Real-World Incidence of Immune-Related Adverse Events Associated with Nivolumab Plus Ipilimumab in Patients with Advanced Renal Cell Carcinoma: A Retrospective Observational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Data Collection
2.2. irAEs
2.3. Laboratory Testing
2.4. Objectives of This Study
2.5. Statistical Analyses
3. Results
3.1. Patient Characteristics and Tumor Location
3.2. Nivolumab Plus Ipilimumab Therapy
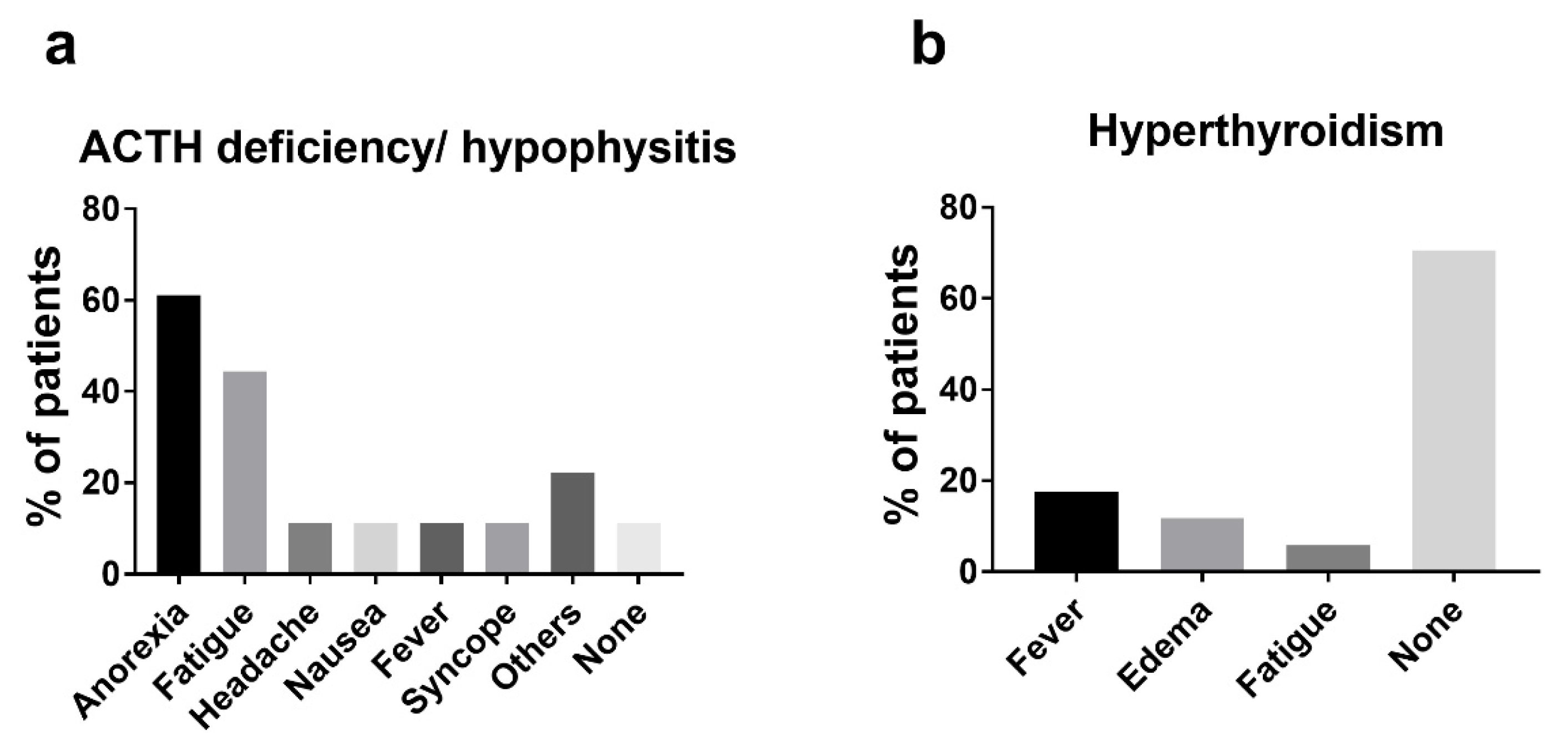
3.3. Endocrine irAEs
3.4. Non-Endocrine irAEs
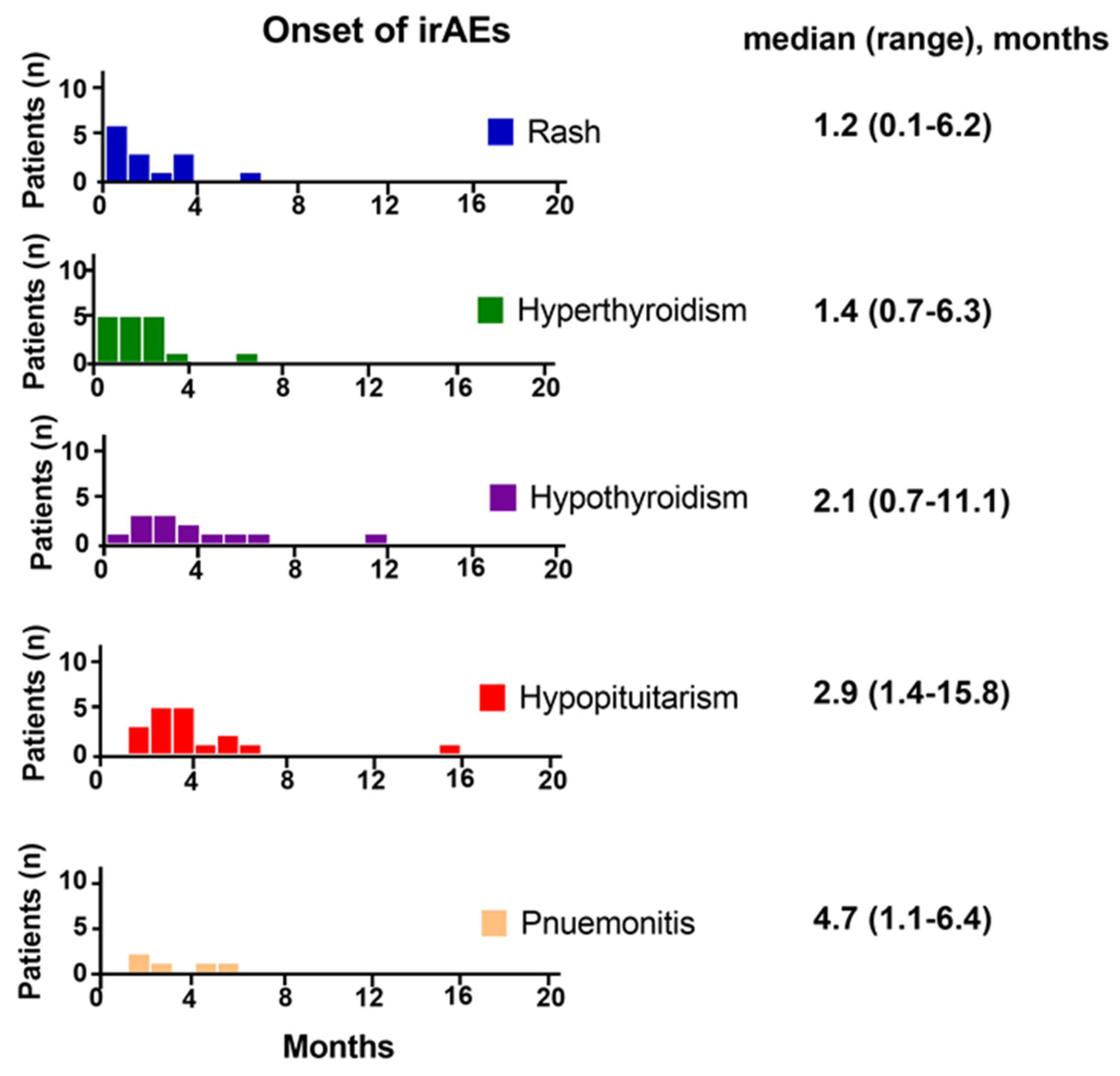
3.5. Onset of irAEs
3.6. Co-Occurrence and Sequence of Endocrine irAEs
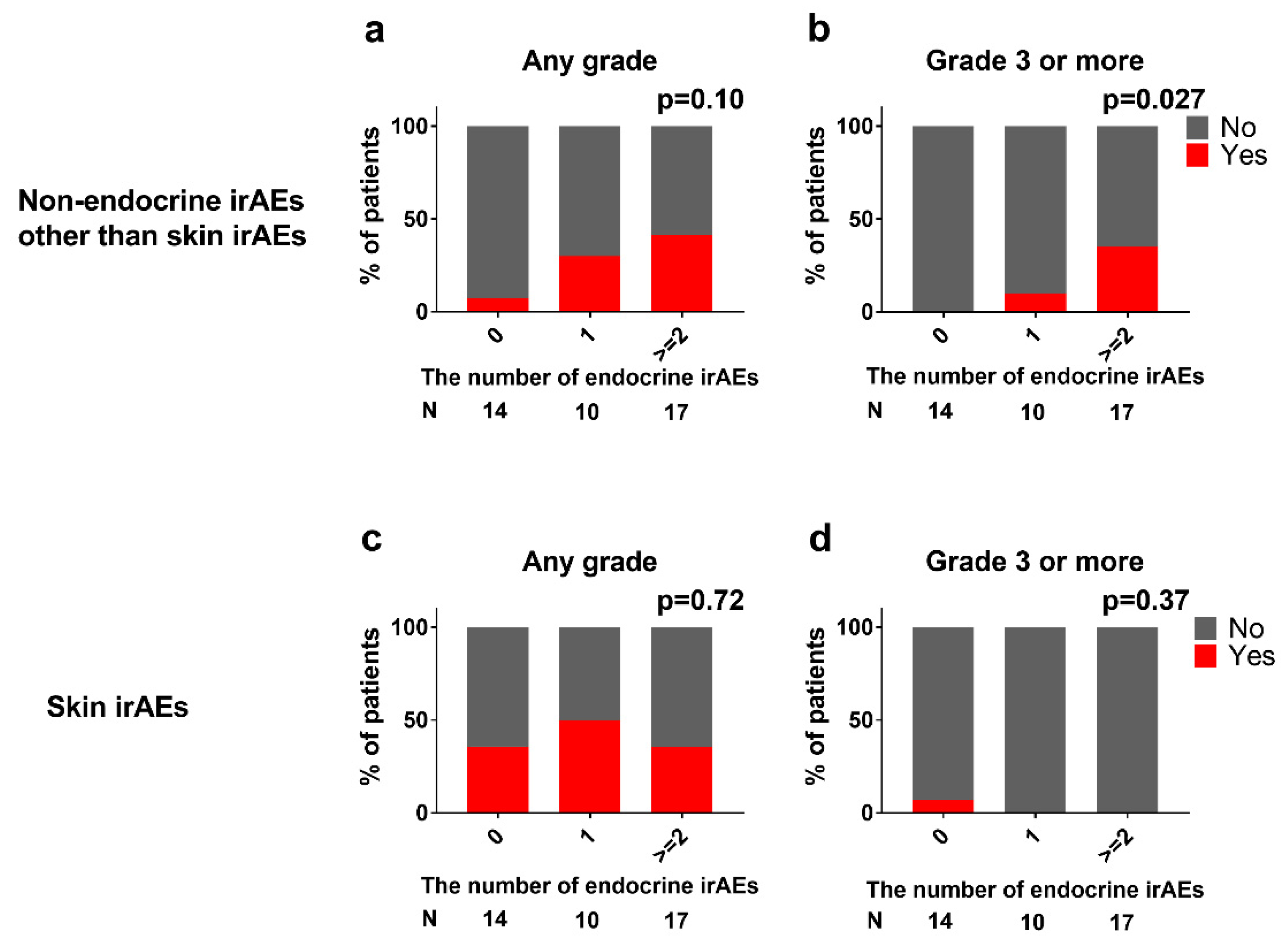
3.7. Association between Endocrine and Non-Endocrine irAEs
3.8. Assessing Predisposing Factors for High Grade irAEs
3.9. Corticosteroid Therapy and Thyroid Hormone Replacement Therapy
4. Discussion
4.1. Hypophysitis and Hypopituitarism
4.2. Thyroid Dysfunction
4.3. Non-Endocrine irAEs
4.4. Ethnic Differences in the Incidence and Severity of irAEs
4.5. Difference in the Number of Endocrine irAEs between CheckMate 214 and This Study
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Frontera, O.A.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Larkin, J.; Sileni, V.C.; Gonzalez, R.; Grob, J.-J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef] [PubMed]
- Postow, M.A.; Callahan, M.K.; Wolchok, J.D. Immune Checkpoint Blockade in Cancer Therapy. J. Clin. Oncol. 2015, 33, 1974–1982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutros, C.; Tarhini, A.; Routier, E.; Lambotte, O.; Ladurie, F.L.; Carbonnel, F.; Izzeddine, H.; Marabelle, A.; Champiat, S.; Berdelou, A.; et al. Safety profiles of anti-CTLA-4 and anti-PD-1 antibodies alone and in combination. Nat. Rev. Clin. Oncol. 2016, 13, 473–486. [Google Scholar] [CrossRef]
- Callahan, M.K.; Wolchok, J.D. At the Bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 41–53. [Google Scholar] [CrossRef] [Green Version]
- De Filette, J.; Andreescu, C.E.; Cools, F.; Bravenboer, B.; Velkeniers, B. A Systematic Review and Meta-Analysis of Endocrine-Related Adverse Events Associated with Immune Checkpoint Inhibitors. Horm. Metab. Res. 2019, 51, 145–156. [Google Scholar] [CrossRef] [Green Version]
- Asher, N.; Ben-Betzalel, G.; Lev-Ari, S.; Shapira-Frommer, R.; Steinberg-Silman, Y.; Gochman, N.; Schachter, J.; Meirson, T.; Markel, G. Real World Outcomes of Ipilimumab and Nivolumab in Patients with Metastatic Melanoma. Cancers 2020, 12, 2329. [Google Scholar] [CrossRef]
- Tanaka, T.; Hatakeyama, S.; Numakura, K.; Kido, K.; Noro, D.; Oikawa, M.; Hosogoe, S.; Tokui, N.; Yamamoto, H.; Narita, S.; et al. Efficacy and safety of first-line nivolumab plus ipilimumab in patients with metastatic renal cell carcinoma: A multicenter retrospective study. Int. J. Urol. 2020, 27, 1095–1100. [Google Scholar] [CrossRef]
- Kobayashi, T.; Iwama, S.; Yasuda, Y.; Okada, N.; Okuji, T.; Ito, M.; Onoue, T.; Goto, M.; Sugiyama, M.; Tsunekawa, T.; et al. Pituitary dysfunction induced by immune checkpoint inhibitors is associated with better overall survival in both malignant melanoma and non-small cell lung carcinoma: A prospective study. J. Immunother. Cancer 2020, 8, e000779. [Google Scholar] [CrossRef]
- Arima, H.; Iwama, S.; Inaba, H.; Ariyasu, H.; Makita, N.; Otsuki, M.; Kageyama, K.; Imagawa, A.; Akamizu, T. Management of immune-related adverse events in endocrine organs induced by immune checkpoint inhibitors: Clinical guidelines of the Japan Endocrine Society. Endocr. J. 2019, 66, 581–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motzer, R.J.; Rini, B.I.; McDermott, D.F.; Frontera, O.A.; Hammers, H.J.; Carducci, M.A.; Salman, P.; Escudier, B.; Beuselinck, B.; Amin, A.; et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019, 20, 1370–1385. [Google Scholar] [CrossRef]
- Faje, A. Hypophysitis: Evaluation and Management. Clin. Diabetes Endocrinol. 2016, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Faje, A.T.; Sullivan, R.; Lawrence, D.; Tritos, N.A.; Fadden, R.; Klibanski, A.; Nachtigall, L. Ipilimumab-Induced Hypophysitis: A Detailed Longitudinal Analysis in a Large Cohort of Patients With Metastatic Melanoma. J. Clin. Endocrinol. Metab. 2014, 99, 4078–4085. [Google Scholar] [CrossRef] [Green Version]
- Haanen, J.; Carbonnel, F.; Robert, C.; Kerr, K.; Peters, S.; Larkin, J.; Jordan, K. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv119–iv142. [Google Scholar] [CrossRef]
- Caturegli, P.; Di Dalmazi, G.; Lombardi, M.; Grosso, F.; Larman, H.B.; Larman, T.; Taverna, G.; Cosottini, M.; Lupi, I. Hypophysitis Secondary to Cytotoxic T-Lymphocyte–Associated Protein 4 Blockade. Am. J. Pathol. 2016, 186, 3225–3235. [Google Scholar] [CrossRef] [Green Version]
- Iwama, S.; De Remigis, A.; Callahan, M.K.; Slovin, S.F.; Wolchok, J.D.; Caturegli, P. Pituitary Expression of CTLA-4 Mediates Hypophysitis Secondary to Administration of CTLA-4 Blocking Antibody. Sci. Transl. Med. 2014, 6, 230ra45. [Google Scholar] [CrossRef]
- Gough, S.C.L.; Walker, L.S.K.; Sansom, D. CTLA4 gene polymorphism and autoimmunity. Immunol. Rev. 2005, 204, 102–115. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, K.; Scotland, R.; Lee, P.; Liu, D.; Groshen, S.; Snively, J.; Sian, S.; Nichol, G.; Davis, T.; Keler, T.; et al. Autoimmunity in a Phase I Trial of a Fully Human Anti-Cytotoxic T-Lymphocyte Antigen-4 Monoclonal Antibody With Multiple Melanoma Peptides and Montanide ISA 51 for Patients With Resected Stages III and IV Melanoma. J. Clin. Oncol. 2005, 23, 741–750. [Google Scholar] [CrossRef]
- Breunis, W.B.; Tarazona, E.; Chen, R.; Kiley, M.; Rosenberg, S.A.; Chanock, S.J. Influence of Cytotoxic T Lymphocyte-associated Antigen 4 (CTLA4) Common Polymorphisms on Outcome in Treatment of Melanoma Patients With CTLA-4 Blockade. J. Immunother. 2008, 31, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corsello, S.M.; Barnabei, A.; Marchetti, P.; De Vecchis, L.; Salvatori, R.; Torino, F. Endocrine Side Effects Induced by Immune Checkpoint Inhibitors. J. Clin. Endocrinol. Metab. 2013, 98, 1361–1375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, I.; Sakane, Y.; Fukuda, Y.; Fujii, T.; Taura, D.; Hirata, M.; Hirota, K.; Ueda, Y.; Kanai, Y.; Yamashita, Y.; et al. Clinical Features of Nivolumab-Induced Thyroiditis: A Case Series Study. Thyroid 2017, 27, 894–901. [Google Scholar] [CrossRef]
- Angell, T.E.; Min, L.; Wieczorek, T.J.; Hodi, F.S. Unique cytologic features of thyroiditis caused by immune checkpoint inhibitor therapy for malignant melanoma. Genes Dis. 2018, 5, 46–48. [Google Scholar] [CrossRef]
- Yang, J.; He, X.; Lv, Q.; Jing, J.; Shi, H. Management of Adverse Events in Cancer Patients Treated With PD-1/PD-L1 Blockade: Focus on Asian Populations. Front. Pharmacol. 2019, 10, 726. [Google Scholar] [CrossRef] [Green Version]
- Nishio, M.; Hida, T.; Atagi, S.; Sakai, H.; Nakagawa, K.; Takahashi, T.; Nogami, N.; Saka, H.; Takenoyama, M.; Maemondo, M.; et al. Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non-squamous non-small cell lung cancer. ESMO Open 2017, 2, e000108. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.S.; Lee, K.H.; Cho, E.K.; Kim, D.-W.; Kim, S.-W.; Kim, J.-H.; Cho, B.C.; Kang, J.H.; Han, J.-Y.; Min, Y.J.; et al. Nivolumab in advanced non-small-cell lung cancer patients who failed prior platinum-based chemotherapy. Lung Cancer 2018, 122, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osawa, M.; Hasegawa, M.; Bello, A.; Roy, A.; Hruska, M.W. Population pharmacokinetics analysis of nivolumab in Asian and non-Asian patients with gastric and gastro-esophageal junction cancers. Cancer Chemother. Pharmacol. 2019, 83, 705–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, Y.; Kondo, T.; Kimura, G.; Inoue, T.; Wakumoto, Y.; Yao, M.; Sugiyama, T.; Oya, M.; Fujii, Y.; Obara, W.; et al. Nivolumab plus ipilimumab versus sunitinib in previously untreated advanced renal-cell carcinoma: Analysis of Japanese patients in CheckMate 214 with extended follow-up. Jpn. J. Clin. Oncol. 2020, 50, 12–19. [Google Scholar] [CrossRef] [Green Version]


| N | (%) | ||
|---|---|---|---|
| Age | Median (range) | 68 | (44–87) |
| Sex | Man | 28 | (68) |
| Woman | 13 | (32) | |
| Prior Nephrectomy | Yes | 17 | (41) |
| No | 24 | (59) | |
| IMDC risk | Intermediate | 15 | (37) |
| Poor | 26 | (63) | |
| Histology | Clear cell | 32 | (78) |
| Non-clear cell | 7 | (17) | |
| Unknown | 2 | (5) |
| Grade 1–2 | Grade 3–4 | All Grade | ||||||
|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |||
| Endocrine | Pituitary | Hypopituitarism | 4 | (10) | 14 | (34) | 18 | (44) |
| Thyroid | Hyperthyroidism/ thyroiditis | 15 | (37) | 2 | (5) | 17 | (41) | |
| Primary hypothyroidism | 9 | (22) | 0 | (0) | 9 | (22) | ||
| Non-endocrine | Respiratory | Pneumonitis | 2 | (5) | 3 | (7) | 5 | (12) |
| Cardiac | Myocarditis | 0 | (0) | 1 | (2) | 1 | (2) | |
| Gastrointestinal | Colitis | 1 | (2) | 0 | (0) | 1 | (2) | |
| Renal | Acute kidney injury/ creatinine increased | 2 | (5) | 1 | (2) | 3 | (7) | |
| Proteinuria | 1 | (2) | 0 | (0) | 1 | (2) | ||
| Hepatobiliary | Hepatitis/ ALT increased | 1 | (2) | 2 | (5) | 3 | (7) | |
| Nervous system | Radiculitis | 1 | (2) | 0 | (0) | 1 | (2) | |
| Skin | Rash/dermatitis | 12 | (29) | 1 | (2) | 13 | (32) | |
| Pruritus | 2 | (5) | 0 | (0) | 2 | (5) | ||
| Hypopigmentation | 1 | (2) | 0 | (0) | 1 | (2) | ||
| Musculoskeletal | Arthritis | 1 | (2) | 0 | (0) | 1 | (2) | |
| ≥Grade 3 irAEs (N = 24) | ≤Grade 2 or No irAEs (N = 17) | p Values | ||
|---|---|---|---|---|
| Age | median (range) | 68 (44–87) | 69 (56–83) | 0.43 |
| Sex | Man | 15 | 13 | 0.50 |
| Woman | 9 | 4 | ||
| Prior Nephrectomy | Yes | 7 | 10 | 0.11 |
| No | 17 | 7 | ||
| IMDC risk | Intermediate | 14 | 12 | 0.51 |
| Poor | 10 | 5 | ||
| Histology | Clear cell | 18 | 15 | 0.40 |
| Non-clear cell | 4 | 2 | ||
| Unknown | 2 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Washino, S.; Takeshita, H.; Inoue, M.; Kagawa, M.; Soma, T.; Yamada, H.; Kageyama, Y.; Miyagawa, T.; Kawakami, S. Real-World Incidence of Immune-Related Adverse Events Associated with Nivolumab Plus Ipilimumab in Patients with Advanced Renal Cell Carcinoma: A Retrospective Observational Study. J. Clin. Med. 2021, 10, 4767. https://doi.org/10.3390/jcm10204767
Washino S, Takeshita H, Inoue M, Kagawa M, Soma T, Yamada H, Kageyama Y, Miyagawa T, Kawakami S. Real-World Incidence of Immune-Related Adverse Events Associated with Nivolumab Plus Ipilimumab in Patients with Advanced Renal Cell Carcinoma: A Retrospective Observational Study. Journal of Clinical Medicine. 2021; 10(20):4767. https://doi.org/10.3390/jcm10204767
Chicago/Turabian StyleWashino, Satoshi, Hideki Takeshita, Masaharu Inoue, Makoto Kagawa, Takahiko Soma, Hodaka Yamada, Yukio Kageyama, Tomoaki Miyagawa, and Satoru Kawakami. 2021. "Real-World Incidence of Immune-Related Adverse Events Associated with Nivolumab Plus Ipilimumab in Patients with Advanced Renal Cell Carcinoma: A Retrospective Observational Study" Journal of Clinical Medicine 10, no. 20: 4767. https://doi.org/10.3390/jcm10204767
APA StyleWashino, S., Takeshita, H., Inoue, M., Kagawa, M., Soma, T., Yamada, H., Kageyama, Y., Miyagawa, T., & Kawakami, S. (2021). Real-World Incidence of Immune-Related Adverse Events Associated with Nivolumab Plus Ipilimumab in Patients with Advanced Renal Cell Carcinoma: A Retrospective Observational Study. Journal of Clinical Medicine, 10(20), 4767. https://doi.org/10.3390/jcm10204767






