Minimally Invasive Surgery and Surgical Volume-Specific Survival and Perioperative Outcome: Unmet Need for Evidence in Gynecologic Malignancy
Abstract
1. Introduction
2. Materials and Methods
2.1. Approach for Systematic Literature Review
2.2. Article Retrieval
2.3. Study Selection
2.4. Data Extraction
2.5. Outcome Measures Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias of Included Studies
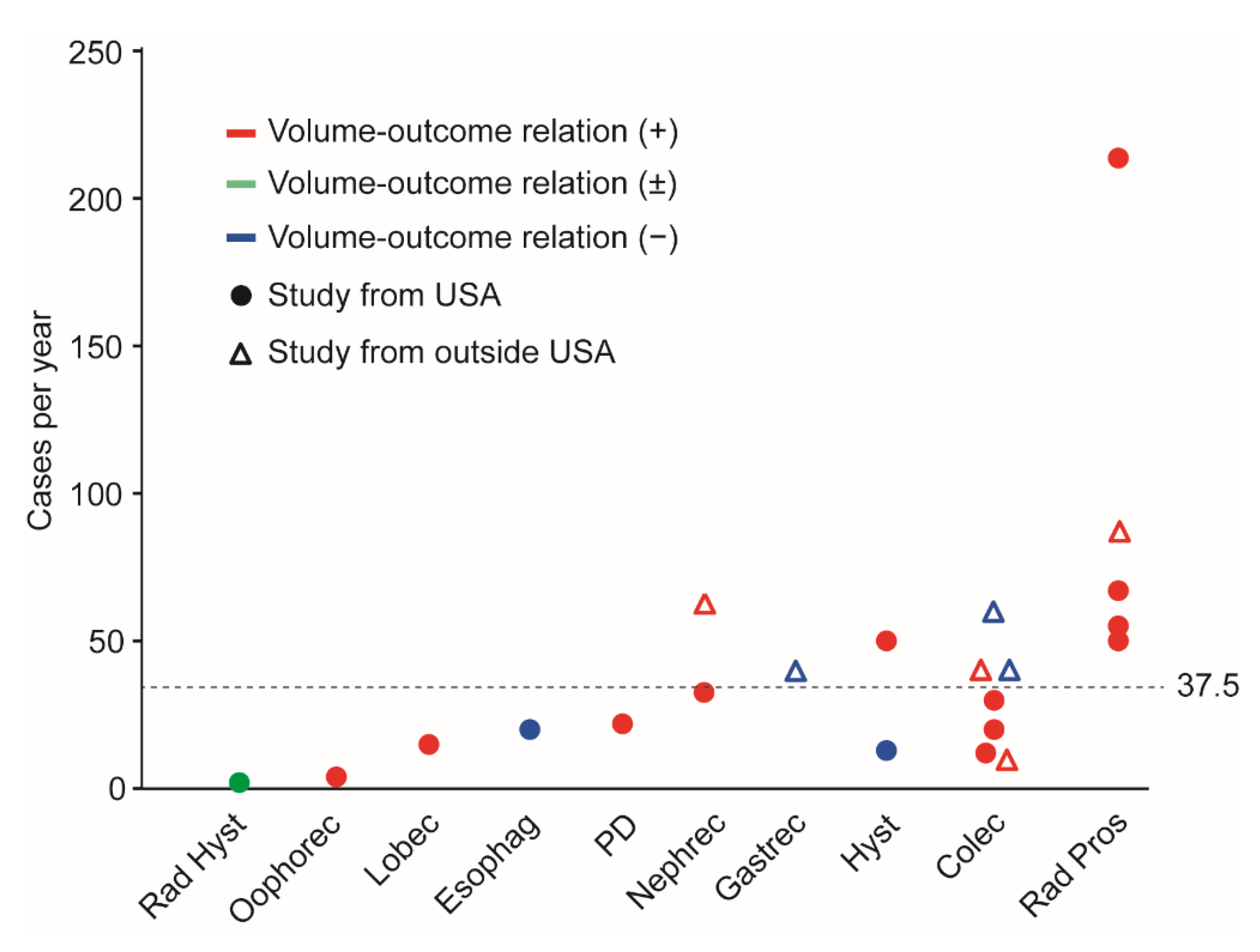
3.4. Definition of High-Volume Center
3.5. Perioperative Outcomes
3.6. Oncologic Outcomes
3.7. Robotic-Assisted Minimally Invasive Surgery
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Epstein, A.J.; Groeneveld, P.W.; Harhay, M.O.; Yang, F.; Polsky, D. Impact of minimally invasive surgery on medical spending and employee absenteeism. JAMA Surg. 2013, 148, 641–647. [Google Scholar] [CrossRef]
- Minig, L.; Achilarre, M.T.; Garbi, A.; Zanagnolo, V. Minimally Invasive Surgery to Treat Gynecological Cancer: Conventional Laparoscopy and/or Robot-Assisted Surgery. Int. J. Gynecol. Cancer 2017, 27, 562–574. [Google Scholar] [CrossRef]
- Kroft, J.; Li, Q.; Saskin, R.; Elit, L.; Bernardini, M.Q.; Gien, L.T. Trends over time in the use of laparoscopic hysterectomy for the treatment of endometrial cancer. Gynecol. Oncol. 2015, 138, 536–541. [Google Scholar] [CrossRef]
- Wright, J.D.; Hershman, D.L.; Burke, W.M.; Lu, Y.S.; Neugut, A.I.; Lewin, S.N.; Herzog, T.J. Influence of surgical volume on outcome for laparoscopic hysterectomy for endometrial cancer. Ann. Surg. Oncol. 2012, 19, 948–958. [Google Scholar] [CrossRef]
- Matsuo, K.; Chang, E.J.; Matsuzaki, S.; Mandelbaum, R.S.; Matsushima, K.; Grubbs, B.H.; Klar, M.; Roman, L.D.; Sood, A.K.; Wright, J.D. Minimally invasive surgery for early-stage ovarian cancer: Association between hospital surgical volume and short-term perioperative outcomes. Gynecol. Oncol. 2020, 158, 59–65. [Google Scholar] [CrossRef]
- Wright, J.D.; Ananth, C.V.; Tergas, A.I.; Herzog, T.J.; Burke, W.M.; Lewin, S.N.; Lu, Y.-S.; Neugut, A.I.; Hershman, D.L. An economic analysis of robotically assisted hysterectomy. Obstet. Gynecol. 2014, 123, 1038–1048. [Google Scholar] [CrossRef]
- Matsuo, K.; Matsuzaki, S.; Mandelbaum, R.S.; Chang, E.J.; Klar, M.; Matsushima, K.; Grubbs, B.H.; Roman, L.D.; Wright, J.D. Minimally invasive radical hysterectomy for early-stage cervical cancer: Volume-outcome relationship in the early experience period. Gynecol. Oncol. 2020, 158, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Nassour, I.; Choti, M.A.; Porembka, M.R.; Yopp, A.C.; Wang, S.C.; Polanco, P.M. Robotic-assisted versus laparoscopic pancreaticoduodenectomy: Oncological outcomes. Surg. Endosc. 2018, 32, 2907–2913. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Endo, M.; Ueda, Y.; Mimura, K.; Kakigano, A.; Egawa-Takata, T.; Kumasawa, K.; Yoshino, K.; Kimura, T. A case of acute Sheehan’s syndrome and literature review: A rare but life-threatening complication of postpartum hemorrhage. BMC Pregnancy Childbirth 2017, 17, 188. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Nagase, Y.; Ueda, Y.; Lee, M.; Matsuzaki, S.; Maeda, M.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Endo, M.; et al. The association of endometriosis with placenta previa and postpartum hemorrhage: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2021, 3, 100417. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Matsuzaki, S.; Chang, E.J.; Yasukawa, M.; Roman, L.D.; Matsuo, K. Surgical and oncologic outcomes of hyperthermic intraperitoneal chemotherapy for uterine leiomyosarcoma: A systematic review of literature. Gynecol. Oncol. 2021, 161, 70–77. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Jitsumori, M.; Hara, T.; Matsuzaki, S.; Nakagawa, S.; Miyake, T.; Takiuchi, T.; Kakigano, A.; Kobayashi, E.; Tomimatsu, T.; et al. Systematic review on the needle and suture types for uterine compression sutures: A literature review. BMC Surg. 2019, 19, 196. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- van Loveren, C.; Aartman, I.H. The PICO (Patient-Intervention-Comparison-Outcome) question. Ned. Tijdschr. Tandheelkd. 2007, 114, 172–178. [Google Scholar] [PubMed]
- Sterne, J.A.; Hernan, M.A.; Reeves, B.C.; Savovic, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Danna, S.M.; Graham, E.; Burns, R.J.; Deschenes, S.S.; Schmitz, N. Association between Depressive Symptoms and Cognitive Function in Persons with Diabetes Mellitus: A Systematic Review. PLoS ONE 2016, 11, e0160809. [Google Scholar] [CrossRef] [PubMed]
- ROBINS-I detailed guidance 2016. Available online: https://www.riskofbias.info/welcome/home/current-version-of-robins-i/robins-i-detailed-guidance-2016 (accessed on 16 September 2021).
- Matsuzaki, S.; Lee, M.; Nagase, Y.; Jitsumori, M.; Matsuzaki, S.; Maeda, M.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Ueda, Y.; et al. A systematic review and meta-analysis of obstetric and maternal outcomes after prior uterine artery embolization. Sci. Rep. 2021, 11, 16914. [Google Scholar] [CrossRef]
- Matsuzaki, S.; Nagase, Y.; Takiuchi, T.; Kakigano, A.; Mimura, K.; Lee, M.; Matsuzaki, S.; Ueda, Y.; Tomimatsu, T.; Endo, M.; et al. Antenatal diagnosis of placenta accreta spectrum after in vitro fertilization-embryo transfer: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 9205. [Google Scholar] [CrossRef]
- Concors, S.J.; Murken, D.R.; Hernandez, P.T.; Mahmoud, N.N.; Paulson, E.C. The volume-outcome relationship in robotic protectectomy: Does center volume matter? Results of a national cohort study. Surg. Endosc. 2020, 34, 4472–4480. [Google Scholar]
- Salfity, H.; Timsina, L.; Su, K.; Ceppa, D.; Birdas, T. Case Volume-to-Outcome Relationship in Minimally Invasive Esophagogastrectomy. Ann. Thorac. Surg. 2019, 108, 1491–1497. [Google Scholar] [CrossRef]
- Gietelink, L.; Henneman, D.; van Leersum, N.J.; de Noo, M.; Manusama, E.; Tanis, P.J.; Tollenar, R.A.E.M.; Wouters, M.W.J.M. The Influence of Hospital Volume on Circumferential Resection Margin Involvement: Results of the Dutch Surgical Colorectal Audit. Ann. Surg. 2016, 263, 745–750. [Google Scholar] [CrossRef]
- Murata, A.; Muramatsu, K.; Ichimiya, Y.; Kubo, T.; Fujino, Y.; Matsuda, S. Influence of hospital volume on outcomes of laparoscopic gastrectomy for gastric cancer in patients with comorbidity in Japan. Asian J. Surg. 2015, 38, 33–39. [Google Scholar] [CrossRef]
- Zheng, Z.; Hanna, N.; Onukwugha, E.; Bikov, K.A.; Mullins, C.D. Hospital center effect for laparoscopic colectomy among elderly stage I-III colon cancer patients. Ann. Surg. 2014, 259, 924–929. [Google Scholar] [CrossRef]
- Keller, D.S.; Hashemi, L.; Lu, M.; Delaney, C.P. Short-term outcomes for robotic colorectal surgery by provider volume. J. Am. Coll. Surg. 2013, 217, 1063–1069. [Google Scholar] [CrossRef]
- Kuwabara, K.; Matsuda, S.; Fushimi, K.; Ishikawa, K.B.; Horiguchi, H.; Fujimori, K. Impact of hospital case volume on the quality of laparoscopic colectomy in Japan. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2009, 13, 1619–1626. [Google Scholar] [CrossRef]
- Yasunaga, H.; Matsuyama, Y.; Ohe, K. Effects of hospital and surgeon volumes on operating times, postoperative complications, and length of stay following laparoscopic colectomy. Surg. Today 2009, 39, 955–961. [Google Scholar] [CrossRef]
- Kuhry, E.; Bonjer, H.J.; Haglind, E.; Hop, W.C.; Veldkamp, R.; Cuesta, M.A.; Msika, S.; Morino, M.; Lacy, A.M. Impact of hospital case volume on short-term outcome after laparoscopic operation for colonic cancer. Surg. Endosc. 2005, 19, 687–692. [Google Scholar] [PubMed]
- Adam, M.A.; Thomas, S.; Youngwirth, L.; Pappas, T.; Roman, S.A.; Sosa, J.A. Defining a Hospital Volume Threshold for Minimally Invasive Pancreaticoduodenectomy in the United States. JAMA Surg. 2017, 152, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Sperling, C.D.; Taylor, B.L.; Talwar, R.; Chelluri, R.R.; Raman, J.D.; Lee, D.J.; Lee, D.I.; Guzzo, T.J. Associations between Hospital Volume and Outcomes of Robot-Assisted Radical Prostatectomy. J. Urol. 2020, 203, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Peyronnet, B.; Tondut, L.; Bernhard, J.C.; Vaessen, C.; Doumerc, N.; Sebe, P.; Pradere, B.; Guillonneau, B.; Khene, Z.-E.; Nouhand, F.-X.; et al. Impact of hospital volume and surgeon volume on robot-assisted partial nephrectomy outcomes: A multicentre study. BJU Int. 2018, 121, 916–922. [Google Scholar] [CrossRef] [PubMed]
- Weiner, A.B.; Murthy, P.; Richards, K.A.; Patel, S.G.; Eggener, S.E. Population based analysis of incidence and predictors of open conversion during minimally invasive radical prostatectomy. J. Urol. 2015, 193, 826–831. [Google Scholar] [CrossRef] [PubMed]
- Monn, M.F.; Bahler, C.D.; Flack, C.K.; Dube, H.T.; Sundaram, C.P. The impact of hospital volume on postoperative complications following robot-assisted partial nephrectomy. J. Endourol. 2014, 28, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Hyams, E.S.; Mullins, J.K.; Pierorazio, P.M.; Partin, A.W.; Allaf, M.E.; Matlaga, B.R. Impact of robotic technique and surgical volume on the cost of radical prostatectomy. J. Endourol. 2013, 27, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.Y.; Hevelone, N.D.; Lipsitz, S.R.; Kowalczyk, K.J.; Nguyen, P.L.; Hu, J.C. Hospital volume, utilization, costs and outcomes of robot-assisted laparoscopic radical prostatectomy. J. Urol. 2012, 187, 1632–1637. [Google Scholar] [CrossRef] [PubMed]
- Budaus, L.; Morgan, M.; Abdollah, F.; Zorn, K.C.; Sun, M.; Johal, R.; Thuret, R.; Abdo, A.; Schmitges, J.; Isbarn, H.; et al. Impact of annual surgical volume on length of stay in patients undergoing minimally invasive prostatectomy: A population-based study. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2011, 37, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Tchouta, L.N.; Park, H.S.; Boffa, D.J.; Blasberg, J.D.; Detterbeck, F.C.; Kim, A.W. Hospital Volume and Outcomes of Robot-Assisted Lobectomies. Chest 2017, 151, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, E.W.; Michler, R.E. An Overview of the Intuitive System: The Surgeon’s Perspective. Oper. Tech. Thorac. Cardiovasc. Surg. 2001, 6, 170–176. [Google Scholar] [CrossRef]
- Has the da Vinci Surgical System been cleared by the FDA? Available online: https://www.marinahospital.com/faq/has-the-da-vinci-surgical-system-been-cleared-by-the-fda (accessed on 8 July 2020).
- Matsuo, K.; Matsuzaki, S.; Mandelbaum, R.S.; Matsushima, K.; Klar, M.; Grubbs, B.H.; Roman, L.D.; Wright, J.D. Hospital surgical volume and perioperative mortality of pelvic exenteration for gynecologic malignancies. J. Surg. Oncol. 2020, 121, 402–409. [Google Scholar] [CrossRef]
- Khuri, S.F.; Daley, J.; Henderson, W.; Hur, K.; Hossain, M.; Soybel, D.; Kizer, K.W.; Aust, J.B.; Bell, R.H., Jr.; Chong, V.; et al. Relation of surgical volume to outcome in eight common operations: Results from the VA National Surgical Quality Improvement Program. Ann. Surg. 1999, 230, 414–429; discussion 429–432. [Google Scholar] [CrossRef]
- Matsuo, K.; Shimada, M.; Yamaguchi, S.; Matoda, M.; Nakanishi, T.; Kikkawa, F.; Ohmichi, M.; Okamoto, A.; Sugiyama, T.; Mikami, M.; et al. Association of Radical Hysterectomy Surgical Volume and Survival for Early-Stage Cervical Cancer. Obstet. Gynecol. 2019, 133, 1086–1098. [Google Scholar] [CrossRef]
- Ramirez, P.T.; Frumovitz, M.; Pareja, R.; Lopez, A.; Vieira, M.; Ribeiro, R.; Buda, A.; Yan, X.; Shuzhong, Y.; Chetty, N.; et al. Minimally Invasive versus Abdominal Radical Hysterectomy for Cervical Cancer. N. Engl. J. Med. 2018, 379, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- Caution When using robotically-assisted Surgical Devices in Women’s Health including Mastectomy and Other Cancer-Related Surgeries: FDA Safety Communication. Available online: https://www.fda.gov/medical-devices/safety-communications/caution-when-using-robotically-assisted-surgical-devices-womens-health-including-mastectomy (accessed on 5 August 2020).
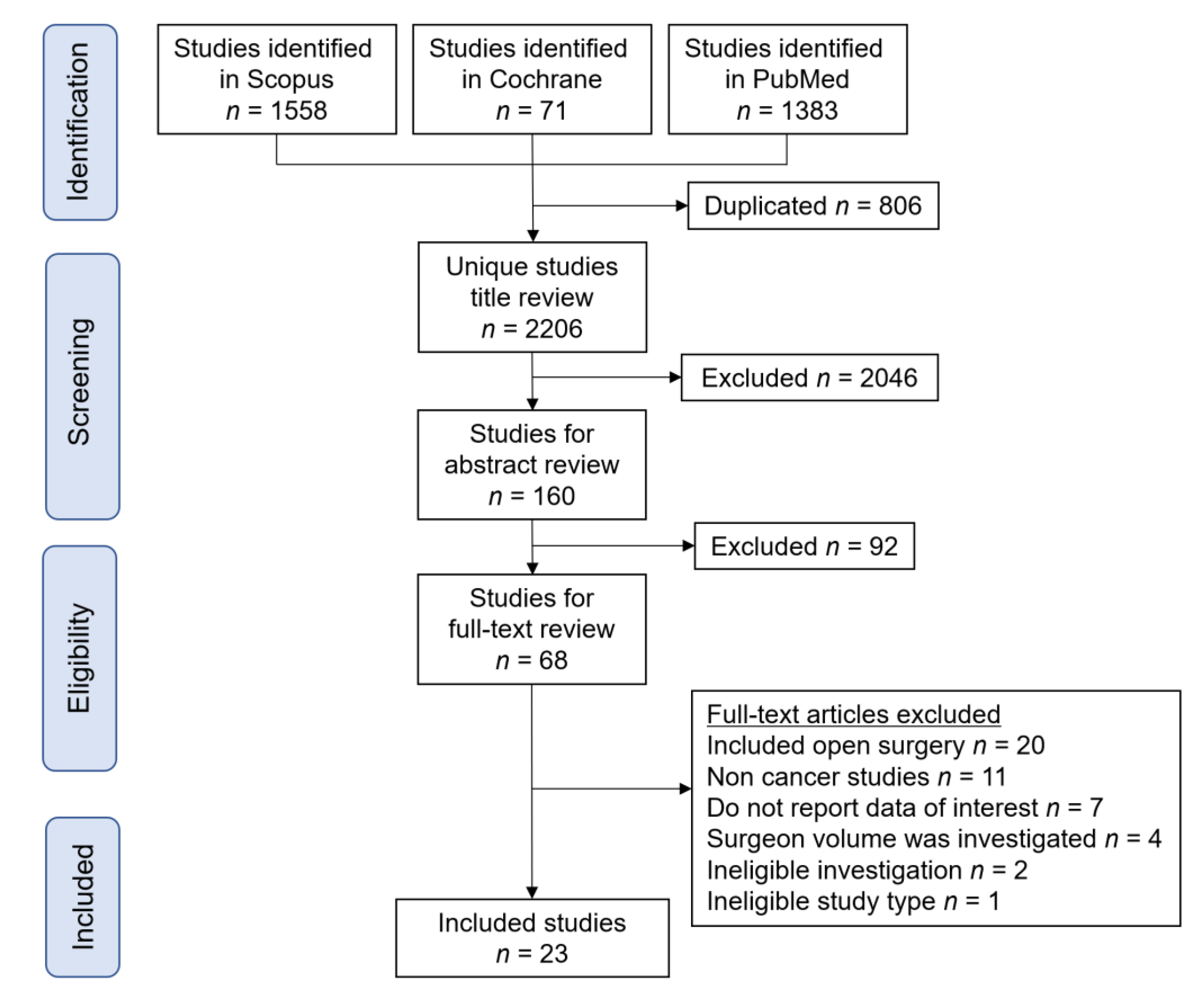

| Author | Year | Study Period | Category | Cancer Type | Surgery Type | HV def (/yr) | HV Classification |
|---|---|---|---|---|---|---|---|
| Matsuo K [5] | 2020 | 2001–2011 | GYN | Ovarian Ca | Oophorectomy | >2 | 90%ile |
| Matsuo K [7] | 2020 | 2007–2011 | GYN | Cervical Ca | Radical hysterectomy | >4 | 90%ile |
| Wright JD [6] | 2014 | 2006–2012 | GYN | EM Ca | Hysterectomy | >50 | Random |
| Wright JD [4] | 2012 | 2000–2010 | GYN | EM Ca | Hysterectomy | >12.8 | Top 3rd |
| Concors SJ [20] | 2019 | 2010–2015 | GI | Colorectal Ca | Colectomy | ≥12 | 90%ile |
| Gietelink L [22] | 2016 | 2011–2012 | GI | Colorectal Ca | Colectomy | ≥40 | Random |
| Zheng Z [24] | 2014 | 2003–2007 | GI | Colorectal Ca | Colectomy | ≥30 | QT1 |
| Keller DS [25] | 2013 | 2010–2012 | GI | NA | Colectomy | >20 | Random |
| Kuwabara K [26] | 2009 | 2007 | GI | Colorectal Ca | Colectomy | ≥60 | Random |
| Yasunaga H [27] | 2009 | 2006–2007 | GI | Colorectal Ca | Colectomy | ≥40 | Random |
| Kuhry E [28] | 2005 | 1997–2003 | GI | Colorectal Ca | Colectomy | ≥10 | Random |
| Murata A [23] | 2015 | 2009–2011 | GI | Gastric Ca | Gastrectomy | ≥40 | Random |
| Salfity H [21] | 2019 | 2010–2013 | GI | Esophag Ca | Esophagectomy | ≥20 | QT1 |
| Nassour I [8] | 2018 | 2010–2013 | HPB | Pancreas Ca | PD | NA | ¶ |
| Adam MA [29] | 2017 | 2000–2012 | HPB | Pancreas Ca | PD | >22 | RCSs * |
| Xia L [30] | 2020 | 2010–2014 | GU | Prostate Ca | Radical prostatectomy | ≥219 | Random |
| Weiner AB [32] | 2015 | 2010–2011 | GU | Prostate Ca | Radical prostatectomy | >72 | QT1 |
| Hyams ES [34] | 2013 | 2008–2011 | GU | NA | Radical prostatectomy | >60 | Random |
| Yu HY [35] | 2012 | 2008 | GU | Prostate Ca | Radical prostatectomy | ≥55 | QT1 |
| Budäus L [36] | 2011 | 2005–2008 | GU | Prostate Ca | Radical prostatectomy | ≥92 | Random |
| Peyronnet B [31] | 2018 | 2009–2015 | GU | Renal Ca | Partial nephrectomy | >70 | QT1 |
| Monn MF [33] | 2014 | 2009–2011 | GU | Renal tumor | Partial nephrectomy | ≥35 | Top 3rd |
| Tchouta LN [37] | 2017 | 2008–2013 | Other | Lung (NA) | Lobectomy | ≥15 | QT1 |
| Surgery Type | Category | Robotic | Author | Year | No. | HV (/yr) | Surgical Outcome | Oncologic Outcome |
|---|---|---|---|---|---|---|---|---|
| Volume–outcome relationship, observed | ||||||||
| Oophorectomy | GYN | - | Matsuo [5] | 2020 | 4822 | >4 | ↓complication | -- |
| PD | HPB | Yes * | Nassour [8] | 2018 | 1623 | ¶ | -- | ↑3-year OS |
| HPB | Yes * | Adam [29] | 2017 | 865 | >22 | ↓complication | -- | |
| Radical prostatectomy | GU | Yes | Xia [30] | 2020 | 114,957 | ≥219 | ↓length of stay, ↓PSM | -- |
| GU | Yes * | Weiner [32] | 2015 | 87,415 | >72 | ↓lap conversion | -- | |
| GU | Yes | Hyams [34] | 2013 | 1489 | >60 | ↓surgical cost | -- | |
| GU | Yes | Yu [35] | 2012 | 2348 | ≥55 | ↓complication | -- | |
| GU | - | Budäus [36] | 2011 | 2108 | ≥92 | ↓length of stay | -- | |
| Nephrectomy ** | GU | Yes | Peyronnet [31] | 2018 | 1222 | >70 | ↓complication, ↓PSM | -- |
| GU | Yes | Monn [33] | 2014 | 17,583 | ≥35 | ↓complication | -- | |
| Lobectomy | Other | Yes | Tchouta [37] | 2017 | 8253 | ≥15 | ↓hospital mortality | -- |
| Volume–outcome relationship, inconsistent | ||||||||
| RH | GYN | Yes * | Matsuo [7] | 2020 | 2202 | >2 | LSC: ↓complication | -- |
| Robotic: no association | -- | |||||||
| Hysterectomy | GYN | Yes * | Wright [6] | 2014 | 10,906 | >50 | ↓cost ‡ | -- |
| GYN | - | Wright [4] | 2012 | 4137 | >12.8 | No association | -- | |
| Colectomy | GI | Yes | Concors [20] | 2019 | 8107 | ≥12 | ↓lap conversion, ↓PSM | -- |
| GI | - | Gietelink [22] | 2016 | 5161 | ≥40 | ↓PSM | -- | |
| GI | - | Zheng [24] | 2014 | 4617 | ≥30 | ↓length of stay, ↓hospital mortality | -- | |
| GI | Yes | Keller [25] | 2013 | 1428 | >20 | ↓complication | -- | |
| GI | - | Kuwabara [26] | 2009 | 3765 | ≥60 | No association | -- | |
| GI | - | Yasunaga [27] | 2009 | 1212 | ≥40 | Complication: no association | -- | |
| GI | - | Kuhry [28] | 2005 | 627 | ≥10 | ↓resp complication | -- | |
| Volume–outcome relationship, not observed | ||||||||
| Gastrectomy | GI | - | Murata [23] | 2015 | 5941 | ≥40 | Complication: no association | -- |
| Esophagectomy | GI | No | Salfity [21] | 2019 | 2371 | ≥20 | Mortality: no association | No associationfor OS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsuzaki, S.; Klar, M.; Chang, E.J.; Matsuzaki, S.; Maeda, M.; Zhang, R.H.; Roman, L.D.; Matsuo, K. Minimally Invasive Surgery and Surgical Volume-Specific Survival and Perioperative Outcome: Unmet Need for Evidence in Gynecologic Malignancy. J. Clin. Med. 2021, 10, 4787. https://doi.org/10.3390/jcm10204787
Matsuzaki S, Klar M, Chang EJ, Matsuzaki S, Maeda M, Zhang RH, Roman LD, Matsuo K. Minimally Invasive Surgery and Surgical Volume-Specific Survival and Perioperative Outcome: Unmet Need for Evidence in Gynecologic Malignancy. Journal of Clinical Medicine. 2021; 10(20):4787. https://doi.org/10.3390/jcm10204787
Chicago/Turabian StyleMatsuzaki, Shinya, Maximilian Klar, Erica J. Chang, Satoko Matsuzaki, Michihide Maeda, Renee H. Zhang, Lynda D. Roman, and Koji Matsuo. 2021. "Minimally Invasive Surgery and Surgical Volume-Specific Survival and Perioperative Outcome: Unmet Need for Evidence in Gynecologic Malignancy" Journal of Clinical Medicine 10, no. 20: 4787. https://doi.org/10.3390/jcm10204787
APA StyleMatsuzaki, S., Klar, M., Chang, E. J., Matsuzaki, S., Maeda, M., Zhang, R. H., Roman, L. D., & Matsuo, K. (2021). Minimally Invasive Surgery and Surgical Volume-Specific Survival and Perioperative Outcome: Unmet Need for Evidence in Gynecologic Malignancy. Journal of Clinical Medicine, 10(20), 4787. https://doi.org/10.3390/jcm10204787







