Incidence and Transition of Acute Kidney Injury, Acute Kidney Disease to Chronic Kidney Disease after Acute Type A Aortic Dissection Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source
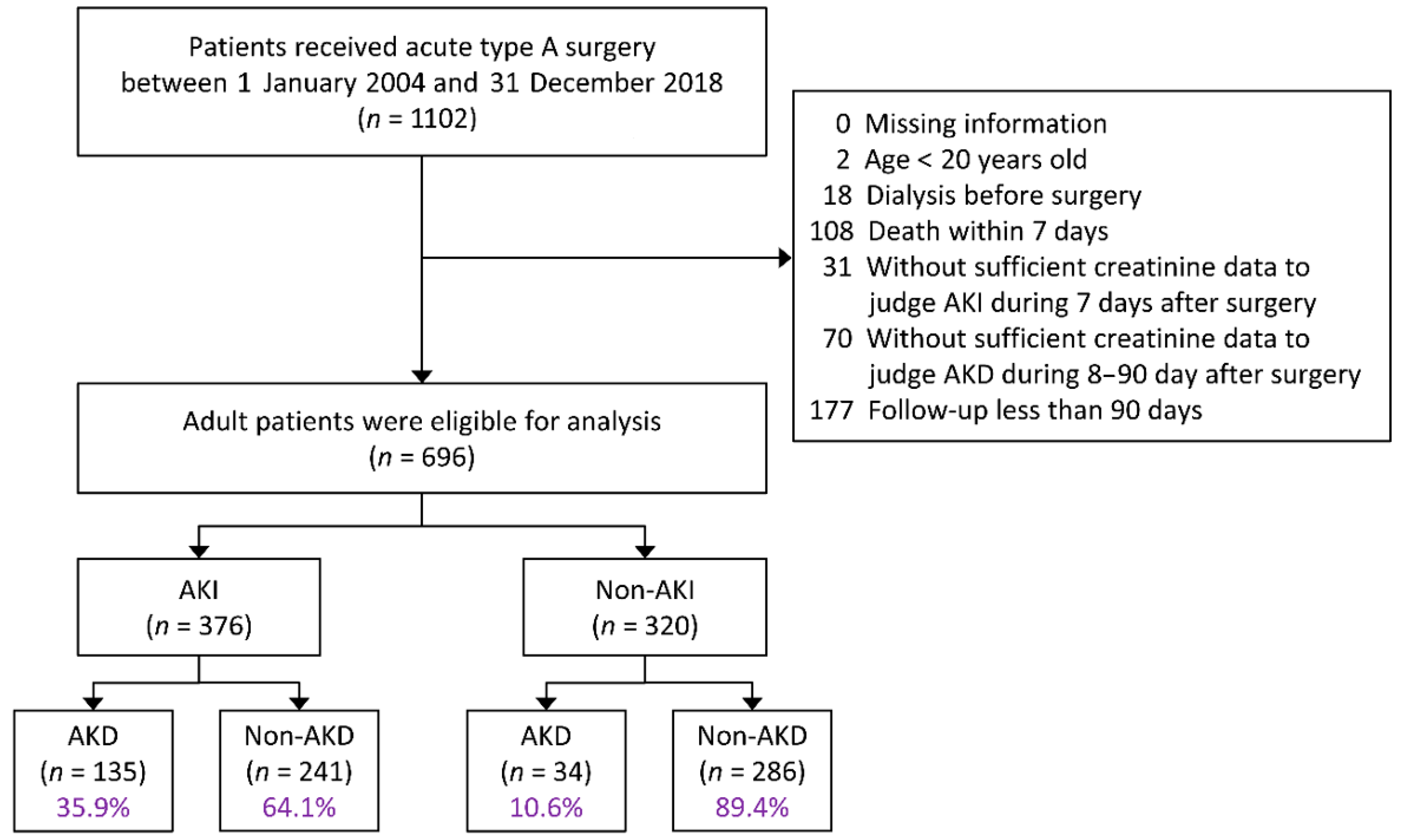
2.2. Study Population
2.3. Definition of AKI and AKD
2.4. Covariates
2.5. Outcomes
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Incidence of AKD after Surgery
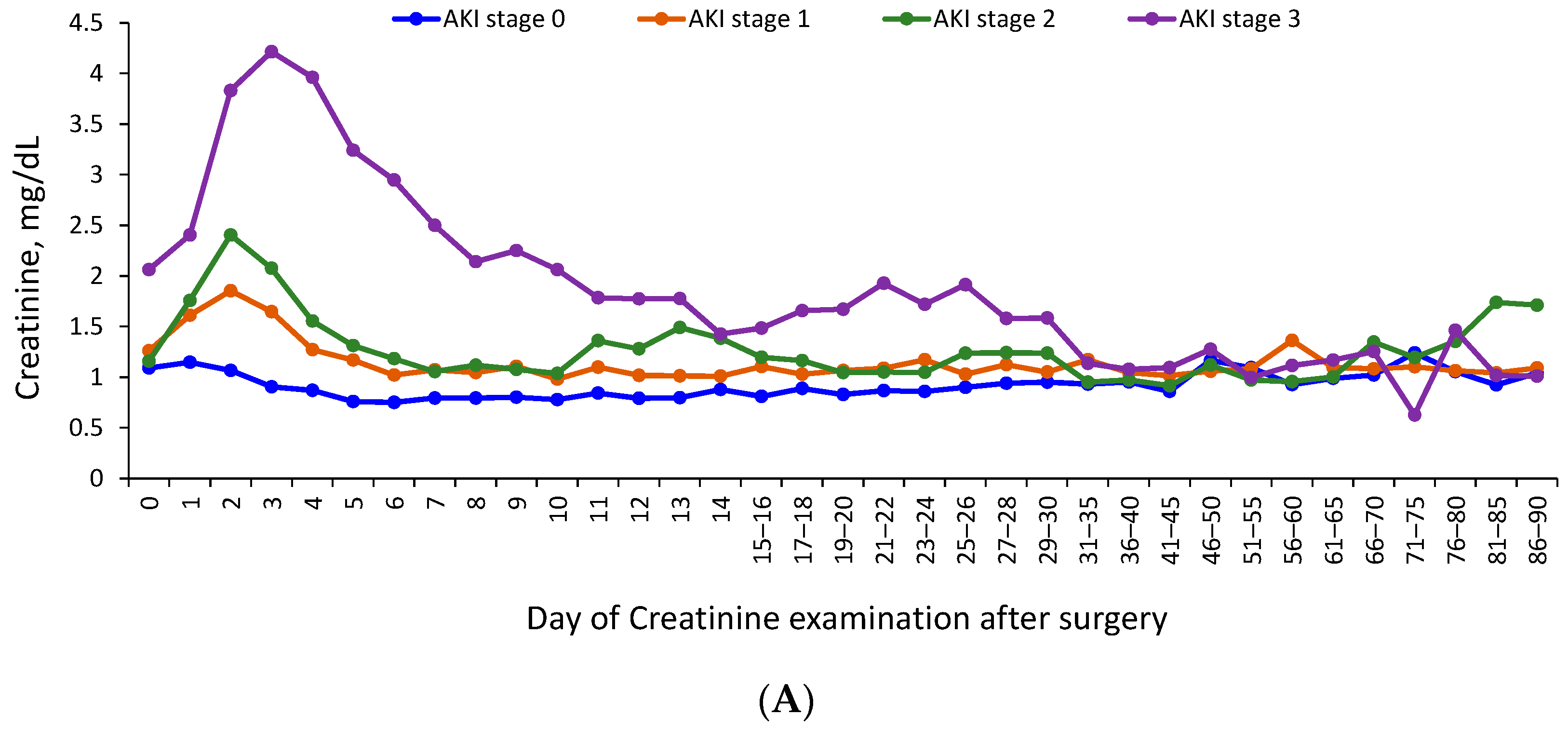
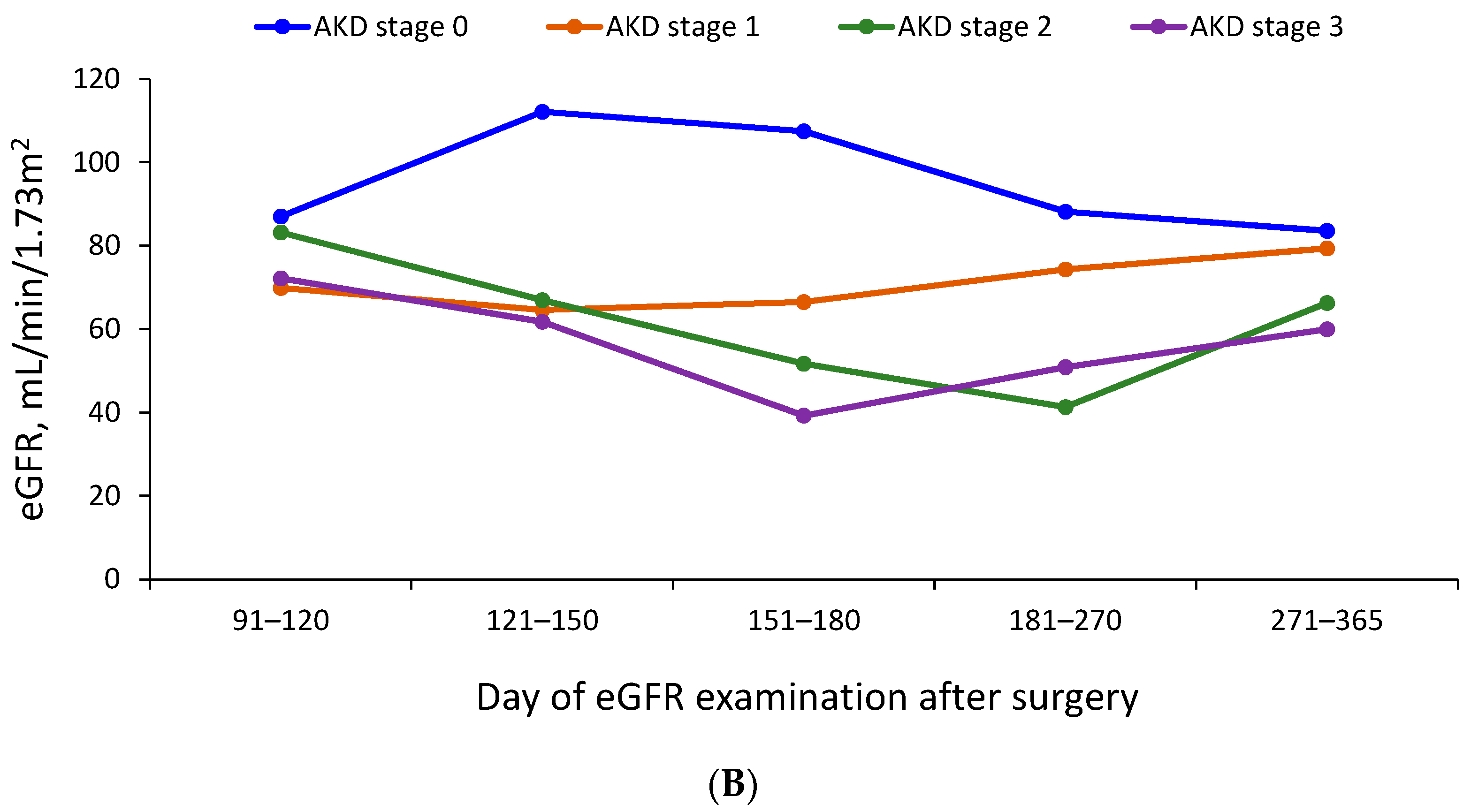
3.3. Trajectory of Renal Function
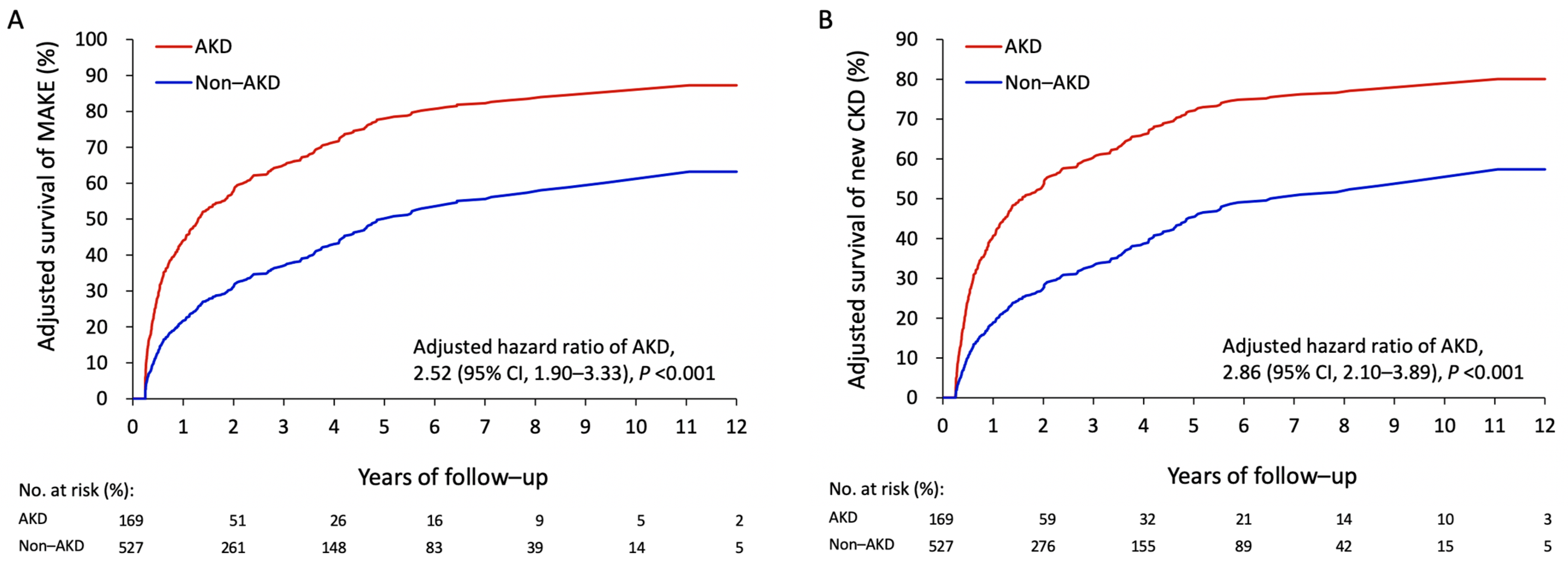
3.4. Association between AKD and the Risk of Late Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mussa, F.F.; Horton, J.D.; Moridzadeh, R.; Nicholson, J.; Trimarchi, S.; Eagle, K.A. Acute Aortic Dissection and Intramural Hematoma: A Systematic Review. JAMA 2016, 316, 754–763. [Google Scholar] [CrossRef] [PubMed]
- Pape, L.A.; Awais, M.; Woznicki, E.M.; Suzuki, T.; Trimarchi, S.; Evangelista, A.; Myrmel, T.; Larsen, M.; Harris, K.M.; Greason, K.; et al. Presentation, Diagnosis, and Outcomes of Acute Aortic Dissection: 17-Year Trends From the International Registry of Acute Aortic Dissection. J. Am. Coll. Cardiol. 2015, 66, 350–358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jormalainen, M.; Raivio, P.; Biancari, F.; Mustonen, C.; Honkanen, H.P.; Venermo, M.; Vento, A.; Juvonen, T. Late Outcome after Surgery for Type-A Aortic Dissection. J. Clin. Med. 2020, 9, 2731. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Zhai, G.; Liu, N.; Sun, L.; Zhu, J. Independent risk factors for postoperative AKI and the impact of the AKI on 30-day postoperative outcomes in patients with type A acute aortic dissection: An updated meta-analysis and meta-regression. J. Thorac. Dis. 2018, 10, 2590–2598. [Google Scholar] [CrossRef] [PubMed]
- Thakar, C.V.; Liangos, O.; Yared, J.P.; Nelson, D.; Piedmonte, M.R.; Hariachar, S.; Paganini, E.P. ARF after open-heart surgery: Influence of gender and race. Am. J. Kidney Dis. 2003, 41, 742–751. [Google Scholar] [CrossRef]
- Arora, P.; Kolli, H.; Nainani, N.; Nader, N.; Lohr, J. Preventable risk factors for acute kidney injury in patients undergoing cardiac surgery. J. Cardiothorac. Vasc. Anesth 2012, 26, 687–697. [Google Scholar] [CrossRef]
- Lagny, M.G.; Jouret, F.; Koch, J.N.; Blaffart, F.; Donneau, A.F.; Albert, A.; Roediger, L.; Krzesinski, J.M.; Defraigne, J.O. Incidence and outcomes of acute kidney injury after cardiac surgery using either criteria of the RIFLE classification. BMC Nephrol. 2015, 16, 76. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.W.; Tsai, F.C.; Lin, Y.S.; Chang, C.H.; Chen, D.Y.; Chou, A.H.; Chen, T.H. Long-term outcomes of extracorporeal membrane oxygenation support for postcardiotomy shock. J. Thorac. Cardiovasc. Surg. 2017, 154, 469–477. [Google Scholar] [CrossRef]
- Chawla, L.S.; Bellomo, R.; Bihorac, A.; Goldstein, S.L.; Siew, E.D.; Bagshaw, S.M.; Bittleman, D.; Cruz, D.; Endre, Z.; Fitzgerald, R.L.; et al. Acute kidney disease and renal recovery: Consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat. Rev. Nephrol. 2017, 13, 241–257. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, R.; Iwagami, M.; Moriya, H.; Ohtake, T.; Hamasaki, Y.; Nangaku, M.; Doi, K.; Kobayashi, S.; Noiri, E. The Clinical Course of Acute Kidney Disease after Cardiac Surgery: A Retrospective Observational Study. Sci. Rep. 2020, 10, 6490. [Google Scholar] [CrossRef] [Green Version]
- Shao, S.C.; Chan, Y.Y.; Kao Yang, Y.H.; Lin, S.J.; Hung, M.J.; Chien, R.N.; Lai, C.C.; Lai, E.C. The Chang Gung Research Database-A multi-institutional electronic medical records database for real-world epidemiological studies in Taiwan. Pharmacoepidemiol. Drug Saf. 2019, 28, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.S.; Lin, M.H.; Lee, C.P.; Yang, Y.H.; Chen, W.C.; Chang, G.H.; Tsai, Y.T.; Chen, P.C.; Tsai, Y.H. Chang Gung Research Database: A multi-institutional database consisting of original medical records. Biomed J. 2017, 40, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Section 2: AKI Definition. Kidney Int. Suppl. 2012, 2, 19–36. [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Loberiza, F.R.; Klein, J.P.; Zhang, M.J. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput. Methods Programs Biomed. 2007, 88, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Siew, E.D.; Abdel-Kader, K.; Perkins, A.M.; Greevy, R.A., Jr.; Parr, S.K.; Horner, J.; Vincz, A.J.; Denton, J.; Wilson, O.D.; Hung, A.M.; et al. Timing of Recovery From Moderate to Severe AKI and the Risk for Future Loss of Kidney Function. Am. J. Kidney Dis. 2020, 75, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Moledina, D.G.; Luciano, R.L.; Kukova, L.; Chan, L.; Saha, A.; Nadkarni, G.; Alfano, S.; Wilson, F.P.; Perazella, M.A.; Parikh, C.R. Kidney Biopsy-Related Complications in Hospitalized Patients with Acute Kidney Disease. Clin. J. Am. Soc. Nephrol. 2018, 13, 1633–1640. [Google Scholar] [CrossRef] [Green Version]
- Chu, R.; Li, C.; Wang, S.; Zou, W.; Liu, G.; Yang, L. Assessment of KDIGO definitions in patients with histopathologic evidence of acute renal disease. Clin. J. Am. Soc. Nephrol. 2014, 9, 1175–1182. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.J.; Lee, C.C.; Kuo, G.; Fan, P.C.; Lin, C.Y.; Chang, S.W.; Tian, Y.C.; Chen, Y.C.; Chang, C.H. Comparison between watchful waiting strategy and early initiation of renal replacement therapy in the critically ill acute kidney injury population: An updated systematic review and meta-analysis. Ann. Intensive Care 2020, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Molnar, A.O.; Parikh, C.R.; Sint, K.; Coca, S.G.; Koyner, J.; Patel, U.D.; Butrymowicz, I.; Shlipak, M.; Garg, A.X. Association of postoperative proteinuria with AKI after cardiac surgery among patients at high risk. Clin. J. Am. Soc. Nephrol. 2012, 7, 1749–1760. [Google Scholar] [CrossRef] [Green Version]
- Gayat, E.; Hollinger, A.; Cariou, A.; Deye, N.; Vieillard-Baron, A.; Jaber, S.; Chousterman, B.G.; Lu, Q.; Laterre, P.F.; Monnet, X.; et al. Impact of angiotensin-converting enzyme inhibitors or receptor blockers on post-ICU discharge outcome in patients with acute kidney injury. Intensive Care Med. 2018, 44, 598–605. [Google Scholar] [CrossRef]
- Chen, Y.T.; Chan, C.K.; Li, W.Y.; Huang, T.M.; Lai, T.S.; Wu, V.C.; Chu, T.S.; National Taiwan University Hospital Study Group on Acute Renal Failure (NSARF). Renin-angiotensin-aldosterone system inhibition decreased contrast-associated acute kidney injury in chronic kidney disease patients. J. Formos. Med. Assoc. 2020, 120, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.; Ye, F.; James, M.T.; Hemmelgarn, B.; Klarenbach, S.; Pannu, N.; Interdisciplinary Chronic Disease, C. Association of Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use With Outcomes After Acute Kidney Injury. JAMA Intern. Med. 2018, 178, 1681–1690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.W.; Lin, Y.S.; Wu, V.C.; Lin, M.S.; Chou, A.H.; Chu, P.H.; Chen, T.H. Effect of beta-blocker therapy on late outcomes after surgical repair of type A aortic dissection. J. Thorac. Cardiovasc. Surg. 2020, 159, 1694–1703. [Google Scholar] [CrossRef]
- Scarton, M.; Oppenheimer, A.; Chaibi, K.; Dreyfuss, D.; Gaudry, S. Renin-angiotensin-aldosterone system blockers after KDIGO stage 3 acute kidney injury: Use and impact on 2-year mortality in the AKIKI trial. Crit. Care 2019, 23, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.Y.; Tsai, I.J.; Pan, H.C.; Liao, H.W.; Neyra, J.A.; Wu, V.C.; Chueh, J.S. The Impact of Angiotensin-Converting Enzyme Inhibitors or Angiotensin II Receptor Blockers on Clinical Outcomes of Acute Kidney Disease Patients: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2021, 12, 665250. [Google Scholar] [CrossRef]
- Thomas, M.E.; Blaine, C.; Dawnay, A.; Devonald, M.A.; Ftouh, S.; Laing, C.; Latchem, S.; Lewington, A.; Milford, D.V.; Ostermann, M. The definition of acute kidney injury and its use in practice. Kidney Int. 2015, 87, 62–73. [Google Scholar] [CrossRef] [PubMed]
| Late Outcomes (>90 Days) | AKD (n = 169) | Non-AKD (n = 527) | Unadjusted Analysis | Multivariable Analysis * | ||
|---|---|---|---|---|---|---|
| HR (95% CI) for AKD | p-Value | HR (95% CI) for AKD | p-Value | |||
| MAKEs | 111 (65.7) | 222 (42.1) | 2.24 (1.76–2.86) | <0.001 | 2.52 (1.90–3.33) | <0.001 |
| Recurrent AKI | 35 (20.7) | 77 (14.6) | 1.57 (1.05–2.35) | 0.029 | 1.24 (0.77–2.01) | 0.372 |
| Newly diagnosed CKD | 93 (55.0) | 201 (38.1) | 1.95 (1.50–2.55) | <0.001 | 2.86 (2.10–3.89) | <0.001 |
| ESRD | 8 (4.7) | 15 (2.8) | 1.66 (0.72–3.85) | 0.236 | 1.51 (0.65–3.48) | 0.336 |
| All-cause mortality | 17 (10.1) | 32 (6.1) | 1.67 (0.92–3.03) | 0.093 | 1.58 (0.81–3.09) | 0.179 |
| All-cause readmission | 101 (59.8) | 236 (44.8) | 1.47 (1.16–1.86) | 0.002 | 1.45 (1.09–1.92) | 0.010 |
| Respiratory failure | 11 (6.5) | 23 (4.4) | 1.62 (0.79–3.33) | 0.190 | 1.54 (0.61–3.90) | 0.365 |
| Ischemic stroke | 14 (8.3) | 31 (5.9) | 1.39 (0.73–2.66) | 0.322 | 1.10 (0.57–2.11) | 0.786 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, C.-H.; Chen, S.-W.; Chen, J.-J.; Chan, Y.-H.; Yen, C.-L.; Lee, T.H.; Cheng, Y.-T. Incidence and Transition of Acute Kidney Injury, Acute Kidney Disease to Chronic Kidney Disease after Acute Type A Aortic Dissection Surgery. J. Clin. Med. 2021, 10, 4769. https://doi.org/10.3390/jcm10204769
Chang C-H, Chen S-W, Chen J-J, Chan Y-H, Yen C-L, Lee TH, Cheng Y-T. Incidence and Transition of Acute Kidney Injury, Acute Kidney Disease to Chronic Kidney Disease after Acute Type A Aortic Dissection Surgery. Journal of Clinical Medicine. 2021; 10(20):4769. https://doi.org/10.3390/jcm10204769
Chicago/Turabian StyleChang, Chih-Hsiang, Shao-Wei Chen, Jia-Jin Chen, Yi-Hsin Chan, Chieh-Li Yen, Tao Han Lee, and Yu-Ting Cheng. 2021. "Incidence and Transition of Acute Kidney Injury, Acute Kidney Disease to Chronic Kidney Disease after Acute Type A Aortic Dissection Surgery" Journal of Clinical Medicine 10, no. 20: 4769. https://doi.org/10.3390/jcm10204769
APA StyleChang, C.-H., Chen, S.-W., Chen, J.-J., Chan, Y.-H., Yen, C.-L., Lee, T. H., & Cheng, Y.-T. (2021). Incidence and Transition of Acute Kidney Injury, Acute Kidney Disease to Chronic Kidney Disease after Acute Type A Aortic Dissection Surgery. Journal of Clinical Medicine, 10(20), 4769. https://doi.org/10.3390/jcm10204769





