Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility and Selection Criteria
2.2. Search Strategy
2.3. Data Extraction
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
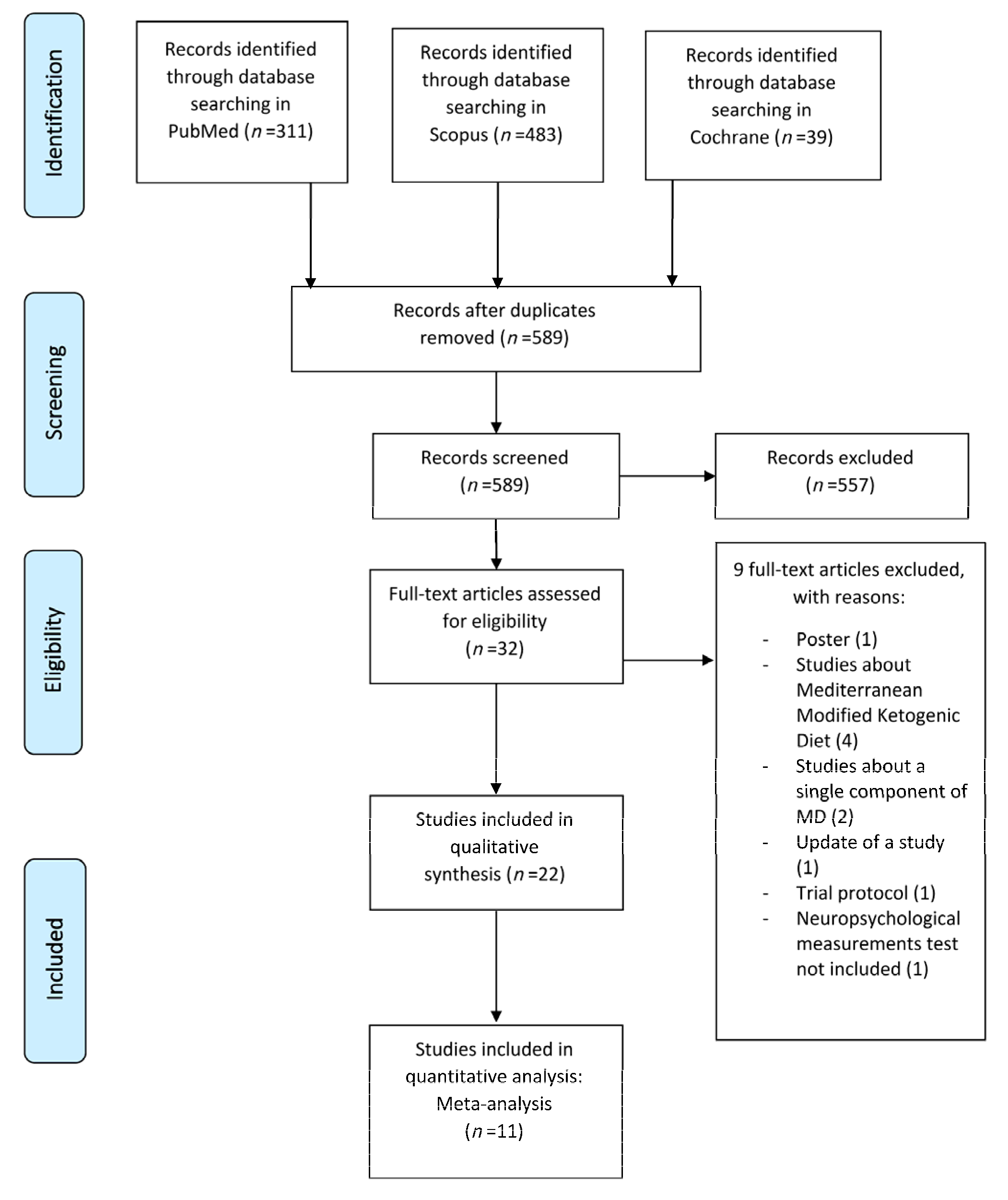
3. Results
3.1. Qualitative Synthesis
3.1.1. Participants Demographic and Clinical Characteristics
3.1.2. Study Design and Mediterranean Diet Adherence
3.1.3. Effects of Mediterranean Diet Adherence on Neuropsychological Tests and Cognitive Function
3.1.4. Effects of Mediterranean Diet Adherence on Magnetic Resonance Imaging Volumetry
3.1.5. Effects of Mediterranean Diet Adherence on Glucose Metabolism in Brain
3.1.6. Effects of Mediterranean Diet Adherence on Brain Alzheimer’s Disease β-amyloid and Tau Tangles Deposition
3.1.7. Effects of Mediterranean Diet Adherence on Alzheimer’s Disease Risk, Incidence, or Progression from Mild Cognitive Impairment
3.1.8. Vascular Risk and Lifestyle Variables Included in the Studies
3.2. Quantitative Synthesis
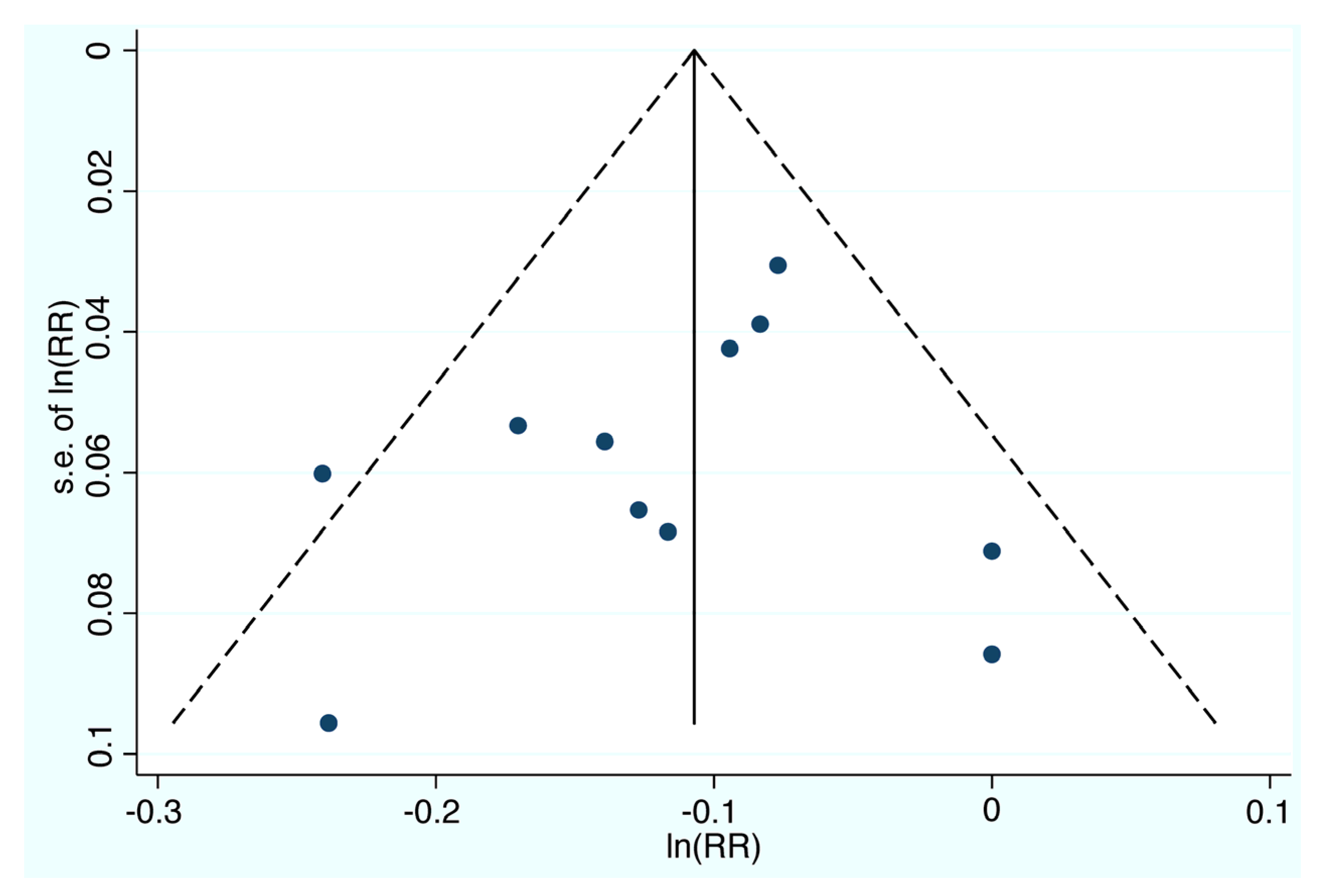
3.2.1. Risk of Bias
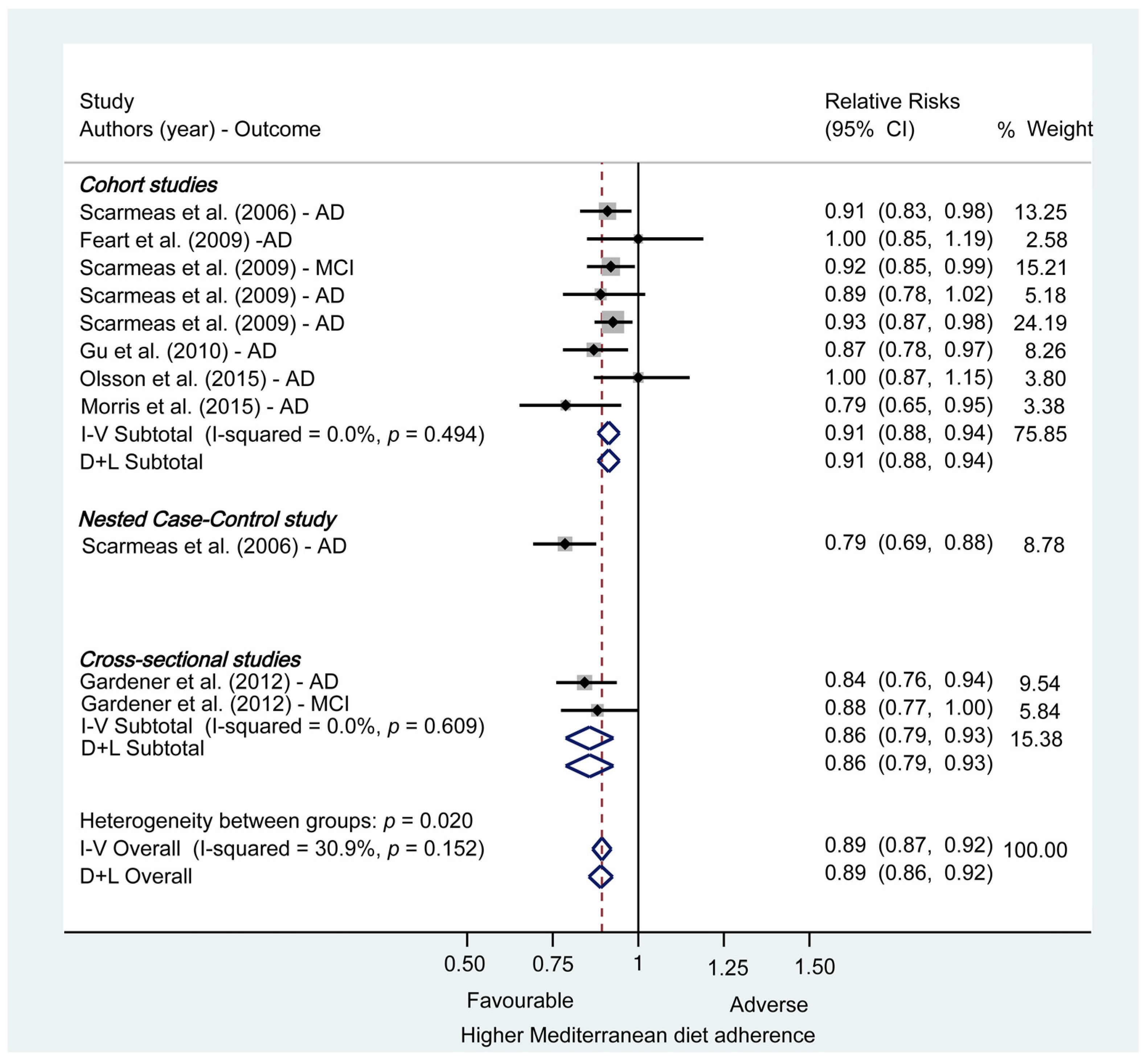
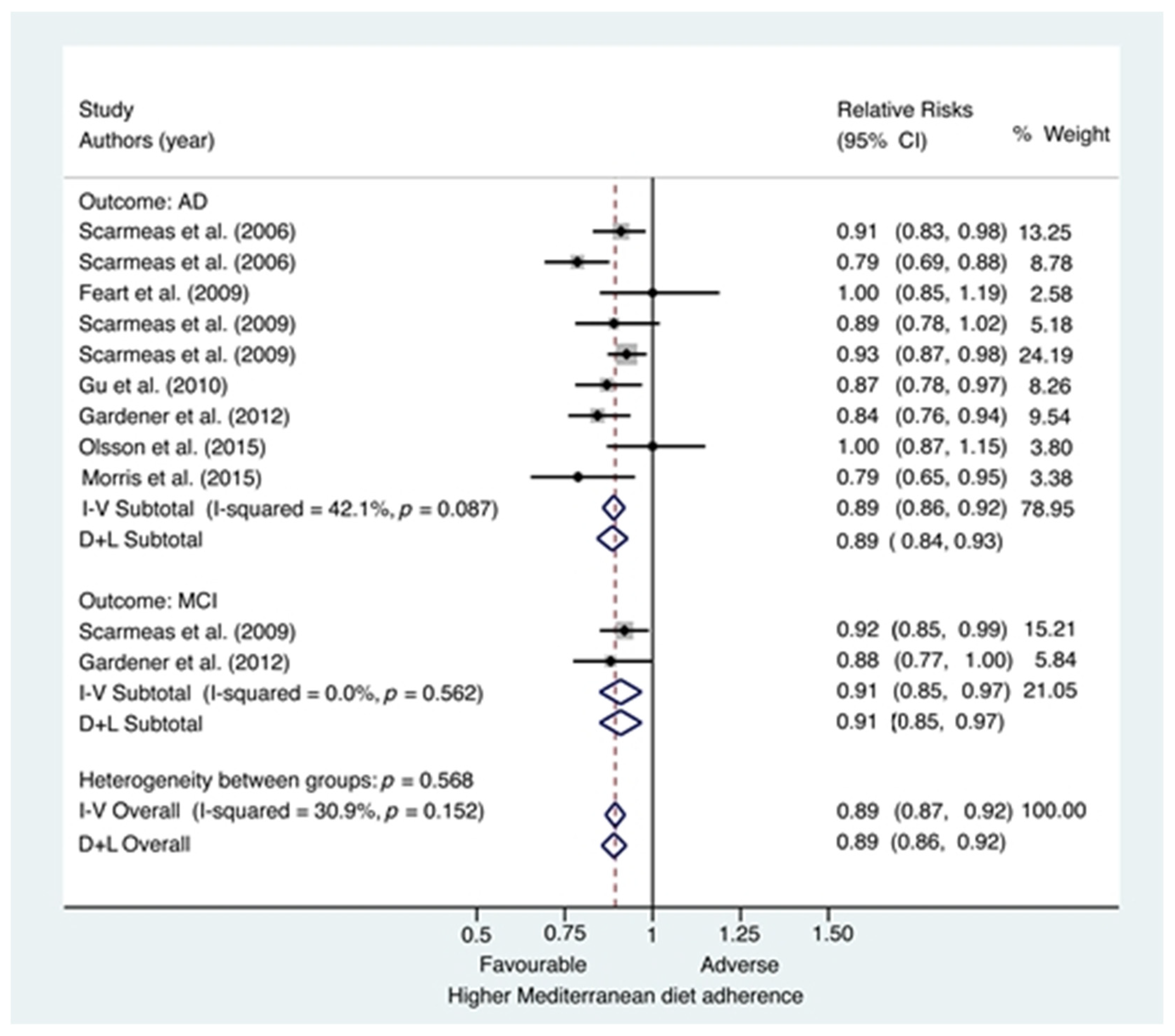
3.2.2. Meta-analysis: Mediterranean Diet and Risk of Mild Cognitive Impairment and Alzheimer’s Disease
3.2.3. Subgroup and Sensibility Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
- (1)
- Diet, Mediterranean [Mesh] OR Mediterranean Diet;
- (2)
- Alzheimer Disease [Mesh] OR Mild Cognitive impairment OR Cognitive Dysfunction [Mesh] OR Cognitive Decline OR Alzheimer OR Cognitive Aging [Mesh];
- (3)
- (1) and (2).
Appendix B
| Authors (Year) | Type of Study | Selection | Comparability | Outcome/Exposure | Overall | Risk of Bias |
|---|---|---|---|---|---|---|
| Ouctome: AD | ||||||
| Scarmeas et. al. (2006) | Nested Case-control | 3 | 2 | 2 | 7 | Low |
| Scarmeas et. al. (2006) | Prospective cohort | 4 | 2 | 2 | 8 | Low |
| Feart et. al. (2009) | Prospective cohort | 4 | 2 | 2 | 8 | Low |
| Scarmeas et. al. (2009) | Prospective cohort | 3 | 2 | 3 | 8 | Low |
| Scarmeas et. al. (2009) | Prospective cohort | 3 | 1 | 3 | 7 | Low |
| Gu et. al. (2010) | Prospective cohort | 3 | 2 | 3 | 8 | Low |
| Gardener et. al. (2012) | Cross-sectional | 3 | 2 | 1 | 6 | Moderate |
| Olsson et. al. (2015) | Prospective cohort | 3 | 2 | 2 | 7 | Low |
| Morris et. al. (2015) | Prospective cohort | 3 | 2 | 3 | 8 | Low |
| Ouctome: MCI | ||||||
| Scarmeas et. al. (2009) | Prospective cohort | 4 | 2 | 3 | 9 | Low |
| Gardener et. al. (2012) | Cross-sectional | 3 | 2 | 1 | 6 | Moderate |
References
- Walters, M.J.; Sterling, J.; Quinn, C.; Ganzer, C.; Osorio, R.S.; Andrews, R.D.; Matthews, D.C.; Vallabhajosula, S.; de Leon, M.; Isaacson, R.S.; et al. Associations of lifestyle and vascular risk factors with Alzheimer’s brain biomarker changes during middle age: A 3-year longitudinal study in the broader New York City area. BMJ Open 2018, 8, e023664. [Google Scholar] [CrossRef] [Green Version]
- Organización Mundial de la Salud. Demencia. 2019. Available online: https://www.who.int/es/news-room/fact-sheets/detail/dementia (accessed on 7 January 2021).
- Mosconi, L.; Walters, M.; Sterling, J.; Quinn, C.; McHugh, P.; E Andrews, R.; Matthews, D.C.; Ganzer, C.; Osorio, R.S.; Isaacson, R.S.; et al. Lifestyle and vascular risk effects on MRI-based biomarkers of Alzheimer’s disease: A cross-sectional study of middle-aged adults from the broader New York City area. BMJ Open 2018, 8, e019362. [Google Scholar] [CrossRef] [PubMed]
- Global Action Plan on the Public Health Response to Dementia 2017–2025; World Health Organization: Geneva, Switherlands, 2017; Available online: https://apps.who.int/iris/bitstream/handle/10665/259615/9789241513487-eng.pdf;jsessionid=93194072084D7FB27389487E82081E22?sequence=1 (accessed on 7 January 2021).
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and Future Treatments in Alzheimer Disease: An Update. J. Central Nerv. Syst. Dis. 2020, 12, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iadecola, C.; Duering, M.; Hachinski, V.; Joutel, A.; Pendlebury, S.T.; Schneider, J.A.; Dichgans, M. Vascular Cognitive Impairment and Dementia: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019, 73, 3326–3344. [Google Scholar] [CrossRef] [PubMed]
- Berti, V.; Walters, M.; Sterling, J.; Quinn, C.G.; Logue, M.; Andrews, R.; Matthews, D.C.; Osorio, R.S.; Pupi, A.; Vallabhajosula, S.; et al. Mediterranean diet and 3-year Alzheimer brain biomarker changes in middle-aged adults. Neurology 2018, 90, e1789–e1798. [Google Scholar] [CrossRef]
- Vassilaki, M.; Aakre, J.A.; Syrjanen, J.A.; Mielke, M.M.; Geda, Y.E.; Kremers, W.K.; Machulda, M.M.; Alhurani, R.E.; Staubo, S.C.; Knopman, D.S.; et al. Mediterranean Diet, Its Components, and Amyloid Imaging Biomarkers. J. Alzheimer’s Dis. 2018, 64, 281–290. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch. Neurol. 2006, 63, 1709–1717. [Google Scholar] [CrossRef] [Green Version]
- Karstens, A.J.; Tussing-Humphreys, L.; Zhan, L.; Rajendran, N.; Cohen, J.; Dion, C.; Zhou, X.J.; Lamar, M. Associations of the Mediterranean diet with cognitive and neuroimaging phenotypes of dementia in healthy older adults. Am. J. Clin. Nutr. 2019, 109, 361–368. [Google Scholar] [CrossRef]
- Calil, S.R.; Brucki, S.M.; Nitrini, R.; Yassuda, M.S. Adherence to the Mediterranean and MIND diets is associated with better cognition in healthy seniors but not in MCI or AD. Clin. Nutr. ESPEN 2018, 28, 201–207. [Google Scholar] [CrossRef]
- Singh, B.; Parsaik, A.K.; Mielke, M.M.; Erwin, P.J.; Knopman, D.S.; Petersen, R.C.; Roberts, R.O. Association of mediterranean diet with mild cognitive impairment and Alzheimer’s disease: A systematic review and meta-analysis. J. Alzheimers Dis. 2014, 39, 271–282. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Rainey-Smith, S.R.; Gu, Y.; Gardener, S.L.; Doecke, J.D.; Villemagne, V.L.; Brown, B.M.; Taddei, K.; Laws, S.M.; Sohrabi, H.R.; Weinborn, M.; et al. Mediterranean diet adherence and rate of cerebral Aβ-amyloid accumulation: Data from the Australian Imaging, Biomarkers and Lifestyle Study of Ageing. Transl. Psychiatry 2018, 8, 238. [Google Scholar] [CrossRef] [Green Version]
- Coelho-Júnior, H.J.; Trichopoulou, A.; Panza, F. Cross-sectional and longitudinal associations between adherence to Mediterranean diet with physical performance and cognitive function in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2021, 70, 101395. [Google Scholar] [CrossRef]
- Gardener, S.; Gu, Y.; Rainey-Smith, S.R.; Keogh, J.B.; Clifton, P.M.; Mathieson, S.L.; Taddei, K.; Mondal, A.; Ward, V.K.; Scarmeas, N.; et al. Adherence to a Mediterranean diet and Alzheimer’s disease risk in an Australian population. Transl. Psychiatry 2012, 2, e164. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Luchsinger, J.A.; Schupf, N.; Brickman, A.M.; Cosentino, S.; Tang, M.X.; Stern, Y. Physical activity, diet, and risk of Alzheimer disease. JAMA 2009, 302, 627–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Sun, D. Adherence to Mediterranean diet and risk of developing cognitive disorders: An updated systematic review and meta-analysis of prospective cohort studies. Sci. Rep. 2017, 7, 41317. [Google Scholar] [CrossRef]
- Petersson, S.D.; Philippou, E. Mediterranean Diet, Cognitive Function, and Dementia: A Systematic Review of the Evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosconi, L.; Murray, J.; Tsui, W.H.; Li, Y.; Davies, M.; Williams, S.; Pirraglia, E.; Spector, N.; Osorio, R.S.; Glodzik, L.; et al. Mediterranean Diet and Magnetic Resonance Imaging-Assessed Brain Atrophy in Cognitively Normal Individuals at Risk for Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2014, 1, 23–32. [Google Scholar] [PubMed]
- Hill, E.; Goodwill, A.M.; Gorelik, A.; Szoeke, C. Diet and biomarkers of Alzheimer’s disease: A systematic review and meta-analysis. Neurobiol. Aging 2019, 76, 45–52. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ 2009, 339, b2535. [Google Scholar] [CrossRef] [Green Version]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 31 July 2021).
- Zhang, J.; Yu, K.F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenland, S. Model-based estimation of relative risks and other epidemiologic measures in studies of common outcomes and in case-control studies. Am. J. Epidemiol. 2004, 160, 301–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNutt, L.A.; Wu, C.; Xue, X.; Hafner, J.P. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am. J. Epidemiol. 2003, 157, 940–943. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimers Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenland, S.; Longnecker, M. Methods for Trend Estimation from Summarized Dose-Response Data, with Applications to Meta-Analysis. Am. J. Epidemiol. 1992, 135, 1301–1309. [Google Scholar] [CrossRef] [PubMed]
- Orsini, N.; Bellocco, R.; Greenland, S. Generalized Least Squares for Trend Estimation of Summarized Dose–response Data. Stata J. Promot. Commun. Stat. Stata 2006, 6, 40–57. [Google Scholar] [CrossRef] [Green Version]
- Ballarini, T.; van Lent, D.M.; Brunner, J.; Schröder, A.; Wolfsgruber, S.; Altenstein, S.; Brosseron, F.; Buerger, K.; Dechent, P.; Dobisch, L.; et al. DELCODE study group. Mediterranean Diet, Alzheimer Disease Biomarkers and Brain Atrophy in Old Age. Neurology 2021, 96, e2920–e2932. [Google Scholar] [CrossRef] [PubMed]
- Hill, E.; Clifton, P.; Goodwill, A.M.; Dennerstein, L.; Campbell, S.; Szoeke, C. Dietary patterns and Œ≤-amyloid deposition in aging Australian women. Alzheimers Dement. 2018, 4, 535–541. [Google Scholar] [CrossRef]
- Hill, E.; Szoeke, C.; Dennerstein, L.; Campbell, S.; Clifton, P. Adherence to the Mediterranean Diet Is not Related to Beta-Amyloid Deposition: Data from the Women’s Healthy Ageing Project. J. Prev. Alzheimers Dis. 2018, 5, 137–141. [Google Scholar]
- Merrill, D.A.; Siddarth, P.; Raji, C.A.; Emerson, N.D.; Rueda, F.; Ercoli, L.M.; Miller, K.J.; Lavretsky, H.; Harris, L.M.; Burggren, A.C.; et al. Modifiable Risk Factors and Brain Positron Emission Tomography Measures of Amyloid and Tau in Nondemented Adults with Memory Complaints. Am. J. Geriatr. Psychiatry 2016, 24, 729–737. [Google Scholar] [CrossRef] [Green Version]
- Olsson, E.; Karlstr√∂m, B.; Kilander, L.; Byberg, L.; Cederholm, T.; Sjögren, P. Dietary patterns and cognitive dysfunction in a 12-year follow-up study of 70 year old men. J. Alzheimers Dis. 2015, 43, 109–119. [Google Scholar] [CrossRef]
- Matthews, D.C.; Davies, M.; Murray, J.; Williams, S.; Tsui, W.H.; Li, Y.; Andrews, R.D.; Lukic, A.; McHugh, P.; Vallabhajosula, S.; et al. Physical Activity, Mediterranean Diet and Biomarkers-Assessed Risk of Alzheimer’s: A Multi-Modality Brain Imaging Study. Adv. J. Mol. Imaging 2014, 4, 43–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, Y.; Luchsinger, J.A.; Stern, Y.; Scarmeas, N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer’s disease. J. Alzheimers Dis. 2010, 22, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feart, C. Adherence to a Mediterranean Diet, Cognitive Decline, and Risk of Dementia. JAMA 2009, 302, 638–648. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Stern, Y.; Tang, M.X.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet and risk for Alzheimer’s disease. Ann. Neurol. 2006, 59, 912–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benítez-Arciniega, A.A.; Mendez, M.A.; Baena-Díez, J.M.; Rovira Martori, M.A.; Soler, C.; Marrugat, J.; Covas, M.I.; Sanz, H.; Llopis, A.; Schröder, H. Concurrent and construct validity of Mediterranean diet scores as assessed by an FFQ. Public Health Nutr. 2011, 14, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C. Nutritional Epidemiology; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Charisis, S.; Ntanasi, E.; Yannakoulia, M.; Anastasiou, C.A.; Kosmidis, M.H.; Dardiotis, E.; Hadjigeorgiou, G.; Sakka, P.; Scarmeas, N. Mediterranean diet and risk for dementia and cognitive decline in a Mediterranean population. J. Am. Geriatr. Soc. 2021, 69, 1548–1559. [Google Scholar] [CrossRef]
| First Author, Year and Country | Sample Size | Mean Age (Years) | Study Desing | Diet Measures: FFQ → Diet Score (Scale)/Pattern | Other Tests and Measures | Results |
|---|---|---|---|---|---|---|
| Ballarini et al., 2021 Germany [30] | N = 512 | 69.5 | CS Groups: CN (N = 169) MCI (N = 81) SCD (N = 209) AD (N = 53) | EPIC-FFQ → MDs (1–9) -Low MDA ( 0–3) -Medium MDA (4–5) -High MDA (6–8) | -MRI volumetry -NPT (ADAS-COG; WMS; CERAD; SDMT; FNT; FCSRT; VF; BNT; TMT, CDT) -Cerebrospinal fluid (Aβ42/40 ratio, pTau181) | Higher MDA related to larger mediotemporal gray matter volume (p < 0.05), better memory (p = 0.038), and less amyloid (p = 0.008) and pTau181 pathology (p = 0.004) |
| Karstens et al., 2019 USA [10] | N = 82 | 68.8 | CS Groups: ND (N = 82) | BFFQ 2005 → MDs (0–55) -Median split: -Low MDA (N = 39) -High MDA (N = 43) | -MRI volumetry -NPT (CVLT-II, TMT, WAIS-IV digit symbol coding, WAIS-IV letter number sequencing subtest, WTAR, MMSE, BDI, BAI) -MFSRP -BMI | High adherence to MD is related with better learning and memory in NPT (p = 0.007) and with larger dentate gyrus volumes compared with low MDA (p = 0.03). MDA is not related with information processing or executive functioning in NPT and neither with white matter hyperintensity. |
| Walters et al., 2018 USA [1] | N = 70 | 49 | L for 3 years Groups: CN (N = 70) | HWSQFFQ → MDs (0–9) - Continuous variable | -MRI volumetry -FDG-PET -PiB-PET -NPT (WAIS digit symbol substitution, WAIS vocabulary, MMSE, paragraph recall, paired associates recall, object naming, design tests) - Vascular risk measures (BMI, blood pressure, plasma cholesterol/HDL ratio, plasma homocysteine QUICKI) -APO-E G -MLTAQ -Intellectual activity through life interview | Lower adherence to MD is related with faster decline in FDG-PET (p < 0.05). Adherence to MD is not related with NPT, PiB-PET or MRI measures. Exercise and intellectual activity are not related with changes in AD biomarkers or NPT. |
| Calil et al., 2018 Brazil [11] | N = 96 | 75.2 | CS Groups: CN (N = 36) MCI (N = 30) AD (N = 30) | FFQ → MDs (0–55) -Tertiles: -Low MDA -Middle MDA -High MDA | -NPT (MMSE, BCSB, VF, CDT, GDS) -BMI | Higher adherence to MD is related with higher MMSE and BCSB Learning scores in CN group (p < 0.05). No associations are found between other NPT outcomes and MDs. No associations are found between dietary patterns and NPT outcomes in MCI or AD participants. |
| Hill et al., 2018 Australia [31] | N = 115 | 70 | CS Groups: Women of the Women’s Health Ageing Project (N = 115) | DQESv2 → -High fat pattern (N = 24) -MD pattern (N = 31) -Junk food pattern (N = 24) -Low fat pattern (N = 35) | -F18F-PET -NPT (CERAD) -BMI -APO-E G | Adherence to junk food pattern was related with higher F18F-PET measures (p = 0.03). Other dietary patterns are not related with F18-PET. |
| Rainey-Smith et al., 2018 Australia [14] | N = 77 | 71.5 | L for 3 years Groups: CN (N = 77) | CCVFFQ → MDs (0–9) -Continuous variable | -PiB-PET -APO-E G -BMI -NPT (MMSE) | Higher MDs is related with lower PiB-PET measures (p = 0.007). |
| Vassilaki et al., 2018 USA [8] | N = 278 | 77.7 | CS Groups: CN (N = 278) | MB1995RHHFFQ → MDs (0–9) -Continuous variable | -PiB-PET -BMI -NPT | Higher MDs (p = 0.012), vegetable consumption (p = 0.002), Vitamin A (p = 0.003) and β-carotene intakes (p = 0.005) and moderate alcohol consumption (p = 0.03) are related with lower PiB-PET measures. |
| Berti et al., 2018 USA [7] | N = 70 | 50 | CS and L for 3 years Groups: CN (N = 70) | HFFQ → MDs (0–9) -Median split: -Low MDA (N = 36) -High MDA (N = 34) | -NPT (GDS, HDRS, MMSE, CDR, WAIS digit symbol substitution, paired associates recall, paragraph recall, design tests, object naming) -MRI volumetry -FDG-PET -PiB-PET -Vascular risk measures (BMI, blood pressure, plasma cholesterol, triglycerides, plasma homocysteine, fasting glucose, hip-to-waist ratio, QUICKI, fasting glucose) | Low MDA is related with lower FDG-PET measures and higher PiB-PET measures compared with high MDA at baseline (p < 0.001). Low MDA is related with greater FDG-PET declines and PiB-PET increases compared with high MDA longitudinally (p < 0.001). No relation is observed between MDA and MRI volumes. |
| Hill et al., 2018 Australia [32] | N = 111 | 69.7 | CS Groups: Women of the Women’s Health Ageing Project (N = 111) | DQESv2→ MDs (0–18 ) -Continuous variable -Tertiles: -Low MDA (N = 56) -Middle MDA (N = 32) -High MDA (N= 23) | -NPT -MRI -F18F-PET -IPAQ -BMI -IPAQ-E -AACVRs | There is no significant relation between MDA and F18F-PET measures. |
| Mosconi et al., 2018 USA [3] | N = 116 | 50 | CS Groups: CN (N = 116) | BFFQ y HFFQ → MDs -Continuous variable | -MRI volumetry -NPT (CDR, GDetS, HDRS, memory, WAIS digit symbol substitution, WAIS vocabulary) -Intellectual activity through life 25-item interview -Vascular risk measures (BMI, blood pressure, plasma cholesterol, plasma homocysteine, QUICKI) -Baecke and Minnesota leisure time physical activity questionnaires | Higher MDA and higher insulin sensitivity are both significant related with higher MRI volumetry measures (p < 0.08). No other lifestyle and vascular risk variables are significant related with MRI volumetry measures. Higher MRI volumes are significant related with better cognitive performance. Intellectual enrichment is related with better cognition (p < 0.01) |
| Merrill et al., 2016 USA [33] | N = 44 | 62.6 | CS Groups: SMI (N = 24) MCI (N = 20) | 5 points Likert scale of Mediterranean-type diet -Often adherence to MD -Rarely adherence to MD | -FDDNP-PET -BMI -IPAQ-E -NPT (MMSE, HRSD, HRSA) -MRI | MCI group with above normal BMI have higher FDDNP-PET binding than MCI group with normal BMI (p = 0.02). Higher physical activity is related with lower FDDNP-PET binding in MCI group (p = 0.04) but not in SMI group. Higher consume of MD is related with lower FDDNP-PET binding in both groups (p = 0.04) |
| Morris et al., 2015 USA [27] | N = 923 | 58–98 (Range) | L for 4.5 years Groups: ND (N = 923) | HFFQ → MDs (0–55) -Continuous variables -Tertiles: -Low MDA -Middle MDA -High MDA | -NPT (CESDS) -APO-E G -BMI -Cognitively stimulating activities self-reported -Physical activity time spent self-reported | High adherence to MD have significant lower rates of AD incidence than low adherence to it (p for trend = 0.006). |
| Olsson et al., 2015 Sweeden [34] | N = 1038 | 71 | L for 12 years Groups: Men CN (N = 1038) | Seven days food record prepared by Swedish National Food Administration→ modified MDs(0–8) -Continuous variables -Tertiles: -Low MDA -Middle MDA -High MDA | -NPT (MMSE) -APO-E G -Vascular risk measures (BMI, blood pressure, plasma cholesterol, HDL and LDL cholesterol, serum triglycerides, insulin sensitivity) -CRP levels | Higher MDA is potentially- not significantly- related with lower risk of developing all-type cognitive impairment (but not with AD or all-type dementia risk). |
| Matthews et al., 2014 USA [35] | N = 45 | 54 | CS Groups: CN (N = 45) | HFFQ →MDs (0–9) -Median split: -Low MDA -High MDA | -MLTAQ -MRI volumetry -FDG-PET -PiB-PET -NPT (CDR, MMSE, HDRS, MHIS, WAIS vocabulary, WAIS digit symbol substitution, paired associated recall, paragraph recall, designs, object naming) -APO-E G -Vascular risk measures (BMI, HTWR, blood pressure, plasma cholesterol, HDL and LDL cholesterol, blood glucose, serum triglycerides, insulin sensitivity) | Lower physical activity is related with higher PiB-PET measures, lower FDG-PET measures and reduced MRI measures than higher physical activity (p < 0.001). Low MDA is related with higher PiB-PET measures, lower FDG-PET measures and reduced MRI measures than high adherence (p < 0.001). Significant interactions effects between physical activity and MDA are seen in FDG-PET measures (p = 0.003). |
| Mosconi et al., 2014 USA [20] | N = 52 | 54 | CS Groups: CN (N = 52) | HFFQ → MDs (0–9) -Continuous variable -Median split: -Low MDA -High MDA | -MRI volumetry -NPT (CDR, MMSE, HDRS, MHIS, GDetS, WAIS vocabulary, WAIS digit symbol substitution, paired associated recall, paragraph recall, designs, object naming) -Vascular risk measures (BMI, HTWR, blood pressure, plasma cholesterol, HDL and LDL cholesterol, blood glucose, serum triglycerides, plasma homocysteine, insulin sensitivity) -APO-E G | High MDA is related with greater MRI measures in left hemisphere AD-vulnerable regions compared with low MDA (p = 0.026). MDA is not related with cognitive performance. |
| Gardener et al., 2012 Australia [16] | N = 970 | 71.72 | CS Groups: CN (N = 723) MCI (N = 98) AD (N = 149) | CCVFFQ →MDs (0–9) -Continuous variable | -NPT (MMSE, LM II, D-KEFS Verbal Fluency, CVLT II Long Delay) -BMI -APO-E G | AD group has lower MDA than CN group (p < 0.001). MCI group has lower MDA than CN group (p < 0.05). MDs is related with changes in MMSE over 18 months period in CN group (p < 0.05). |
| Gu et al., 2010 USA [36] | N = 1219 | 76.7 | CS and L for 4 years Groups: ND (N = 1219) | HFFQ → MDs (0–9) -Continuous variable -Tertiles: -Low MDA -Middle MDA -High MDA | -NPT (memory, language, processing speed and visual-spatial ability) -High sensitivity CRP plasma levels -Fasting insulin serum levels -Serum total adiponectin levels -APO-E G -BMI -Modified CIC | Higher MDA is related with lower levels of hsCRP (p = 0.003). Higher MDA is not related with levels of fasting insulin or total adiponectin. Higher MDA is related with lower risk of developing AD (p for trend = 0.04). Association between MDA and AD risk of incidence did not seem to be mediated by high sensitivity CRP, fasting insulin or total adiponectin levels. |
| Scarmeas et al., 2009 USA [17] | N = 1880 | 77.2 | L for 14 years Groups: ND (N = 1880) | HFFQ →MDs (0–9) -Continuous variable -Tertiles: -Low MDA -Middle MDA -High MDA -Median split: -Low MDA -Middle MDA -High MDA | -NPT (memory, orientation, abstract reasoning, language, visual-spatial abilities, CDR) -GLTEQ -BMI -CIC -APO-E G | Middle MDA compared with low MDA reduces AD risk with HR= 0.98 (95% CI 0.72–1.33), while high MDA compared with low MDA reduces AD risk with HR = 0.6 (95% CI 0.42–0.87), (p for trend= 0.008). Some physical activity compared with no physical activity reduces AD risk with HR = 0.75 (95% CI 0.54–1.04), while much physical activity compared with no physical activity reduces AD risk with HR = 0.67 (95% CI 0.47–0.95) (p for trend = 0.03). Much physical activity and high MDA compared with no physical activity and low MDA reduces AD risk with HR = 0.65 (95% CI 0.44–0.96) (p for trend = 0.03). |
| Scarmeas et al., 2009 USA [13] | N = 1875 | 76.9 | L for 10 years Groups: CN (N = 1393) MCI (N = 482) | HFFQ →MDs (0–9) -Continuous variable -Tertiles: -Low MDA -Middle MDA -High MDA | -NPT (memory, orientation, abstract reasoning, language, visual-spatial abilities, CDR) -BMI -APO-E G | Middle MDA compared with low MDA is borderline related with lower risk of developing MCI (p = 0.24). High MDA compared with low MDA is related with lower risk of developing MCI (p = 0.05). Middle MDA compared with low MDA is related with lower risk of developing AD from MCI (p = 0.01). High MDA compared with low MDA is related with lower risk of developing AD from MCI (p = 0.02). |
| Feart et al., 2009 France [37] | N = 1410 | 75.9 | L for 5 years Groups: CN (N = 1410) AD (N = 66) | HFFQ →MDs (0–9) -Continuous variable -Tertiles: -Low MDA -Middle MDA -High MDA | -NPT (MMSE, IST, BVRT, FCSRT) -APO-E G | Higher MDA, was associated with slower MMSE cognitive decline but not with other cognitive tests this relationship was attenuated when adjusting for stroke. Higher MDA was not associated with risk for incident dementia. |
| Scarmeas et al., 2006 USA [9] | N = 1984 | 76.3 | Nested Case-control Groups: ND (N = 1790) AD (N = 194) | HFFQ →MDs (0–9) -Continuous variable -Tertiles: -Low MDA -Middle MDA -High MDA | -NPT (memory, orientation, abstract reasoning, language, visual-spatial abilities, CDR) -APO-E G -BMI -Modified CIC -Vascular risk measures (BMI, plasma cholesterol, HDL and LDL cholesterol, blood glucose, serum triglycerides, plasma homocysteine, insulin sensitivity) | Higher MDA is related with lower risk of AD (p < 0.001). High and middle MDA are related with lower risk of AD compared with low MDA (p for trend < 0.001). Vascular variables do not change de magnitude of the association. |
| Scarmeas et al., 2006 USA [38] | N = 2258 | 77.2 | L for 10 years Groups: ND (N = 2258) | HFFQ →MDs (0–9 pts.) -Continuous variable -Tertiles: -Low MDA -Middle MDA -High MDA | -NPT (memory, orientation, abstract reasoning, language, visual-spatial abilities, CDR) -APO-E G -BMI | Higher MDA is related with lower risk of AD incidence (p = 0.003). High and middle MDA are related with lower risk of AD incidence compared with low MDA (p for trend = 0.007). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Casares, N.; Gallego Fuentes, P.; Barbancho, M.Á.; López-Gigosos, R.; García-Rodríguez, A.; Gutiérrez-Bedmar, M. Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis. J. Clin. Med. 2021, 10, 4642. https://doi.org/10.3390/jcm10204642
García-Casares N, Gallego Fuentes P, Barbancho MÁ, López-Gigosos R, García-Rodríguez A, Gutiérrez-Bedmar M. Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis. Journal of Clinical Medicine. 2021; 10(20):4642. https://doi.org/10.3390/jcm10204642
Chicago/Turabian StyleGarcía-Casares, Natalia, Paloma Gallego Fuentes, Miguel Ángel Barbancho, Rosa López-Gigosos, Antonio García-Rodríguez, and Mario Gutiérrez-Bedmar. 2021. "Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis" Journal of Clinical Medicine 10, no. 20: 4642. https://doi.org/10.3390/jcm10204642
APA StyleGarcía-Casares, N., Gallego Fuentes, P., Barbancho, M. Á., López-Gigosos, R., García-Rodríguez, A., & Gutiérrez-Bedmar, M. (2021). Alzheimer’s Disease, Mild Cognitive Impairment and Mediterranean Diet. A Systematic Review and Dose-Response Meta-Analysis. Journal of Clinical Medicine, 10(20), 4642. https://doi.org/10.3390/jcm10204642






