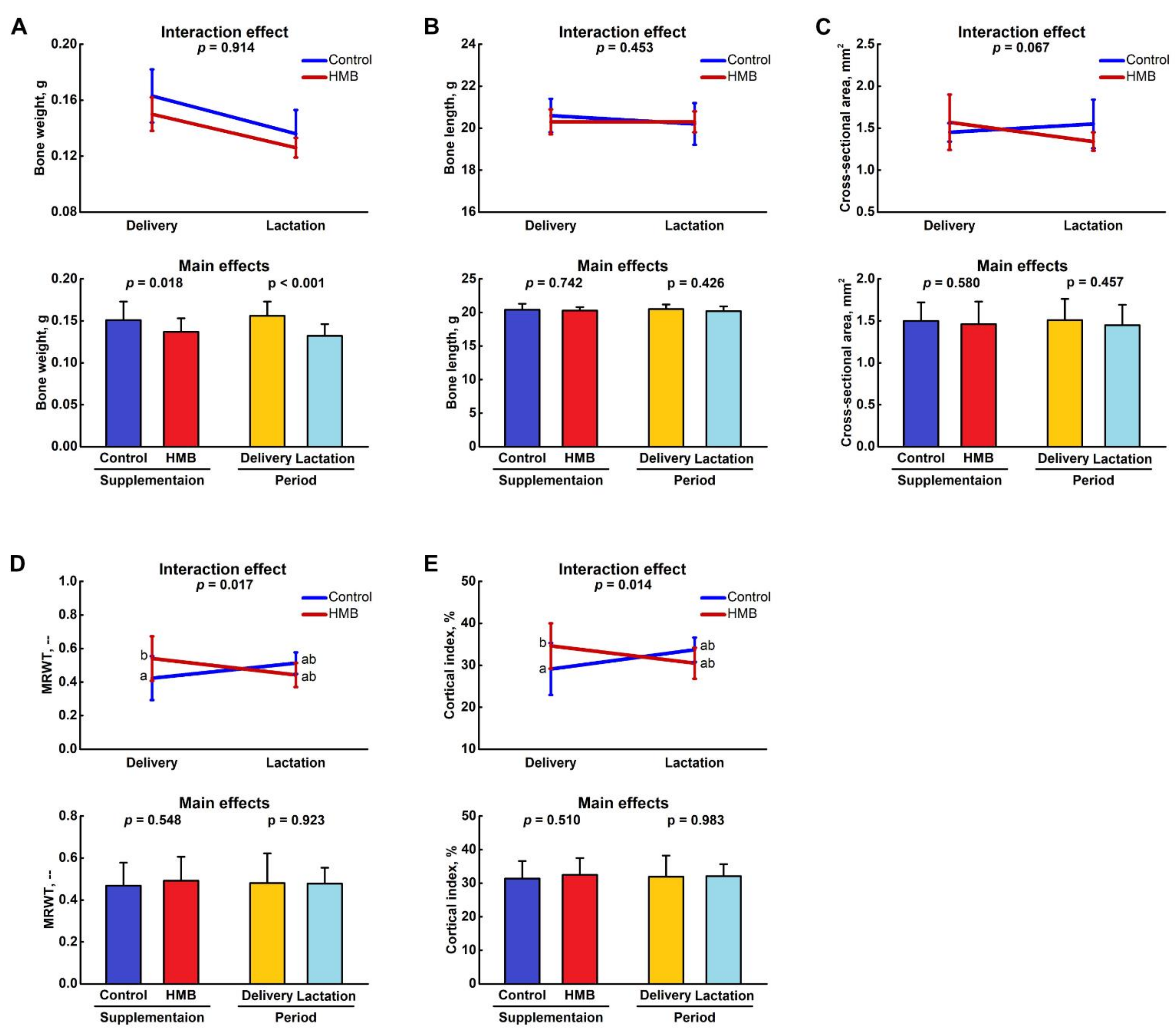
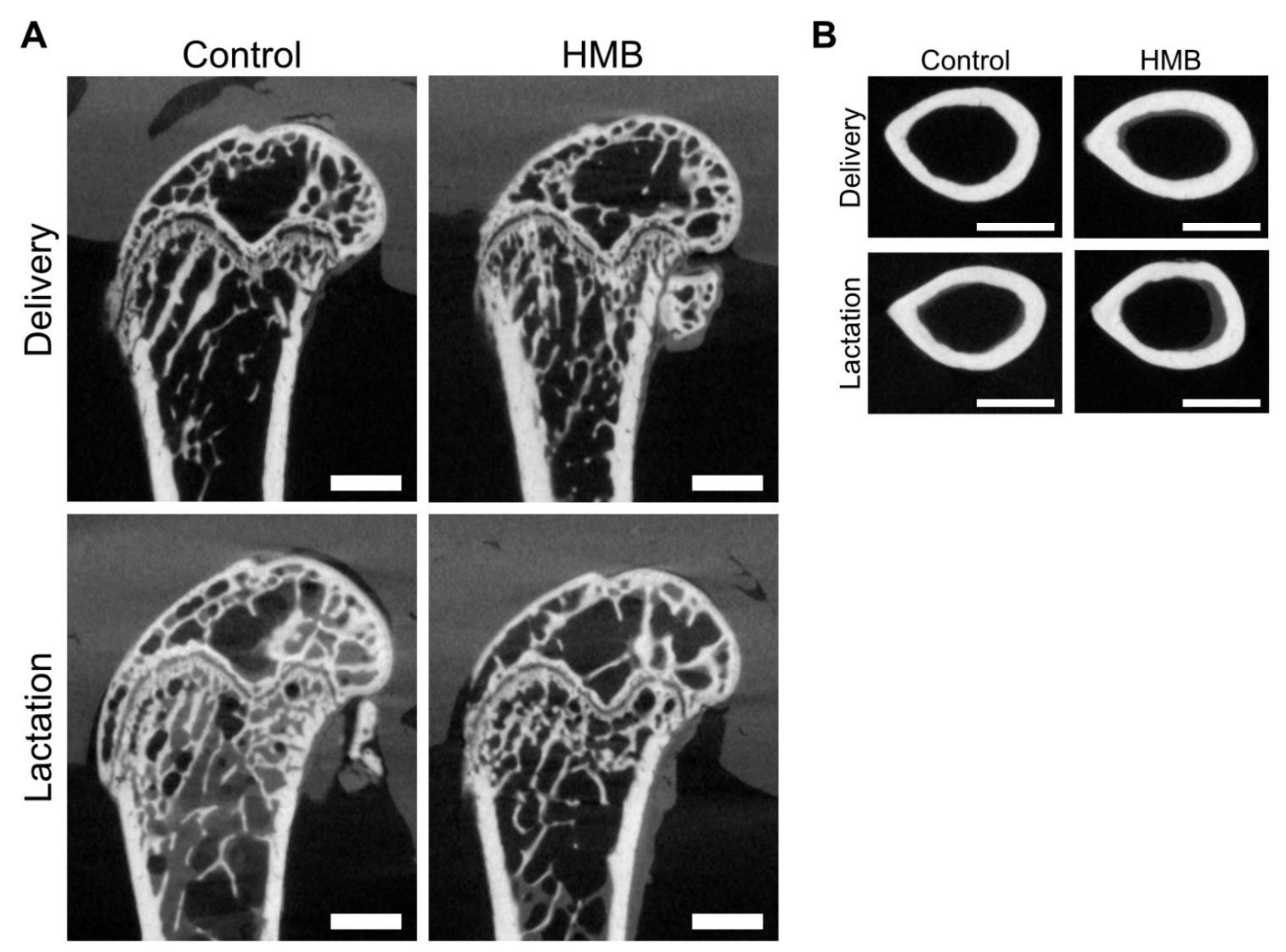
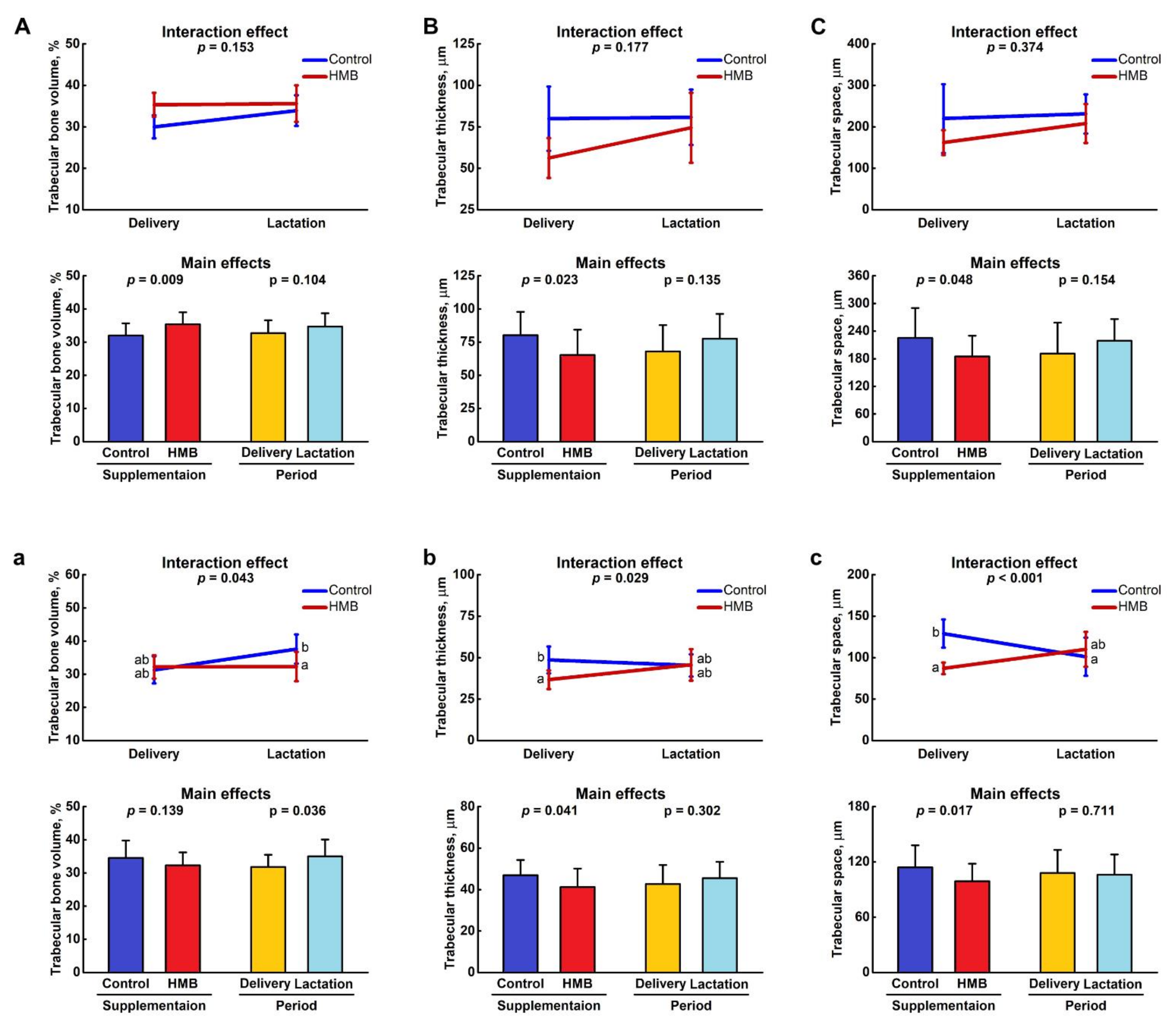
3.1. Bone Analysis
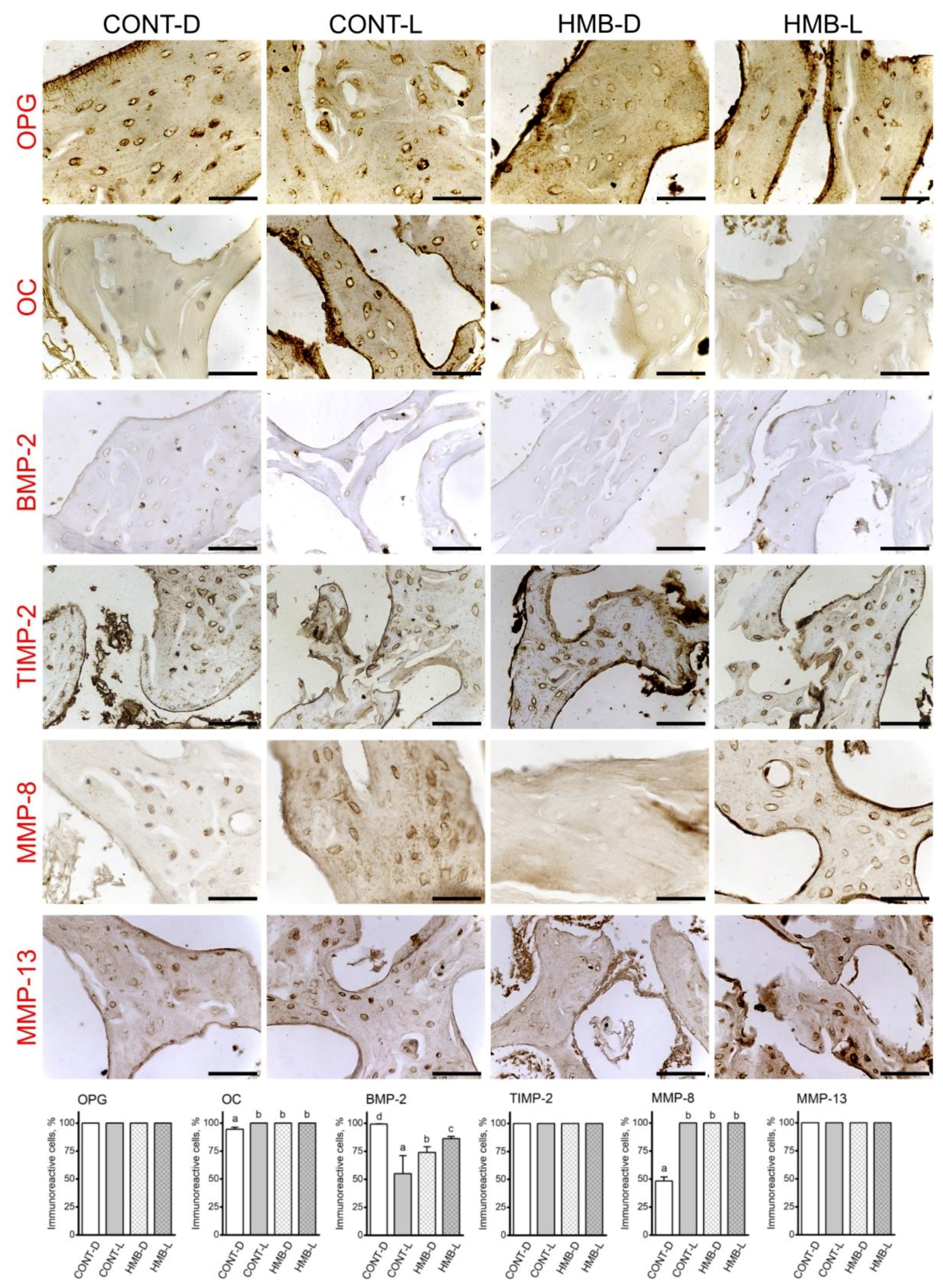
3.2. OPG, OC, BMP-2, TIMP-2, MMP-8 and MMP-13 Immunoreaction in Growth Plate Cartilage
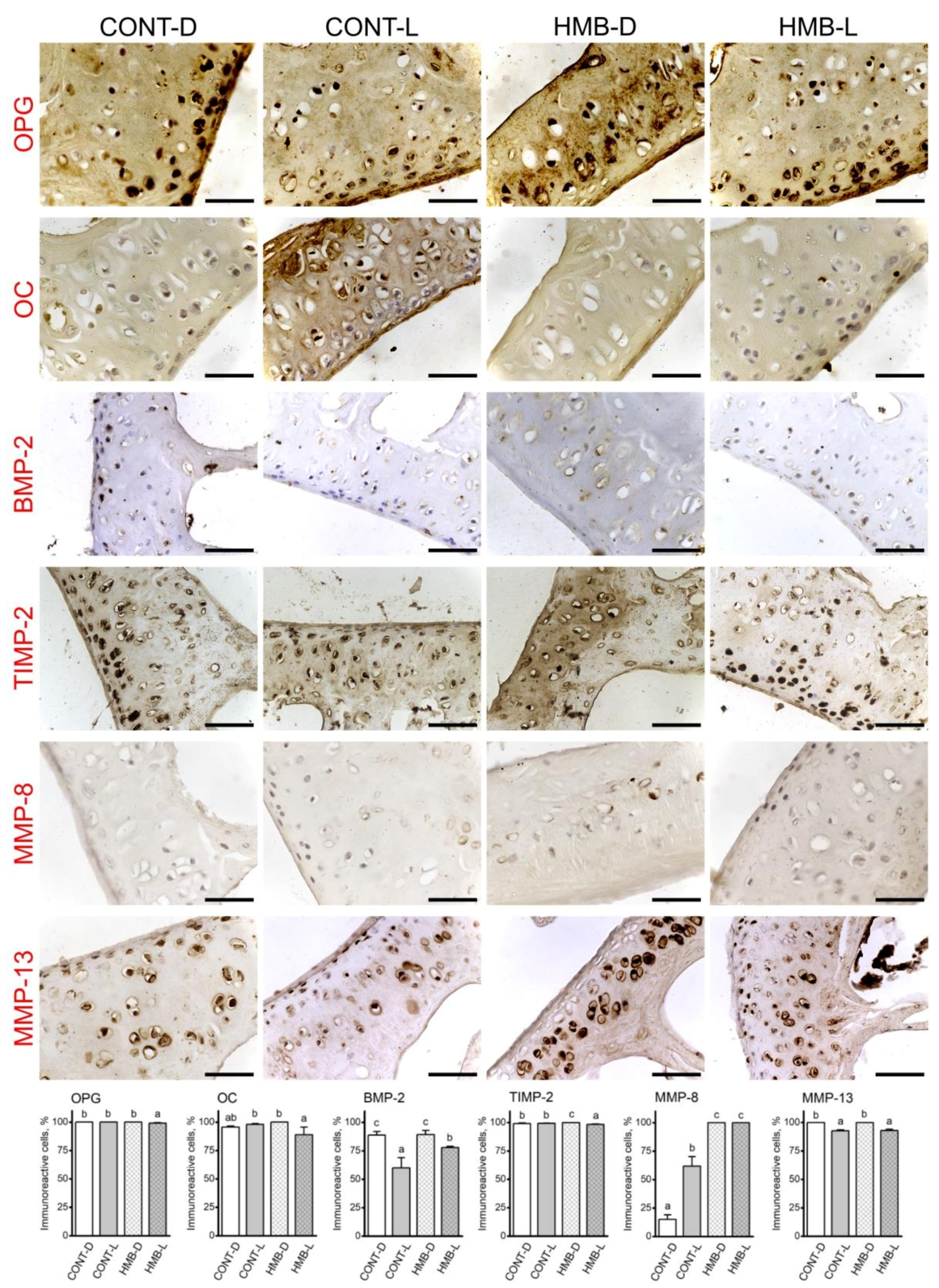
3.3. OPG, OC, BMP-2, TIMP-2, MMP-8 and MMP-13 Immunoreaction in Trabecular Bone
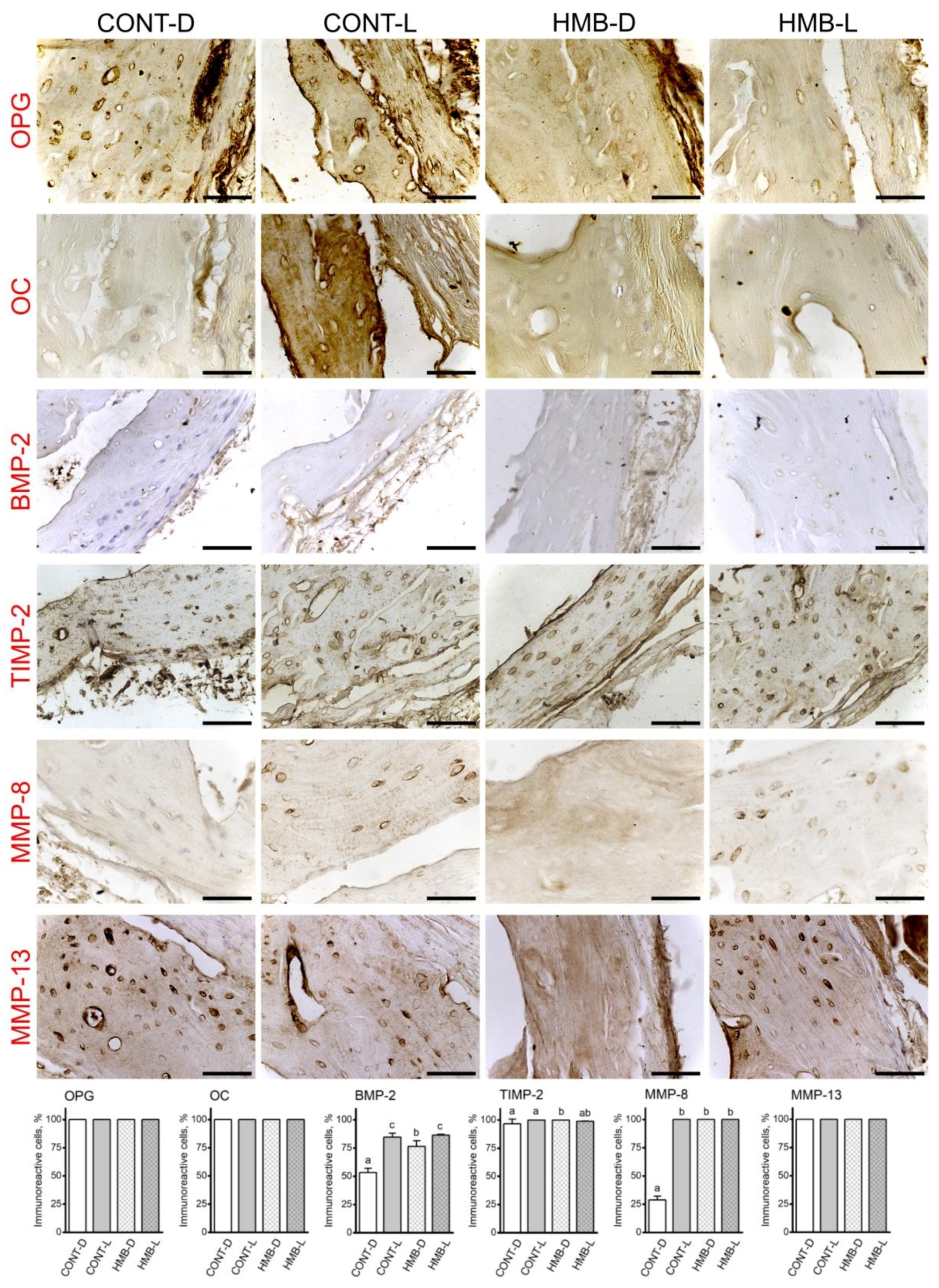
3.4. OPG, OC, BMP-2, TIMP-2, MMP-8 and MMP-13 Immunoreaction in Compact Bone
3.5. OPG, OC, BMP-2, TIMP-2, MMP-8 and MMP-13 Immunoreaction in Articular Cartilage
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rabijewski, M. Risk factors for osteoporosis, with particular emphasis on the correct development and metabolism of bone tissue. Inf. Forum 2017, 8, 77–82. [Google Scholar]
- Maggioli, C.; Stagi, S. Bone modeling, remodeling, and skeletal health in children and adolescents: Mineral accrual, assessment and treatment. Ann. Pediatr. Endocrinol. Metab. 2017, 22, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis and Therapy. Osteoporosis, prevention and treatment. J. Am. Med. Assoc. 2001, 285, 765–795. [Google Scholar]
- Kovacs, C.S.; Kronenberg, H.M. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr. Rev. 1997, 18, 832–872. [Google Scholar]
- Kovacs, C.S.; Fuleihan Gel, H. Calcium and bone disorders during pregnancy and lactation. Endocrinol. Metab. Clin. North Am. 2006, 35, 21–51. [Google Scholar] [CrossRef]
- Kalkwarf, H.J.; Specker, B.L. Bone mineral changes during pregnancy and lactation. Endocrine 2002, 17, 49–53. [Google Scholar] [CrossRef]
- Urban-Mocek, M.; Szymczak, J. Pregnancy and lactation-associated osteoporosis. Forum Reumatol. 2019, 2, 97–104. [Google Scholar] [CrossRef]
- Cohen, A.; Kamanda-Kosseh, M.; Dempster, D.W.; Zhou, H.; Müller, R.; Goff, E.; Colon, I.; Bucovsky, M.; Stubby, J.; Nickolas, T.L.; et al. Women with pregnancy and lactation–associated osteoporosis (PLO) have low bone remodeling rates at the tissue level. J. Bone Miner. Res. 2019, 34, 1552–1561. [Google Scholar] [CrossRef]
- Khastgir, G.; Studd, J.W.W.; King, H.; Abdaila, H.; Jones, J.; Carter, G.; Alahband-Zadeh, J. Changes in bone density and biochemical markers of bone turnover in pregnancy-associated osteoporosis. Br. J. Obstet. Gynaecol. 1996, 103, 716–718. [Google Scholar] [CrossRef]
- Smith, R.; Winearls, C.G.; Stevenson, J.C.; Woods, C.G.; Wordsworth, B.P. Osteoporosis of pregnancy. Lancet 1985, 325, 1178–1180. [Google Scholar] [CrossRef]
- Kovacs, C.S.; Ralston, S.H. Presentation and management of osteoporosis presenting in association with pregnancy or lactation. Osteoporos. Int. 2015, 26, 2223–2241. [Google Scholar] [CrossRef]
- Molfino, A.; Gioia, G.; Rossi Fanelli, F.; Muscaritoli, M. Beta-hydroxy-beta-methylbutyrate supplementation in health and disease: A systematic review of randomized trials. Amino Acids 2013, 45, 1273–1292. [Google Scholar] [CrossRef]
- Duan, Y.; Li, F.; Li, Y.; Tang, Y.; Kong, X.; Feng, Z.; Antoony, T.G.; Watford, M.; Hou, Y.; Wu, G.; et al. The role of leucine and its metabolites in protein and energy metabolism. Amino Acids 2016, 48, 41–51. [Google Scholar] [CrossRef]
- Henning, P.C.; Park, B.S.; Kim, J.S. Beta-Hydroxy-beta-methylbutyrate improves bone properties and attenuates the depression of protein synthesis during a simulated sustained operation. Mil. Med. 2014, 179, 679–685. [Google Scholar] [CrossRef] [Green Version]
- Hankosky, E.R.; Sherrill, L.K.; Ruvola, L.A.; Haake, R.M.; Kim, T.; Hammerslag, L.R.; Kougias, D.G.; Juraska, J.M.; Gulley, J.M. Effects of beta-hydroxy-beta-methyl butyrate on working memory and cognitive flexibility in an animal model of aging. Nutr. Neurosci. 2017, 20, 379–387. [Google Scholar] [CrossRef]
- Bruckbauer, A.; Zemel, M.B.; Thorpe, T.; Akula, M.R.; Stuckey, A.C.; Osborne, D.; Martin, E.B.; Kennel, S.; Wall, J.S. Synergistic effects of leucine and resveratrol on insulin sensitivity and fat metabolism in adipocytes and mice. Nutr. Metab. 2012, 9, 77. [Google Scholar] [CrossRef] [Green Version]
- Wan, H.F.; Zhu, J.T.; Shen, Y.; Xiang, X.; Yin, H.J.; Fang, Z.F.; Che, L.Q.; Lin, Y.; Xu, S.Y.; Feng, B.; et al. Effects of dietary supplementation of beta-hydroxy-beta-methylbutyrate on sow performance and mRNA expression of myogenic markers in skeletal muscle of neonatal piglets. Reprod. Domest. Anim. 2016, 51, 135–142. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Muszyński, S.; Dobrowolski, P.; Wiącek, D.; Tomczyk-Warunek, A.; Świetlicka, I.; Pierzynowski, S.G. Maternal HMB treatment affects bone and hyaline cartilage development in their weaned piglets via the leptin/osteoprotegerin system. J. Anim. Physiol. Anim. Nutr. 2019, 103, 626–643. [Google Scholar] [CrossRef]
- Szcześniak, K.A.; Ostaszewski, P.; Fuller, J.C., Jr.; Ciecierska, A.; Sadkowski, T. Dietary supplementation of b-hydroxy-b-methylbutyrate in animals—A review. J. Anim. Physiol. Anim. Nutr. 2015, 99, 405–417. [Google Scholar] [CrossRef]
- Tatara, M.R.; Śliwa, E.; Krupski, W. Prenatal programming of skeletal development in the offspring: Effects of maternal treatment with β-hydroxy-β-methylbutyrate (HMB) on femur properties in pigs at slaughter age. Bone 2007, 40, 1615–1622. [Google Scholar] [CrossRef]
- Blicharski, T.; Tomaszewska, E.; Dobrowolski, P.; Hułas-Stasiak, M.; Muszyński, S. A metabolite of leucine (β-hydroxy-β-methylbutyrate) given to sows during pregnancy alters bone development of their newborn offspring by hormonal modulation. PLoS ONE 2017, 12, e0179693. [Google Scholar] [CrossRef]
- Nissen, S.; Sharp, R.L.; Panton, L.; Vukovich, M.; Trappe, S.; Fuller, J.C., Jr. Betahydroxy-beta-methylbutyrate (HMB) supplementation in humans is safe and may decrease cardiovascular risk factors. J. Nutr. 2000, 130, 1937–1945. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.M.; Fischen, P.J.; Campbell, B.; Wilson, G.J.; Znachi, N.; Taylor, L.; Willborn, C.; Kalman, D.S.; Stout, J.R.; Hoffman, J.R.; et al. International Society of Sports Nutrition Position Stand: Beta-hydroxy-beta-methylbutyrate (HMB). J. Int. Soc. Sports Nutr. 2013, 10, 6. [Google Scholar] [CrossRef] [Green Version]
- Lamers, W.H.; Mooren, P.G.; Griep, H.; Endert, E.; Degenhart, H.J.; Charles, R. Hormones in perinatal at and spiny mouse: Relation to altricial and precocial timing of birth. Am. J. Physiol. 1986, 251, E78–E85. [Google Scholar] [CrossRef]
- Carter, A.M. Animal models of human pregnancy and placentation: Alternatives to the mouse. Reproduction 2020, 160, R129–R143. [Google Scholar] [CrossRef]
- Pinhero, G.; Prata, D.F.; Araujo, I.M.; Tiscornia, G. The African spiny mouse (Acomys spp.) as an emerging model for development and regeneration. Lab. Anim. 2018, 52, 565–576. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Donaldson, J.; Kosiński, J.; Dobrowolski, P.; Tomczyk-Warunek, A.; Hułas-Stasiak, M.; Lamorski, K.; Laskowska-Woźniak, D.; Muszyński, S.; Blicharski, R.; et al. β-hydroxy β-methylbutyrate (HMB) supplementation prevents bone loss during pregnancy—Novel evidence from a spiny mouse (Acomys cahirinus) model. Int. J. Mol. Sci. 2021, 22, 3047. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Świetlicka, I.; Muszyński, S.; Kostro, K.; Jakubczak, A.; Taszkun, I.; Żmuda, A.; Rycerz, K.; Blicharski, T.; et al. Effects of maternal treatment with β-hydroxy-β-metylbutyrate and 2-oxoglutaric acid on femur development in offspring of minks of the standard dark brown type. J. Anim. Physiol. Anim. Nutr. 2018, 102, e299–e308. [Google Scholar] [CrossRef] [Green Version]
- Tomczyk-Warunek, A.; Blicharski, T.; Jarecki, J.; Dobrowolski, P.; Muszyński, S.; Tomaszewska, E.; Rovati, L.C. The effect of maternal HMB supplementation on bone mechanical and geometrical properties, as well as histomorphometry and immunolocalization of VEGF, TIMP2, MMP13, BMP2 in the bone and cartilage tissue of the humerus of their newborn piglets. PLoS ONE 2021, 16, e0240642. [Google Scholar] [CrossRef]
- Mizokami, A.; Kawakubo-Yasukochi, T.; Hirata, M. Osteocalcin and its endocrine functions. Biochem. Pharmacol. 2017, 132, 1–8. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Kuhne, C.A.; Viereck, V. The OPG/RANKL/RANK system in metabolic bone diseases. J. Musculoskelet. Neuronal Interact. 2004, 4, 268–475. [Google Scholar] [PubMed]
- Sasano, Y.; Zhu, J.Z.; Tsubota, M.; Takahashi, I.; Onodera, K.; Mizoguchi, I.; Kagayama, M. Gene expression of MMP8 and MMP13 during embryonic development of bone and cartilage in the rat mandible and hind limb. J. Histochem. Cytochem. 2002, 50, 325–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 purified diets for laboratory rodents: Final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the substantiation of health claims related to β-hydroxy β-methylbutyrate monohydrate (HMB) alone or in combination with α-ketoisocaproic acid (KIC) and reduction of muscle tissue damage during exercise (ID 1577, 1584), increase in lean body mass (ID 1579, 1582, 1583), increase in muscle strength (ID 1578, 1583, 1587), increase in endurance performance (ID 1580, 1581), skeletal muscle tissue repair (ID 1586) and faster recovery from muscle fatigue after exercise (ID 1576, 1585) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2227. [Google Scholar]
- Tatara, M.R.; Krupski, W.; Tymczyna, B.; Studziński, T. Effects of combined maternal administration with alpha-ketoglutarate (AKG) and β-hydroxy-β-methylbutyrate (HMB) on prenatal programming of skeletal properties in the offspring. Nutr. Metab. 2012, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Muszyński, S.; Tomczyk, A.; Dobrowolski, P.; Tomaszewska, E.; Hułas-Stasiak, M. Preliminary study of time dependent influence of maternal nutrition with addition of β-hydroxy- β-methlbutyrate on body weight and selected organs weight in the new born spiny mice (Acomys cahrinus) offspring. Electron. J. Pol. Agricult. Univ. 2016, 19, 08. [Google Scholar]
- Wilson, J.M.; Lowery, R.P.; Joy, J.M.; Andersen, J.C.; Wilson, S.M.C.; Stout, J.R.; Duncan, N.; Fuller, J.C.; Baier, S.M.; Naimo, M.A.; et al. The effects of 12 weeks of beta-hydroxy-beta-methylbutyrate free acid supplementation on muscle mass, strength, and power in resistance-trained individuals: A randomized, double-blind, placebo-controlled study. Eur. J. Appl. Physiol. 2014, 114, 1217–1227. [Google Scholar] [CrossRef] [Green Version]
- Świetlicka, I.; Arczewska, M.; Muszyński, S.; Tomaszewska, E.; Świetlicki, M.; Kuc, D.; Mielnik-Błaszczak, M.; Gołacki, K.; Cieślak, K. Surface analysis of etched enamel modified during the prenatal period. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 222, 117271. [Google Scholar] [CrossRef]
- Hułas-Stasiak, M.; Jakubowicz-Gil, J.; Dobrowolski, P.; Tomaszewska, E.; Muszyński, S. Maternal β-hydroxy-β-methylbutyrate (HMB) supplementation during pregnancy affects early folliculogenesis in the ovary of newborn piglets. Theriogenology 2019, 128, 91–100. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Hułas-Stasiak, M.; Tomczyk, A. Maternal nutrition with Β-hydroxy-Β-methylbutyrate as strong determinants of the development of newborn offspring in pigs. J. Neonat. Biol. 2015, 4, 3. [Google Scholar]
- Rudyk, H.; Tomaszewska, E.; Kotsyumbas, I.; Muszyński, S.; Tomczyk-Warunek, A.; Szymańczyk, S.; Dobrowolski, P.; Wiącek, D.; Kamiński, D.; Brezvyn, O. Bone homeostasis in experimental fumonisins intoxication of rats. Ann. Anim. Sci. 2019, 19, 403–419. [Google Scholar] [CrossRef] [Green Version]
- Tomaszewska, E.; Dobrowolski, P.; Puzio, I.; Donaldson, J.; Muszyński, S. Acrylamide-induced prenatal programming of bone structure in mammal model. Ann. Anim. Sci. 2020, 20, 1257–1287. [Google Scholar] [CrossRef]
- Hułas-Stasiak, M.; Jakubowicz-Gil, J.; Dobrowolski, P.; Grzesiak, M.; Muszyński, S.; Świątkiewicz, M.; Tomaszewska, E. Regulation of folliculogenesis by growth factors in piglet ovary exposed prenatally to β-hydroxy-β-methylbutyrate (HMB). Ann. Anim. Sci. 2020, 20, 899–917. [Google Scholar] [CrossRef]
- Kasacka, I.; Piotrowska, Ż.; Niezgoda, M.; Lewnadowska, A.; Łebkowski, W. Ageing-related changes in the levels of β-catenin, CacyBP/SIP, galectin-3 and immunoproteasome subunit LMP7 in the heart of men. PLoS ONE 2020, 15, e0229462. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordelieres, F.P.; Dougherty, R.; Jackson, J.S.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [Green Version]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, M.A. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [Green Version]
- Löfman, O.; Larsson, L.; Toss, G. Bone mineral density in diagnosis of osteoporosis: Reference population, definition of peak bone mass, and measured site determine prevalence. J. Clin. Densitom. 2000, 3, 177–186. [Google Scholar] [CrossRef]
- Sarli, M.; Hakim, C.; Rey, P.; Zanchetta, J. Osteoporosis during pregnancy and lactation. Report of eight cases. Medicina B. Aires 2005, 65, 489–494. [Google Scholar]
- Tuna, F.; Akleylek, C.; Özdemir, H.; Demirbağ Kabayel, D. Risk factors, fractures, and management of pregnancy-associated osteoporosis: A retrospective study of 14 Turkish patients. Gynecol. Endocrinol. 2020, 36, 238–242. [Google Scholar] [CrossRef]
- Hardcastle, S.A. Pregnancy and lactation associated osteoporosis. Calcif. Tissue Int. 2021. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hardcastle, S.A.; Yahya, F.; Bhalla, A.K. Pregnancy-associated osteoporosis: A UK case series and literature review. Osteoporos. Int. 2019, 30, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Kyvernitakis, I.; Reuter, T.C.; Hellmeyer, L.; Hars, O.; Hadji, P. Subsequent fracture risk of women with pregnancy and lactation-associated osteoporosis after a median of 6 years of follow-up. Osteoporos. Int. 2018, 29, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Anai, T.; Urata, K.; Mori, A.; Miyazaki, F.; Okamoto, S. Transient osteoporosis of the hip in pregnancy associated with generalized low bone mineral density-a case report. Gynecol. Obstet. Invest. 2013, 76, 133–138. [Google Scholar] [CrossRef]
- Bonacker, J.; Janousek, M.; Kröber, M. Pregnancy-associated osteoporosis with eight fractures in the vertebral column treated with kyphoplasty and bracing: A case report. Arch. Orthop. Trauma. Surg. 2014, 134, 173–179. [Google Scholar] [CrossRef]
- Tomaszewska, E.; Dobrowolski, P.; Kostro, K.; Jakubczak, A.; Taszkun, I.; Jaworska-Adamu, J.; Żmuda, A.; Rycerz, K.; Muszyński, K. Effect of HMB and 2-Ox administered during pregnancy on bone properties in primiparous and multiparous minks (Neivison vison). Bull. Vet. Inst. Pulawy 2015, 59, 563–568. [Google Scholar] [CrossRef] [Green Version]
- Rycerz, K.; Krawczyk, A.; Jaworska-Adamu, J.; Szelak, J.; Tomaszewska, E.; Dobrowolski, P. Influence of oral administration of HMB to pregnant dams on calbindin expression in the dentate gyrus of the hippocampus during postnatal development in spiny mice offspring. Med. Wet. 2017, 73, 341–345. [Google Scholar] [CrossRef] [Green Version]
- Dobrowolski, P.; Muszyński, S.; Donaldson, J.; Jakubczak, A.; Żmuda, A.; Taszkun, I.; Rycerz, K.; Mielnik-Błaszczak, M.; Kuc, D.; Tomaszewska, E. The effects of prenatal supplementation with β-hydroxy-β-methylbutyrate and/or alpha-ketoglutaric acid on the development and maturation of mink intestines are dependent on the number of pregnancies and the sex of the offspring. Animals 2021, 11, 1468. [Google Scholar] [CrossRef]
- Cohen, N.P.; Foster, R.J.; Mow, V.C. Composition and dynamics of articular cartilage: Structure, function, and maintaining healthy state. J. Orthop. Sports Phys. Ther. 1998, 28, 203–215. [Google Scholar] [CrossRef]
- Krajewska-Włodarczyk, M. Age-related modifications in articular cartilage. Geriatria 2017, 11, 135–141. [Google Scholar]
- Van der Meulen, M.C.H.; Jepsen, K.J.; Miki´c, B. Understanding bone strength: Size isn’t everything. Bone 2011, 29, 101–104. [Google Scholar] [CrossRef]
- Main, R.P.; Lynch, M.E.; Van der Meulen, M.C.H. In vivo tibial stiffness is maintained by whole bone morphology and crosssectional geometry in growing female mice. J. Biomech. 2010, 43, 2689–2694. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Bao, Q.; Chen, S.; Liu, H.; Feng, J.; Qin, H.; Li, A.; Liu, D.; Shen, Y.; Zhao, Y.; et al. Different bone remodeling levels of trabecular and cortical bone in response to changes in Wnt/β-catenin signaling in mice. J. Ortop. Res. 2017, 35, 812–819. [Google Scholar] [CrossRef]
- Wheater, G.; Elshahaly, M.; Tuck, S.P.; Datta, H.K.; van Laar, J.M. The clinical utility of bone marker measurements in osteoporosis. J. Transl. Med. 2013, 11, 201. [Google Scholar] [CrossRef] [Green Version]
- Li, L.J.; Zhang, J.; Gao, P.; Lv, F.; Song, Y.W.; Chang, X.Y.; Zhao, D.C.; Wang, O.; Jiang, Y.; Xing, X.P.; et al. Clinical characteristics and bisphosphonates treatment of rare pregnancy- and lactation-associated osteoporosis. Clin. Rheumatol. 2018, 37, 3141–3150. [Google Scholar] [CrossRef]
- Laroche, M.; Talibart, M.; Cormier, C.; Roux, C.; Guggenbuhl, P.; Degboe, Y. Pregnancy-related fractures: A retrospective study of a French cohort of 52 patients and review of the literature. Osteoporos. Int. 2017, 28, 3135–3142. [Google Scholar] [CrossRef]
- Grizzo, F.M.F.; da Silva Martins, J.; Pinheiro, M.M.; Jorgetti, V.; Carvalho, M.D.B.; Pellso, S.M. Pregnancy and lactation-associated osteoporosis: Bone histomorphometric analysis and response to treatment with zoledronic acid. Calcif. Tissue Int. 2015, 97, 421–425. [Google Scholar] [CrossRef]
- Dincel, V.E.; Sepici-Dincel, A. The importance and the differences of bone morphogenetic proteins for osteoporotic hip fractures. Acta Orthop. Belg. 2014, 80, 216–221. [Google Scholar]
- Zarrinkalam, M.R.; Schultz, C.G.; Ardern, D.W.; Vernon-Roberts, B.; Moore, R.J. Recombinant human bone morphogenetic protein-type 2 (rhBMP-2) enhances local bone formation in the lumbar spine of osteoporotic sheep. J. Orthop. Res. 2013, 31, 1390–1397. [Google Scholar] [CrossRef]
- Huntley, R.; Jensen, E.; Gopalakrishnan, R.; Mansky, K.C. Bone morphogenetic proteins: Their role in regulating osteoclast differentiation. Bone Rep. 2019, 10, 100207. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Dunstan, C.R.; Spelsberg, T.C.; Riggs, B.L.; Khosla, S. Osteoprotegerin production by human osteoblast lineage cells is stimulated by vitamin D, bone morphogenetic protein-2, and cytokines. Biochem. Biophys. Res. Commun. 1998, 250, 776–781. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone resorption by osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Prideaux, M.; Staines, K.A.; Jones, E.R.; Riley, G.P.; Pitsillides, A.A.; Farquharson, C. MMP and TIMP temporal gene expression during osteocytogenesis. Gene Expr. Patterns 2015, 18, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Pulling, O.; Weseloh, G.; Ronneberger, D.L.; Käkönen, A.; Swoboda, B. Chondrocyte differentiation in human osteoarthritis: Expression of osteocalcin in normal and osteoarthritic cartilage and bone. Calcif. Tissue Int. 2000, 67, 230–240. [Google Scholar] [CrossRef]
- Temenoff, J.S.; Mikos, A.G. Review: Tissue engineering for regeneration of articular cartilage. Biomaterials 2000, 21, 431–440. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomaszewska, E.; Muszyński, S.; Donaldson, J.; Dobrowolski, P.; Chand, D.K.P.; Tomczyk-Warunek, A.; Hułas-Stasiak, M.; Puzio, I.; Lamorski, K.; Sławiński, C.; et al. Femoral µCT Analysis, Mechanical Testing and Immunolocalization of Bone Proteins in β-Hydroxy β-Methylbutyrate (HMB) Supplemented Spiny Mouse in a Model of Pregnancy and Lactation-Associated Osteoporosis. J. Clin. Med. 2021, 10, 4808. https://doi.org/10.3390/jcm10214808
Tomaszewska E, Muszyński S, Donaldson J, Dobrowolski P, Chand DKP, Tomczyk-Warunek A, Hułas-Stasiak M, Puzio I, Lamorski K, Sławiński C, et al. Femoral µCT Analysis, Mechanical Testing and Immunolocalization of Bone Proteins in β-Hydroxy β-Methylbutyrate (HMB) Supplemented Spiny Mouse in a Model of Pregnancy and Lactation-Associated Osteoporosis. Journal of Clinical Medicine. 2021; 10(21):4808. https://doi.org/10.3390/jcm10214808
Chicago/Turabian StyleTomaszewska, Ewa, Siemowit Muszyński, Janine Donaldson, Piotr Dobrowolski, Deepesh K. P. Chand, Agnieszka Tomczyk-Warunek, Monika Hułas-Stasiak, Iwona Puzio, Krzysztof Lamorski, Cezary Sławiński, and et al. 2021. "Femoral µCT Analysis, Mechanical Testing and Immunolocalization of Bone Proteins in β-Hydroxy β-Methylbutyrate (HMB) Supplemented Spiny Mouse in a Model of Pregnancy and Lactation-Associated Osteoporosis" Journal of Clinical Medicine 10, no. 21: 4808. https://doi.org/10.3390/jcm10214808
APA StyleTomaszewska, E., Muszyński, S., Donaldson, J., Dobrowolski, P., Chand, D. K. P., Tomczyk-Warunek, A., Hułas-Stasiak, M., Puzio, I., Lamorski, K., Sławiński, C., Jabłoński, M., & Blicharski, T. (2021). Femoral µCT Analysis, Mechanical Testing and Immunolocalization of Bone Proteins in β-Hydroxy β-Methylbutyrate (HMB) Supplemented Spiny Mouse in a Model of Pregnancy and Lactation-Associated Osteoporosis. Journal of Clinical Medicine, 10(21), 4808. https://doi.org/10.3390/jcm10214808







