Shared Care and Virtual Clinics for Glaucoma in a Hospital Setting
Abstract
1. Introduction
2. Methods
2.1. Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Terminology
2.3.1. Glaucoma Subtypes
2.3.2. HCP Working in the Clinic
2.3.3. Organization of the Clinic
3. Results of Shared Care Studies
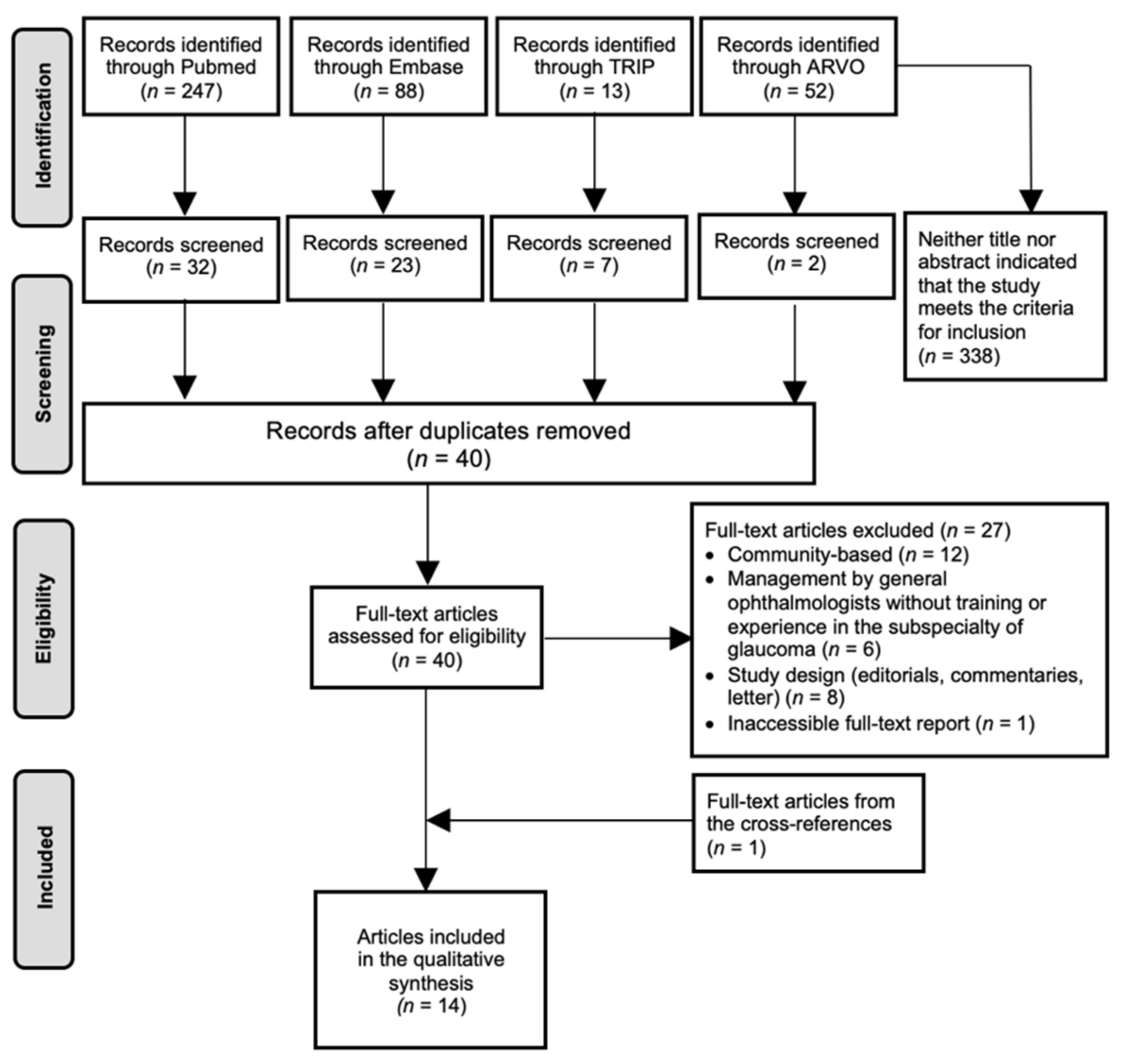
3.1. Study Selection
3.2. Description of the Included Articles
3.2.1. Recommendations for Shared Glaucoma Care
3.2.2. Implementation and Performance of SC Clinics
3.3. The Organization: Implementing SC for Glaucoma
3.3.1. The Role of the GE
3.3.2. The Role of the Non-Medical Staff
3.3.3. Patient Characteristics
- Unsuitable
- Suitable
- Back-referral
3.4. Impact on Glaucoma Care
3.4.1. Quality of Care (QoC)
Performance of the Non-Medical Staff
- Completeness of data collection:
- Accuracy of data collection:
- Management decisions:
Performance of the SC Clinic
- Completeness of data collection:
- Accuracy of data collection:
- Management decisions:
3.4.2. Acceptance
Patients
Staff
3.4.3. Productivity
4. Results of Virtual Clinics’ Studies
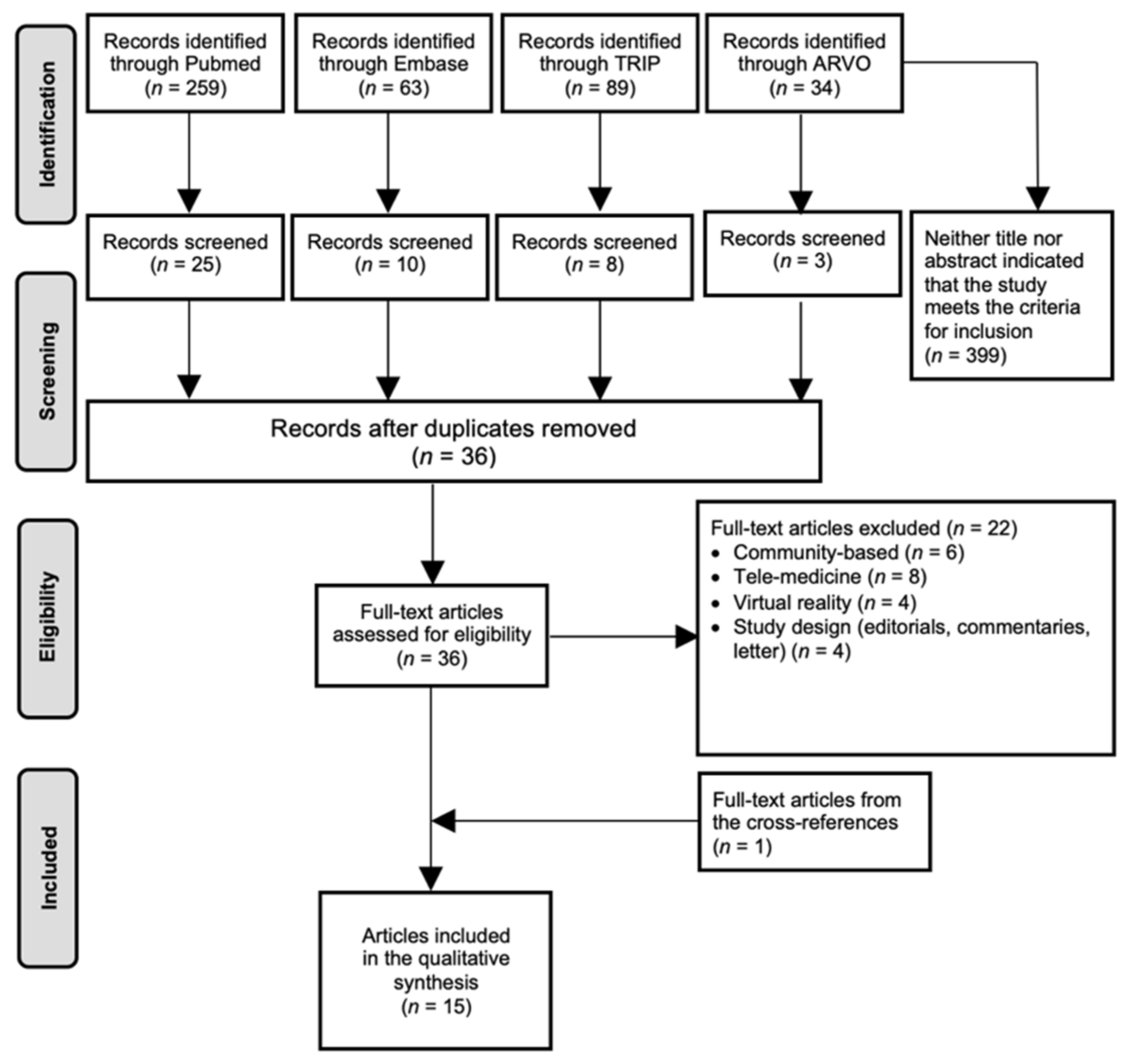
4.1. Literature Search
4.2. Description of the Included Articles
4.3. The Organization: Implementing a VC for Glaucoma
4.3.1. Role of the Staff
4.3.2. Initial Assessment
4.3.3. Follow-Up
4.3.4. Patient Suitability
New Patients
- Suitable:
- Unsuitable:
Follow-Up Patients
- Suitable:
- Unsuitable:
4.4. Impact on Glaucoma Care
4.4.1. QoC
New Patients (GSC and GAC)
Follow-Up Patients (GCC, SMS, VC-PAEP, VC-MREH and VC-BEH, VC-REIP)
4.4.2. Acceptance
Patients
Staff
4.4.3. Productivity
5. Discussion
5.1. Application of SC/VC
5.2. Generalizability to Other Hospitals/Countries
5.3. Skills of the Non-Medical Staff
5.4. Suitable Patients
5.5. Pathway of a High-Risk Patient
5.6. Compliance to Guidelines
5.7. Data Interpretation: Importance of Training
5.8. Quality of Management Decisions
5.9. Acceptance
5.10. Productivity
5.11. Directions for Future Research
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Conlon, R.; Saheb, H.; Ahmed, I.I. Glaucoma treatment trends: A review. Can. J. Ophthalmol. 2017, 52, 114–124. [Google Scholar] [CrossRef]
- Hitchings, R. Shared care for glaucoma. Br. J. Ophthalmol. 1995, 79, 626. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.E.; Klein, R.; Sponsel, W.E.; Franke, T.; Cantor, L.B.; Martone, J.; Menage, M.J. Prevalence of glaucoma. The Beaver Dam Eye Study. Ophthalmology 1992, 99, 1499–1504. [Google Scholar] [CrossRef]
- Constable, I.J.; Yogesan, K.; Eikelboom, R.; Barry, C.; Cuypers, M. Fred Hollows lecture: Digital screening for eye disease. Clin. Exp. Ophthalmol. 2000, 28, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Wollstein, G.; Garway-Heath, D.F.; Fontana, L.; Hitchings, R.A. Identifying early glaucomatous changes. Comparison between expert clinical assessment of optic disc photographs and confocal scanning ophthalmoscopy. Ophthalmology 2000, 107, 2272–2277. [Google Scholar] [CrossRef]
- De Silva, S.R.; Riaz, Y.; Purbrick, R.M.; Salmon, J.F. There is a trend for the diagnosis of glaucoma to be made at an earlier stage in 2010 compared to 2008 in Oxford, United Kingdom. Ophthalmic Physiol. Opt. 2013, 33, 179–182. [Google Scholar] [CrossRef]
- National Collaborating Centre for Acute Care; National Institute for Health and Clinical Excellence. Guidance. Glaucoma: Diagnosis and Management of Chronic Open Angle Glaucoma and Ocular Hypertension; National Collaborating Centre for Acute Care: London, UK, 2009.
- Ratnarajan, G.; Newsom, W.; Vernon, S.A.; Fenerty, C.; Henson, D.; Spencer, F.; Wang, Y.; Harper, R.; McNaught, A.; Collins, L.; et al. The effectiveness of schemes that refine referrals between primary and secondary care-the UK experience with glaucoma referrals: The Health Innovation & Education Cluster (HIEC) Glaucoma Pathways Project. BMJ Open 2013, 3, e002715. [Google Scholar]
- Ratnarajan, G.; Newsom, W.; French, K.; Kean, J.; Chang, L.; Parker, M.; Garway-Heath, D.F.; Bourne, R.R. The impact of glaucoma referral refinement criteria on referral to, and first-visit discharge rates from, the hospital eye service: The Health Innovation & Education Cluster (HIEC) Glaucoma Pathways project. Ophthalmic Physiol. Opt. 2013, 33, 183–189. [Google Scholar]
- Chalk, D.; Smith, M. Guidelines on glaucoma and the demand for services. Br. J. Health Care Manag. 2013, 19, 476–481. [Google Scholar] [CrossRef]
- Smith, R. Our Ophthalmology Service is ‘Failing’, Please Help! Available online: https://www.rcophth.ac.uk/2013/08/our-ophthalmology-service-is-failingplease-help/ (accessed on 30 July 2021).
- Resnikoff, S.; Felch, W.; Gauthier, T.M.; Spivey, B. The number of ophthalmologists in practice and training worldwide: A growing gap despite more than 200,000 practitioners. Br. J. Ophthalmol. 2012, 96, 783–787. [Google Scholar] [CrossRef]
- NPSA. Rapid Response Report NPSA/2009/RRR004: Preventing Delay to Follow Up for Patients with Glaucoma; National Patient Safety Agency: London, UK, 2009.
- Tuck, M.W.; Crick, R.P. Efficiency of referral for suspected glaucoma. BMJ 1991, 302, 998–1000. [Google Scholar] [CrossRef]
- OECD. OECD Health Policy Studies Value for Money in Health Spending; OECD Publishing: Paris, France, 2010. [Google Scholar]
- Morley, A.M.; Murdoch, I. The future of glaucoma clinics. Br. J. Ophthalmol. 2006, 90, 640–645. [Google Scholar] [CrossRef] [PubMed]
- Integrated care for asthma: A clinical, social, and economic evaluation. Grampian Asthma Study of Integrated Care (GRASSIC). BMJ 1994, 308, 559–564.
- Serrano, V.; Rodriguez-Gutierrez, R.; Hargraves, I.; Gionfriddo, M.R.; Tamhane, S.; Montori, V.M. Shared decision-making in the care of individuals with diabetes. Diabet. Med. 2016, 33, 742–751. [Google Scholar] [CrossRef]
- Congalton, A.T.; Oakley, A.M.; Rademaker, M.; Bramley, D.; Martin, R.C. Successful melanoma triage by a virtual lesion clinic (teledermatoscopy). J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2423–2428. [Google Scholar] [CrossRef]
- Harnett, P.; Jones, M.; Almond, M.; Ballasubramaniam, G.; Kunnath, V. A virtual clinic to improve long-term outcomes in chronic kidney disease. Clin. Med. 2018, 18, 356–363. [Google Scholar] [CrossRef]
- Khouri, A.S.; Fechtner, R.D.; Shaarawy, T.M.; Sherwood, M.B.; Hitchings, R.A.; Crowston, J.G. Primary Open-Angle Glaucoma, 2nd ed.; Elsevier/Saunders: London, UK, 2015; Volume 29, pp. 333–345. [Google Scholar]
- Quigley, H.A.; Broman, A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br. J. Ophthalmol. 2006, 90, 262–267. [Google Scholar] [CrossRef]
- Van Melkebeke, L.; Barbosa-Breda, J.; Huygens, M.; Stalmans, I. Optical coherence tomography angiography in glaucoma: A review. Ophthalmic Res. 2018, 60, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Pitha, I.F.; Kass, M.A. Ocular Hypertension. In Glaucoma; Elsevier/Saunders: Philadelphia, PA, USA, 2015; Volume 28, pp. 325–332. Available online: https://www.sciencedirect.com/science/article/pii/B9780702051937000285?via%3Dihub (accessed on 30 July 2021).
- Sunaric-Mégevand, G.; Aclimandos, W.; Creuzot-Garcher, C.; Traverso, C.E.; Tuulonen, A.; Hitchings, R.; Mathysen, D.G. Can ‘Fellow of the European Board of ophthalmology Subspecialty Diploma in Glaucoma’, a subspecialty examination on glaucoma induce the qualification standard of glaucoma clinical practice in Europe? J. Educ. Eval. Health Prof. 2016, 13, 28. [Google Scholar] [CrossRef] [PubMed]
- The Royal College of Ophthalmologists. So You Want to Be an Ophthalmologist? A Short Guide in Ophthalmology in the UK. Available online: https://www.rcophth.ac.uk/wp-content/uploads/2014/07/RCOphth-Ophthalmology-Career-Feb2017.pdf (accessed on 30 July 2021).
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed]
- White, A.; Goldberg, I.; Australian and New Zealand Glaucoma Interest Group and the Royal Australian and New Zealand College of Ophthalmologists. Guidelines for the collaborative care of glaucoma patients and suspects by ophthalmologists and optometrists in Australia. Clin. Exp. Ophthalmol. 2014, 42, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Canadian Glaucoma Society Committee on Interprofessional Collaboration in Glaucoma Care. Model of interprofessional collaboration in the care of glaucoma patients and glaucoma suspects. Can. J. Ophthalmol. 2011, 46, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- NHMRC Guidelines for the screening, prognosis, diagnosis, management and prevention of glaucoma. Available online: https://www.nhmrc.gov.au/sites/default/files/2018-10/cp113_glaucoma_120404.pdf (accessed on 30 July 2021).
- Canadian Ophthalmological Society Glaucoma Clinical Practice Guideline Expert Committee; Canadian Ophthalmological Society. Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of glaucoma in the adult eye. Can. J. Ophthalmol. 2009, 44, S7–S93. [Google Scholar] [CrossRef]
- Damji, K.F.; Behki, R.; Wang, L.; Target IOP Workshop participants. Canadian perspectives in glaucoma management: Setting target intraocular pressure range. Can. J. Ophthalmol. 2003, 38, 189–197. [Google Scholar] [CrossRef]
- Shah, S.M.; Choo, C.; Odden, J.; Zhao, B.; Fang, C.; Schornack, M.; Stalboerger, G.; Bennett, J.R.; Khanna, C.L. Provider agreement in the assessment of glaucoma progression within a team model. J. Glaucoma 2018, 27, 691–698. [Google Scholar] [CrossRef] [PubMed]
- Damento, G.M.; Winkler, N.S.; Hodge, D.O.; Khanna, S.S.; Khanna, C.L. Healthcare utilization by glaucoma patients in a team care model. Semin. Ophthalmol. 2018, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Winkler, N.S.; Damento, G.M.; Khanna, S.S.; Hodge, D.O.; Khanna, C.L. Analysis of a physician-led, team-based care model for the treatment of glaucoma. J. Glaucoma 2017, 26, 702–707. [Google Scholar] [CrossRef]
- Holtzer-Goor, K.M.; Van Vliet, E.J.; Van Sprundel, E.; Plochg, T.; Koopmanschap, M.A.; Klazinga, N.S.; Lemij, H.G. Shared care in monitoring stable glaucoma patients: A randomized controlled trial. J. Glaucoma 2016, 25, e392–e400. [Google Scholar] [CrossRef]
- Holtzer-Goor, K.M.; Klazinga, N.S.; Koopmanschap, M.A.; Lemij, H.G.; Plochg, T.; Van Sprundel, E. Monitoring of Stable Glaucoma Patients: Evaluation of the Effectiveness and Efficiency of a Glaucoma Follow-up Unit, Staffed by Nonphysician Health Care Professionals, as an Intermediate Step Towards Glaucoma Monitoring in Primary Care; Erasmus University Rotterdam: Rotterdam, The Netherlands, 2010. [Google Scholar]
- Holtzer-Goor, K.M.; van Sprundel, E.; Lemij, H.G.; Plochg, T.; Klazinga, N.S.; Koopmanschap, M.A. Cost-effectiveness of monitoring glaucoma patients in shared care: An economic evaluation alongside a randomized controlled trial. BMC Health Serv. Res. 2010, 10, 312. [Google Scholar] [CrossRef]
- Ho, S.; Vernon, S.A. Decision making in chronic glaucoma-optometrists vs ophthalmologists in a shared care service. Ophthalmic Physiol. Opt. 2011, 31, 168–173. [Google Scholar] [CrossRef]
- Banes, M.J.; Culham, L.E.; Crowston, J.G.; Bunce, C.; Khaw, P.T. An optometrist’s role of co-management in a hospital glaucoma clinic. Ophthalmic Physiol. Opt. 2000, 20, 351–359. [Google Scholar] [CrossRef]
- Bentley, S.A.; Green, C.; Malesic, L.; Siggins, T.; Escott, C.; O’Keefe, M.; Clarke, C. Establishing a collaborative model of glaucoma care in an Australian public hospital setting. Invest. Ophthalmol. Vis. Sci. 2019, 60, 1020. [Google Scholar]
- Banes, M.J.; Culham, L.E.; Bunce, C.; Xing, W.; Viswanathan, A.; Garway-Heath, D. Agreement between optometrists and ophthalmologists on clinical management decisions for patients with glaucoma. Br. J. Ophthalmol. 2006, 90, 579–585. [Google Scholar] [CrossRef]
- Bhota, V.; Taylor, S.; Benefield, J.; Ah-Cha, J. Approach to collaborative glaucoma care in New Zealand: An update. Clin. Exp. Ophthalmol. 2019, 47, 798–799. [Google Scholar]
- Phu, J.; Wang, H.; Khuu, S.; Zangerl, B.; Hennessy, M.; Masselos, K.; Kallioniatis, M. Anterior Chamber Angle Evaluation: Consistency and Agreement between Optometrists and Ophthalmologists. Optom. Vis. Sci. 2019, 96, 751–760. [Google Scholar] [CrossRef]
- Prum, B.E., Jr.; Rosenberg, L.F.; Gedde, S.J.; Mansberger, S.L.; Stein, J.D.; Moroi, S.E.; Herndon, L.W.; Lim, M.C.; Williams, R.D. Primary open-angle glaucoma preferred practice pattern® guidelines. Ophthalmology 2016, 123, P41–P111. [Google Scholar] [CrossRef]
- Court, J.H.; Austin, M.W. Virtual glaucoma clinics: Patient acceptance and quality of patient education compared to standard clinics. Clin. Ophthalmol. 2015, 9, 745–749. [Google Scholar] [CrossRef]
- Choong, Y.F.; Devarajan, N.; Pickering, A.; Pickering, S.; Austin, M.W. Initial management of ocular hypertension and primary open-angle glaucoma: An evaluation of the royal college of ophthalmologists’ guidelines. Eye 2003, 17, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Rathod, D.; Win, T.; Pickering, S.; Austin, M.W. Incorporation of a virtual assessment into a care pathway for initial glaucoma management: Feasibility study. Clin. Exp. Ophthalmol. 2008, 36, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, A.; Brookes, J.; Foster, P.J. A technician-delivered ‘virtual clinic’ for triaging low-risk glaucoma referrals. Eye 2017, 31, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Gunn, P.J.G.; Marks, J.R.; Au, L.; Waterman, H.; Spry, P.G.D.; Harper, R.A. Acceptability and use of glaucoma virtual clinics in the UK: A national survey of clinical leads. BMJ Open Ophthalmol. 2018, 3, e000127. [Google Scholar] [CrossRef]
- Wright, H.R.; Diamond, J.P. Service innovation in glaucoma management: Using a Web-based electronic patient record to facilitate virtual specialist supervision of a shared care glaucoma programme. Br. J. Ophthalmol. 2015, 99, 313–317. [Google Scholar] [CrossRef]
- Tatham, J.; Ali, A.; Hillier, N. Knowledge of Glaucoma Among Patients Attending Virtual and Face-to Face Glaucoma Clinics. J. Glaucoma 2021, 30, 325–331. [Google Scholar] [CrossRef]
- Mostafa, I.; Bianchi, E.; Brown, L.; Tatham, A. What is the best way to measure intraocular pressure in a virtual clinic? Eye 2020, 35, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Gunn, P.; Marks, R.; Au, L.; Read, S.; Waterman, H.; Spry, P.; Harper, R. Virtual Clinics for glaucoma care–Patients’ and clinicians’ experiences and perceptions: A qualitative evaluation. Eye 2021, 1–10. [Google Scholar]
- Spackman, W.; Waqar, S.; Booth, A. Patient satisfaction with the virtual glaucoma clinic. Eye 2021, 35, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Clarke, J.; Puertas, R.; Kotecha, A.; Foster, P.J.; Barton, K. Virtual clinics in glaucoma care: Face-to-face versus remote decision-making. Br. J. Ophthalmol. 2017, 101, 892–895. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, A.; Baldwin, A.; Brookes, J.; Foster, P.J. Experiences with developing and implementing a virtual clinic for glaucoma care in an NHS setting. Clin. Ophthalmol. 2015, 9, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Nikita, E.; Kortuem, K.; Fasolo, S.; Tsoukanas, D.; Sim, D. Expanded teleglaucoma clinics. An opportunity to manage the increasing demand for glaucoma care with safety and efficiency. Invest. Ophthalmol. Vis. Sci. 2019, 60, 5480. [Google Scholar]
- Kotecha, A.; Bonstein, K.; Cable, R.; Cammack, J.; Clipston, J.; Foster, P. Qualitative investigation of patients’ experience of a glaucoma virtual clinic in a specialist Ophthalmic Hospital in London, UK. BMJ Open 2015, 5, e009463. [Google Scholar] [CrossRef] [PubMed]
- Nikita, E.; Gazzard, G.; Sim, D.; Fasolo, S.; Kortum, K.; Jayaram, H. Expansion of patient eligibility for virtual glaucoma clinics: A long-term strategy to increase the capacity of high-quality glaucoma care. Br. J. Ophthalmol. 2021, 1–6. [Google Scholar] [CrossRef]
- The Royal College of Ophthalmologists. Commissioning Guide. Glaucoma (Long Version). Available online: https://www.college-optometrists.org/uploads/assets/97a30d3e-f6ba-4504-875d6a91d73ba5e3/Commissioning-Guide-Glaucoma-Full-report.pdf (accessed on 30 July 2021).
- National Institute for Health and Care Excellence. National Institute for Health and Care Excellence: Clinical Guidelines. Glaucoma: Diagnosis and Management; National Institute for Health and Care Excellence: London, UK, 2017.
| First Author, Year | Country | Hospital | SC/Recommendation | Study Sample | NG/OSR vs. FU-Patients |
|---|---|---|---|---|---|
| White et al., 2014 | Australia and New Zealand | / | ANGIG& RANZCO | / | FU-patients |
| Bentley et al., 2019 | Australia | RVEEH | SC-RVAC | 1024 patients | FU-patients |
| Canadian Glaucoma Society Committee, 2011 | Canada | / | CGSC | / | FU-patients |
| Holtzer-Goor et al., 2010 | The Netherlands | REH | CS-GFU | 815 patients (2100 visits) SC-GFU: 405 (1181 visits) StC-GFU: 410 (919 visits) | FU-patients |
| Holtzer-Goor et al., 2016 | The Netherlands | REH | CS-GFU | 815 patients (2100 visits) SC-GFU: 405 (1181 visits) StC-GFU: 410 (919 visits) | FU-patients |
| Lemij et al., 2010 | The Netherlands | REH | CS-GFU | 815 patients (2100 visits) SC-GFU: 405 (1181 visits) StC-GFU: 410 (919 visits) | FU-patients |
| Damento et al.,2018 | USA | MC | SC-MC | 358 patients | FU-patients |
| Winkler et al.,2017 | USA | MC | SC-MC | 591 patients | FU-patients |
| Shah et al., 2018 | USA | MC | SC-MC | 200 patients (299 eyes) | FU-patients |
| Banes et al., 2000 | UK | MEH | SC-MEH | 54 patients (102 eyes) | FU-patients |
| Banes et al., 2006 | UK | MEH | SC-MEH | 349 patients | FU-patients |
| Ho et al., 2011 | UK | QMC | SC-QMC | 140 patients | FU-patients |
| Bhota et al., 2019 | New Zealand | SGC | SC-SGC | 509 patients (760 visits) | FU-patients |
| Phu et al., 2019 | Australia | GMC | SC-GMC | 101 patients | FU-patients |
| SC/ Recommendations | NMS | History Taking | IOP | VA | Slit-Lamp Examination | +Gonio | VF | Fundus Photographs | OCT | HRT | GDx | CCT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ANGIG& RANZCO | NS | x | x | x | x(a&p) | x | x | x | x* | x* | x* | x |
| SC-RVAC | Opto | NS° | NS° | NS° | NS° | NS° | NS° | NS° | NS° | NS° | NS° | NS° |
| CGSC | Opto | x | x | NS | x(a&p) | x | x | x | x** | x** | x** | 0 |
| SC-GFU | Opto OT | x | x | x | 0 | 0 | x | 0 | 0 | 0 | x | 0 |
| SC-MC | Opto | x | x | x | x(a&p) | 0 | x | x | x | 0 | 0 | 0 |
| SC-MEH | Opto | x | x | NS | x(a&p) | x | x | x*** | x*** | x*** | x*** | x*** |
| SC-QMC | Opto | x | x | NS | x(a&p) | x | x | 0 | x | x | 0 | x |
| SC-SGC | Opto | x | x | NS | NS | NS | x | x | 0 | x | 0 | NS |
| SC-GMC | Opto | x | x | x | x(a&p) | x | x | NS | 0 | x | 0 | 0 |
| SC/Recommendations | NMS | Suitable | Unsuitable | Model-Specific Referral | Patient-Specific Referral |
|---|---|---|---|---|---|
| ANGIG& RANZCO | NS | OHT; GS; G: stable & low/moderate risk | High risk of visual loss, e.g., other ocular diseases; advanced glaucoma (both stable and unstable); closed angles | GS: every 3–4 y; G early/moderate stable: every 2 y | Recent diagnosis; start of therapy; unstable disease; acutely raised or very high IOP; narrow angles |
| SC-RVAC | Opto | NS° | NS° | NS° | NS° |
| CGSC | Opto | OHT; GS; G: stable & low risk; Other concurrent eye diseases related to G | G: unstable/moderate/advanced | GS: every 3–4 y; G early: every 2–3 y | Recent diagnosis; start of therapy; GS with high risk (suspected progression); unstable G; acutely raised or very high IOP |
| SC-GFU | Opto OT | OHT; GS; G: stable | Complex cases: other ocular diseases; H laser therapy for DRP | G and GS: every third visit | Recent diagnosis; suspected progression |
| SC-MC | Opto | G: stable (mild/moderate/advanced); Other concurrent eye diseases | G: unstable | Mild G: every 3 y; moderate G: every 2 y; advanced G: every 1 y | Recent diagnosis; suspected progression; significant cataract; intolerant of medications |
| SC-MEH | Opto | OHT; GS; G; Other concurrent eye diseases | Known clinical complication, H laser therapy/surgery | OHT: every 1 y; stable G: every 6 mo; after change in therapy: 1 mo | Recent diagnosis; changes in treatment |
| SC-QMC | Opto | OHT; GS; G; Other concurrent eye diseases | H laser therapy/surgery | NS | Recent diagnosis; changes in treatment |
| SC-GSC | Opto | OHT; GS; G: stable | Other ocular diseases; G: unstable; recent treatment changes | NS | Unstable G |
| SC-QMC | Opto | OHT; GS; G: stable | G: severe and complicated; other ocular diseases | NS | Narrow angles |
| SC/Hospital | First Author | Compliance with Protocol (GFU)/Guidelines | Results of Tests and Examinations | Glaucoma Status | Referral | MD: Clinical Management |
|---|---|---|---|---|---|---|
| SC-RVAC | Bentley et al. [41] | SC vs. AAO PPPg, ANGIG&RANZCO - >85% | Optic nerve assessment skills (% correct diagnosis): -mean increase of 14.0% * | NS | NS | NS |
| SC-GFU | Holtzer-Goor et al. [38] | NMS vs. protocol - >98.8% of the visits | NS | SC vs. STC - % visits stable: SC (17.0%) ≈ StC (16.0%) ** - % visits with shortening of FU-interval: SC (16.0%) ≈ StC (15.1%) ** | NMS: correct referral to GE - 84.4% of the remarkable cases | SC vs. StC - Treatment changes: SC (14.0%) ≈ StC (15.0%) ** |
| Holtzer-Goor et al. [36] | NMS vs. protocol - IOP, VA, GDx: > 97.5% - VF: 25.4% SC/StC vs. protocol - IOP: SC ≈ StC ** - VA: SC > StC * - GDx: SC > StC * - VF: SC ≈ StC ** | SC vs. StC - VA decline (% visits): SC (3.9%) < StC (6.3%) * - IOP: SC ≈ StC ** - VF: SC ≈ StC ** - GDx: SC ≈ StC ** | NMS: correct referral to seek advice from GE - 100.0%: SOF on GDx/VA- 84.6%: IOP > tIOP - 68.2%: VA declined >2 lines | SC vs. StC -Treatment changes: SC (14.0%) ≈ StC (15.0%) ** -Reason for change: SC ≈ StC ** | ||
| Lemij et al. [37] | NMS vs. protocol - IOP, VA, GDx: > 97.5% - VF: 41.2% *** SC/StC vs. protocol - IOP: SC ≈ StC ** - VA: SC > StC * - GDx: SC > StC * - VF: SC ≈ StC ** -Slit-lamp exam: SC < StC * | SC vs. StC - IOP: SC ≈ StC ** - VA: SC ≈ StC ** - GDx: SC ≈ StC ** - VF: SC ≈ StC ** | NMS: correct referral to GE (50.0%) - 92.0%: SOF on GDx - 75.0% SOF on VF - 66.7%: IOP > tIOP - 36.0%: VA declined >2 lines | SC vs. StC -Treatment changes: SC (14.1%) ≈ StC (15.4%) ** -Reason for change: SC ≈ StC ** | ||
| SC-MC | Damento et al. [37] | SC/StC vs. AAO PPPg (mean number of diagnostic tests) - 13 mo: SC > StC * - 25 mo: SC > StC * | NS | SC vs. StC (number of patients visits) - 13 mo: SC > StC * - 25 mo: SC > StC * | NS | NS |
| Winkler et al. [35] | SC/StC vs. AAO PPPg (% of patient visits) - Combined compliance *: SC > StC * - VF: SC ≈ StC ** - Gonio: SC > StC * - Fundus photographs: SC > StC * - OCT: SC > StC * - CCT: SC ≈ StC ** | NS | NS | NS | ||
| Shah et al. [33] | Opto vs. GE (frequency of clinical test data used to assess progression) - IOP: opto > GE * - Disc hemorrhage: opto≈ GE ** -Fundus photographs: opto≈ GE ** - VF: opto < GE (p=0.07, tendency) - OCT: opto < GE * | Among all HCP (GEs and optos); among GEs only: - IOP: κ = 0.57; κ = 0.57 - Disc hemorrhage: κ = 0.65; κ = 0.59 -Fundus photographs: 77%; 89% - VF: κ = 0.45; κ = 0.47 - OCT: κ = 0.26: κ = 0.51 | Among all HCP (GEs and optos); among GEs only: - κ = 0.37; κ = 0.39 | NS | NS | |
| SC-MEH | Banes et al. [40] | NS | Opto vs. GE - IOP: OD median difference = -0.25 mmHg, OS median difference = 0.00 mmH - Slit-lamp exam (cup/disc): median difference = 0, greatest difference = 0.15 - VF: κ = 0.80–0.81 | Opto vs. GE - FU-interval: κ = 0.97 | NS | Opto vs. GE - Medical and surgical treatment: κ = 0.93–1.00 |
| Banes et al. [42] | Opto vs. GE - Slit lamp exam: sensitivity and specificity ≈ 83% Opto vs. GE; GE vs. GE - VF: κ = 0.37–0.33; κ = 0.39 | Opto vs. GE; GE vs. GE - FU-interval: κ = 0.35; κ = 0.41 | Opto vs. GE; GE vs. GE - Correct referral to GE: 72.0% agreement; 72.0% agreement | Opto vs. GE; GE vs. GE - “eye drop” treatment: κ = 0.67; κ = 0.74 - cataract surgery: 94.0%; 93.0% - glaucoma surgery: 95.0%; 97.0% | ||
| SC-QMC | Ho et al. [39] | NS | Opto vs. GE - VF: κ = 0.81–0.93 | Opto vs. GE - next appointment: κ = 0.88–0.97 | Opto vs. GE - Correct referral to GE: κ = 0.96–1.00 | Opto vs. GE - “eye drop” treatment: κ = 0.96–1.00 |
| SC-SGC | Bhota et al. [43] | NS | NS | NS | Opto vs. GE - Correct referral to GE: 66.1% agreement | NS |
| SC-GMC | Phu et al. [44] | NS | Opto vs. GE - Gonio: 59.8% agreement on structures (fair to moderate), 93.4% exact agreement with final diagnosis | NS | NS | NS |
| First Author, Year | Country | Hospital | VC | Study Sample | NG/OSR vs. FU-Patients |
|---|---|---|---|---|---|
| Banes et al., 2018 | UK | Units from the HES | / | / | NG/OSR and FU-patients |
| Wright and Diamond, 2014 | UK | 3 glaucoma clinics: Bristol, Nuneaton and Kingston | GCC | 24257 patients | FU-patients |
| Kotecha et al., 2017 | UK | MEH | GSC (GSMS) | 1380 patients | NG/OSR |
| Clarke et al., 2017 | UK | MEH | SMS (GSMS) | 204 patients | FU-patients |
| Kotecha et al., 2015 | UK | MEH | SMS (GSMS) | 1575 patients | FU-patients |
| Nikita et al., 2019 | UK | MEH | SMS (GSMS) | 2015 patients | FU-patients |
| Kotecha et al., 2005 | UK | MEH | GSMS | 43 patients | NG/OSR and FU-patients |
| Choong et al., 2003 | UK | SH | GAC | 100 patients | NG/OSR |
| Rathod et al., 2008 | UK | SH | GAC | 78 patients | NG/OSR |
| Court and Austin, 2015 | UK | SH | GAC | 170 patients (85 StC and 85 GAC) | NG/OSR |
| Tatham et al., 2021 | UK | PAEP | VC-PAEP | 105 patients (55 StC and 50 VC-PAEP) | FU-patients |
| Gunn et al., 2021 | UK | MREH; BEH | VC-MREH; VC-BEH | 148 patients | FU-patients |
| Mostafa et al., 2020 | UK | PAEP | VC-PAEP | 116 patients | FU-patients |
| Nikita et al., 2021 | UK | MEH | VC-MEH | 2017 patients | NG/OSR and FU-patients |
| Spackman et al., 2020 | UK | REIP | VC-REIP | 68 patients | FU-patients |
| VC | First Author | NMS | History Taking | IOP | VA | Slit-Lamp | Von Herick | OCT (Angle) | VF | Fundus Photographs | OCT | HRT | CCT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GCC | Wright and Diamond | Opto OT | x* x* | x* x* | x* x* | x* x* | x* x* | 0 0 | - x | - x | 0 0 | 0 0 | - x |
| GSC (GSMS) | Kotecha et al. | Clinician; OT | - x* | - x | - x | 0 0 | 0 0 | - x | - x | - x | 0 0 | 0 0 | - x |
| SMS (GSMS) | Clarke et al. Kotecha et al. Nikita et al. | ONP OT OT NS | x* - x* NS | x* - x x | - x x x | x(a) - 0 0 | NS 0 0 0 | 0 0 0 0 | - x x x | - x x x | 0 0 0 x | - x 0 0 | 0 0 0 0 |
| GSMS | Kotecha et al. | Clinician OT | - NS | - NS | - NS | - NS | - NS | - NS | - NS | - NS | - NS | - NS | - NS |
| GAC | Choong et al. Rathod et al. Court and Austin | ONP ONP ONP | x x x | x x x | 0 0 0 | 0 x x | 0 x NS | 0 0 0 | x x x | 0 x x | 0 0 0 | 0 x x | 0 0 0 |
| VC-PAEP | Tatham et al. Mostafa et al. | OT OT ONP | x x x | x x x | NS NS NS | x x x | NS NS NS | 0 0 0 | x x x | x x x | x x x | x x x | x x x |
| VC-MREH; VC-BEH | Gunn et al. | OT | x | x | x | 0 | 0 | 0 | x | 0 | 0 | x | 0 |
| VC-MEH | Nikita et al. | OT | x | x | x | 0 | 0 | x* | x | x | 0 | x | 0 |
| VC-RAEP | Spackman et al. | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| VC | First Author | NG/OSR vs. FU-Patients | Suitable | Unsuitable |
|---|---|---|---|---|
| GCC | Wright and Diamond | FU-patients | General FU-pool (all types and various stages of risk) | NS |
| GSC (GSMS) | Kotecha et al. | NG/OSR | First: low risk of developing G Later: up to three risk factors for G | Definitive signs of G; angle closure suspects; IOP > 32 mmHg |
| SMS (GSMS) | Clarke et al. | FU-patients | OHT; GS; G: stable and low risk; open angle inclusive PDS and PXF | Poor mobility: poor VF; poor disc imaging |
| Kotecha et al. | FU-patients | OHT; GS; G: stable and low/moderate risk | Phakic angle closure/suspects; monocular; coexisting ocular comorbidity; best-corrected VA < Snellen 6/12; H glaucoma filtration surgery; concerns regarding adherence; requirement of hospital transport to attend; signs of cognitive impairment | |
| Nikita et al. | FU-patients | G: most types, various stages of risk | NS | |
| GSMS | Kotecha et al. | NG/OSR; | Low/moderate risk of developing G | NS |
| FU-patients | OHT; GS; G: stable and low/moderate risk, open-angle | NS | ||
| GAC | Choong et al. Rathod et al. Court and Austin | NG/OSR NG/OSR NG/OSR | All NG/OSR * All NG/OSR * All NG/OSR * | NS NS NS |
| VC-PAEP | Tatham et al. Mostafa et al. | FU-patients FU-patients | G: mild to moderate stable GS; OHT | H glaucoma filtration surgery; phakic angle closure/suspects |
| VC-MREH; VC-BEH | Gunn et al. | FU-patients | G; GS; OHT | <18 years of age; unable to speak English |
| VC-MEH | Nikita et al. | NG/OSR and FU-patients | G (most types); GS; H ocular surgery/glaucoma laser/retinal laser | Unstable advanced G |
| VC-REIP | Spackman et al. | FU-patients | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simons, A.-S.; Vercauteren, J.; Barbosa-Breda, J.; Stalmans, I. Shared Care and Virtual Clinics for Glaucoma in a Hospital Setting. J. Clin. Med. 2021, 10, 4785. https://doi.org/10.3390/jcm10204785
Simons A-S, Vercauteren J, Barbosa-Breda J, Stalmans I. Shared Care and Virtual Clinics for Glaucoma in a Hospital Setting. Journal of Clinical Medicine. 2021; 10(20):4785. https://doi.org/10.3390/jcm10204785
Chicago/Turabian StyleSimons, Anne-Sophie, Julie Vercauteren, João Barbosa-Breda, and Ingeborg Stalmans. 2021. "Shared Care and Virtual Clinics for Glaucoma in a Hospital Setting" Journal of Clinical Medicine 10, no. 20: 4785. https://doi.org/10.3390/jcm10204785
APA StyleSimons, A.-S., Vercauteren, J., Barbosa-Breda, J., & Stalmans, I. (2021). Shared Care and Virtual Clinics for Glaucoma in a Hospital Setting. Journal of Clinical Medicine, 10(20), 4785. https://doi.org/10.3390/jcm10204785






