Stratification of Hepatocellular Carcinoma Risk Following HCV Eradication or HBV Control
Abstract
1. Introduction
2. Why Should We Stratify Patients with Viral-Induced Disease According to HCC Risk?
3. Decreased HCC Incidence in Patients with HCV-Related Extensive Fibrosis or Cirrhosis Following SVR
4. Identification of Patients Infected with HCV with Higher Residual HCC Risk Following SVR
5. HCC Incidence in Patients with HBV-Related Liver Disease and Virosuppression Treated by NUCs
5.1. Patients with Cirrhosis
5.2. Patients without Cirrhosis
5.3. Differences Between ETV and TDF
6. HCC Risk Scoring Systems in Controlled Patients Infected with HBV
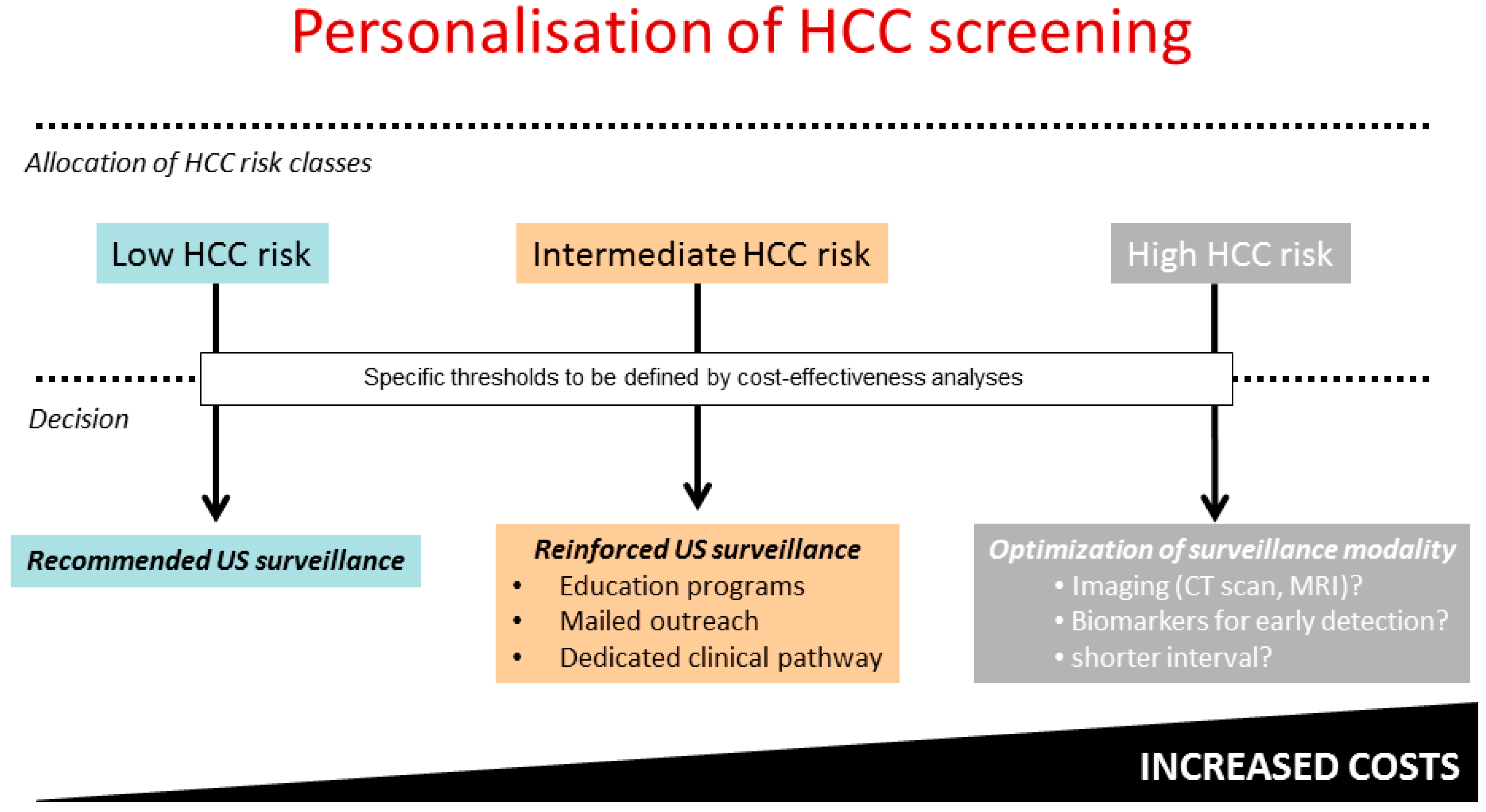
7. Perspectives: Towards Universal HCC Risk Stratification and Precision Medicine?
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- The Lancet. GLOBOCAN 2018: Counting the toll of cancer. Lancet 2018, 392, 985. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.-M.; Negro, F.; Aghemo, A.; Berenguer, M.; Dalgard, O.; Dusheiko, G.; Marra, F.; Puoti, M.; Wedemeyer, H. EASL Recommendations on Treatment of Hepatitis C 2018. J. Hepatol. 2018, 69, 461–511. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef]
- Sherman, M. HCC Risk Scores: Useful or Not? Seminars Liver Dis. 2017, 37, 287–295. [Google Scholar] [CrossRef]
- Prasad, V.; Lenzer, J.; Newman, D.H. Why cancer screening has never been shown to "save lives"—And what we can do about it. BMJ 2016, 352, h6080. [Google Scholar] [CrossRef]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients With Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef]
- Kim, S.Y.; An, J.; Lim, Y.S.; Han, S.; Lee, J.Y.; Byun, J.H.; Won, H.J.; Lee, S.J.; Lee, H.C.; Lee, Y.S. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017, 3, 456–463. [Google Scholar] [CrossRef]
- Berhane, S.; Toyoda, H.; Tada, T.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Schweitzer, N.; Vogel, A.; Manns, M.P.; Benckert, J.; et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin. Gastroenterol. Hepatol. 2016, 14, 875–886.e876. [Google Scholar] [CrossRef]
- Farhang Zangneh, H.; Wong, W.W.L.; Sander, B.; Bell, C.M.; Mumtaz, K.; Kowgier, M.; van der Meer, A.J.; Cleary, S.P.; Janssen, H.L.A.; Chan, K.K.W.; et al. Cost Effectiveness of Hepatocellular Carcinoma Surveillance After a Sustained Virologic Response to Therapy in Patients With Hepatitis C Virus Infection and Advanced Fibrosis. Clin. Gastroenterol. Hepatol. 2019, 17, 1840–1849.e1816. [Google Scholar] [CrossRef]
- Costentin, C.E.; Layese, R.; Bourcier, V.; Cagnot, C.; Marcellin, P.; Guyader, D.; Pol, S.; Larrey, D.; De Ledinghen, V.; Ouzan, D.; et al. Compliance With Hepatocellular Carcinoma Surveillance Guidelines Associated With Increased Lead-Time Adjusted Survival of Patients With Compensated Viral Cirrhosis: A Multi-Center Cohort Study. Gastroenterology 2018, 155, 431–442.e410. [Google Scholar] [CrossRef] [PubMed]
- Del Poggio, P.; Olmi, S.; Ciccarese, F.; Di Marco, M.; Rapaccini, G.L.; Benvegnu, L.; Borzio, F.; Farinati, F.; Zoli, M.; Giannini, E.G.; et al. Factors that affect efficacy of ultrasound surveillance for early stage hepatocellular carcinoma in patients with cirrhosis. Clin. Gastroenterol. Hepatol. 2014, 12, 1927–1933.e1922. [Google Scholar] [CrossRef] [PubMed]
- Goossens, N.; Singal, A.G.; King, L.Y.; Andersson, K.L.; Fuchs, B.C.; Besa, C.; Taouli, B.; Chung, R.T.; Hoshida, Y. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin. Transl. Gastroenterol. 2017, 8, e101. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.L.; An, J.; Park, J.A.; Park, S.H.; Lim, Y.S.; Lee, E.K. Magnetic Resonance Imaging Is Cost-Effective for Hepatocellular Carcinoma Surveillance in High Risk Patients with Cirrhosis. Hepatology 2018. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; Seeff, L.B.; Morgan, T.R.; di Bisceglie, A.M.; Sterling, R.K.; Curto, T.M.; Everson, G.T.; Lindsay, K.L.; Lee, W.M.; Bonkovsky, H.L.; et al. Incidence of hepatocellular carcinoma and associated risk factors in hepatitis C-related advanced liver disease. Gastroenterology 2009, 136, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Nahon, P.; Ganne-Carrie, N. Management of patients with pre-therapeutic advanced liver fibrosis following HCV eradication. JHEP Rep. 2019, 1, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Cervantes, A.; Chau, I.; Daniele, B.; Llovet, J.M.; Meyer, T.; Nault, J.C.; Neumann, U.; Ricke, J.; Sangro, B.; et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 871–873. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, A.J.; Feld, J.J.; Hofer, H.; Almasio, P.L.; Calvaruso, V.; Fernandez-Rodriguez, C.M.; Aleman, S.; Ganne-Carrie, N.; D’Ambrosio, R.; Pol, S.; et al. Risk of cirrhosis-related complications in patients with advanced fibrosis following hepatitis C virus eradication. J. Hepatol. 2017, 66, 485–493. [Google Scholar] [CrossRef]
- Yamashita, N.; Ohho, A.; Yamasaki, A.; Kurokawa, M.; Kotoh, K.; Kajiwara, E. Hepatocarcinogenesis in chronic hepatitis C patients achieving a sustained virological response to interferon: Significance of lifelong periodic cancer screening for improving outcomes. J. Gastroenterol. 2014, 49, 1504–1513. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Kanwal, F.; Richardson, P.; Kramer, J. Risk of hepatocellular carcinoma after sustained virological response in Veterans with hepatitis C virus infection. Hepatology 2016, 64, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.L.; Baack, B.; Smith, B.D.; Yartel, A.; Pitasi, M.; Falck-Ytter, Y. Eradication of hepatitis C virus infection and the development of hepatocellular carcinoma: A meta-analysis of observational studies. Ann. Intern. Med. 2013, 158, 329–337. [Google Scholar] [CrossRef] [PubMed]
- van der Meer, A.J.; Veldt, B.J.; Feld, J.J.; Wedemeyer, H.; Dufour, J.F.; Lammert, F.; Duarte-Rojo, A.; Heathcote, E.J.; Manns, M.P.; Kuske, L.; et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 2012, 308, 2584–2593. [Google Scholar] [CrossRef] [PubMed]
- Nahon, P.; Bourcier, V.; Layese, R.; Audureau, E.; Cagnot, C.; Marcellin, P.; Guyader, D.; Fontaine, H.; Larrey, D.; De Ledinghen, V.; et al. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology 2017, 152, 142–156.e142. [Google Scholar] [CrossRef]
- Innes, H.; Barclay, S.T.; Hayes, P.C.; Fraser, A.; Dillon, J.F.; Stanley, A.; Bathgate, A.; McDonald, S.A.; Goldberg, D.; Valerio, H.; et al. The risk of hepatocellular carcinoma in cirrhotic patients with hepatitis C and sustained viral response: Role of the treatment regimen. J. Hepatol. 2018, 68, 646–654. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Green, P.K.; Berry, K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J. Hepatol. 2017, 68, 25–32. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.; Asch, S.M.; Chayanupatkul, M.; Cao, Y.; El-Serag, H.B. Risk of Hepatocellular Cancer in HCV Patients Treated With Direct-Acting Antiviral Agents. Gastroenterology 2017, 153, 996–1005.e1001. [Google Scholar] [CrossRef]
- Carrat, F.; Fontaine, H.; Dorival, C.; Simony, M.; Diallo, A.; Hezode, C.; De Ledinghen, V.; Larrey, D.; Haour, G.; Bronowicki, J.P.; et al. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: A prospective cohort study. Lancet 2019, 393, 1453–1464. [Google Scholar] [CrossRef]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; Tine, F.; Distefano, M.; Licata, A.; Giannitrapani, L.; et al. Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology 2018, 155, 411–421.e414. [Google Scholar] [CrossRef]
- Trinchet, J.C.; Bourcier, V.; Chaffaut, C.; Ait Ahmed, M.; Allam, S.; Marcellin, P.; Guyader, D.; Pol, S.; Larrey, D.; De Ledinghen, V.; et al. Complications and competing risks of death in compensated viral cirrhosis (ANRS CO12 CirVir prospective cohort). Hepatology 2015, 62, 737–750. [Google Scholar] [CrossRef]
- Nahon, P.; Layese, R.; Bourcier, V.; Cagnot, C.; Marcellin, P.; Guyader, D.; Pol, S.; Larrey, D.; De Ledinghen, V.; Ouzan, D.; et al. Incidence of Hepatocellular Carcinoma After Direct Antiviral Therapy for HCV in Patients With Cirrhosis Included in Surveillance Programs. Gastroenterology 2018, 155, 1436–1450.e1436. [Google Scholar] [CrossRef] [PubMed]
- Nahon, P.; Zucman-Rossi, J. Single nucleotide polymorphisms and risk of hepatocellular carcinoma in cirrhosis. J. Hepatol. 2012, 57, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Green, P.K.; Beste, L.A.; Mun, E.J.; Kerr, K.F.; Berry, K. Development of models estimating the risk of hepatocellular carcinoma after antiviral treatment for hepatitis C. J. Hepatol. 2018, 69, 1088–1098. [Google Scholar] [CrossRef]
- Pons, M.; Rodriguez-Tajes, S.; Esteban, J.I.; Marino, Z.; Vargas, V.; Lens, S.; Buti, M.; Augustin, S.; Forns, X.; Minguez, B.; et al. Non-invasive prediction of liver-related events in patients with HCV-associated compensated advanced chronic liver disease after oral antivirals. J. Hepatol. 2020, 72, 472–480. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, F.; Kramer, J.R.; Asch, S.M.; Cao, Y.; Li, L.; El-Serag, H.B. Long-Term Risk of Hepatocellular Carcinoma in HCV Patients Treated With Direct Acting Antiviral Agents. Hepatology 2019. [Google Scholar] [CrossRef]
- Alonso Lopez, S.; Manzano, M.L.; Gea, F.; Gutierrez, M.L.; Ahumada, A.M.; Devesa, M.J.; Olveira, A.; Polo, B.A.; Marquez, L.; Fernandez, I.; et al. A Model Based on Noninvasive Markers Predicts Very Low Hepatocellular Carcinoma Risk After Viral Response in Hepatitis C Virus-Advanced Fibrosis. Hepatology 2020. [Google Scholar] [CrossRef]
- Lee, H.W.; Yoo, E.J.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Kim, D.Y.; Ahn, S.H.; Han, K.H. Prediction of development of liver-related events by transient elastography in hepatitis B patients with complete virological response on antiviral therapy. Am. J. Gastroenterol. 2014, 109, 1241–1249. [Google Scholar] [CrossRef]
- Papatheodoridis, G.; Dalekos, G.; Sypsa, V.; Yurdaydin, C.; Buti, M.; Goulis, J.; Calleja, J.L.; Chi, H.; Manolakopoulos, S.; Mangia, G.; et al. PAGE-B predicts the risk of developing hepatocellular carcinoma in Caucasians with chronic hepatitis B on 5-year antiviral therapy. J. Hepatol. 2016, 64, 800–806. [Google Scholar] [CrossRef]
- Sohn, W.; Cho, J.Y.; Kim, J.H.; Lee, J.I.; Kim, H.J.; Woo, M.A.; Jung, S.H.; Paik, Y.H. Risk score model for the development of hepatocellular carcinoma in treatment-naive patients receiving oral antiviral treatment for chronic hepatitis B. Clin. Mol. Hepatol. 2017, 23, 170–178. [Google Scholar] [CrossRef]
- Chen, C.H.; Lee, C.M.; Lai, H.C.; Hu, T.H.; Su, W.P.; Lu, S.N.; Lin, C.H.; Hung, C.H.; Wang, J.H.; Lee, M.H.; et al. Prediction model of hepatocellular carcinoma risk in Asian patients with chronic hepatitis B treated with entecavir. Oncotarget 2017, 8, 92431–92441. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Yip, T.C.; Ho, H.J.; Wong, V.W.; Huang, Y.T.; El-Serag, H.B.; Lee, T.Y.; Wu, M.S.; Lin, J.T.; Wong, G.L.; et al. Development of a scoring system to predict hepatocellular carcinoma in Asians on antivirals for chronic hepatitis B. J. Hepatol. 2018, 69, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, Y.D.; Lee, M.; Jun, B.G.; Kim, T.S.; Suk, K.T.; Kang, S.H.; Kim, M.Y.; Cheon, G.J.; Kim, D.J.; et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J. Hepatol. 2018, 69, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.H.; Suh, Y.J.; Jin, Y.J.; Heo, N.Y.; Jang, J.W.; You, C.R.; An, H.Y.; Lee, J.W. Prediction model for hepatocellular carcinoma risk in treatment-naive chronic hepatitis B patients receiving entecavir/tenofovir. Europ. J. Gastroenterol. Hepatol. 2019, 31, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Audureau, E.; Carrat, F.; Layese, R.; Cagnot, C.; Asselah, T.; Guyader, D.; Larrey, D.; De Ledinghen, V.; Ouzan, D.; Zoulim, F.; et al. Personalized surveillance for hepatocellular carcinoma in cirrhosis—Using machine learning adapted to HCV status. J. Hepatol. 2020, 73, 1434–1445. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Papatheodoridis, G.; Sun, J.; Innes, H.; Toyoda, H.; Xie, Q.; Mo, S.; Sypsa, V.; Guha, I.N.; Kumada, T.; et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J. Hepatol. 2020, 73, 1368–1378. [Google Scholar] [CrossRef]
- El-Serag, H.B.; Kanwal, F.; Davila, J.A.; Kramer, J.; Richardson, P. A new laboratory-based algorithm to predict development of hepatocellular carcinoma in patients with hepatitis C and cirrhosis. Gastroenterology 2014, 146, 1249–1255.e1241. [Google Scholar] [CrossRef]
- Ganne-Carrie, N.; Layese, R.; Bourcier, V.; Cagnot, C.; Marcellin, P.; Guyader, D.; Pol, S.; Larrey, D.; de Ledinghen, V.; Ouzan, D.; et al. Nomogram for individualized prediction of hepatocellular carcinoma occurrence in hepatitis C virus cirrhosis (ANRS CO12 CirVir). Hepatology 2016, 64, 1136–1147. [Google Scholar] [CrossRef]
- Singal, A.G.; Mukherjee, A.; Elmunzer, B.J.; Higgins, P.D.; Lok, A.S.; Zhu, J.; Marrero, J.A.; Waljee, A.K. Machine learning algorithms outperform conventional regression models in predicting development of hepatocellular carcinoma. Am. J. Gastroenterol. 2013, 108, 1723–1730. [Google Scholar] [CrossRef]
- Ioannou, G.N.; Tang, W.; Beste, L.A.; Tincopa, M.A.; Su, G.L.; Van, T.; Tapper, E.B.; Singal, A.G.; Zhu, J.; Waljee, A.K. Assessment of a Deep Learning Model to Predict Hepatocellular Carcinoma in Patients With Hepatitis C Cirrhosis. JAMA Netw. Open 2020, 3, e2015626. [Google Scholar] [CrossRef]
- Marcellin, P.; Gane, E.; Buti, M.; Afdhal, N.; Sievert, W.; Jacobson, I.M.; Washington, M.K.; Germanidis, G.; Flaherty, J.F.; Aguilar Schall, R.; et al. Regression of cirrhosis during treatment with tenofovir disoproxil fumarate for chronic hepatitis B: A 5-year open-label follow-up study. Lancet 2013, 381, 468–475. [Google Scholar] [CrossRef]
- Chen, C.J.; Yang, H.I.; Su, J.; Jen, C.L.; You, S.L.; Lu, S.N.; Huang, G.T.; Iloeje, U.H.; Group, R.-H.S. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006, 295, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Iloeje, U.H.; Yang, H.I.; Su, J.; Jen, C.L.; You, S.L.; Chen, C.J.; Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006, 130, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F.; Sung, J.J.; Chow, W.C.; Farrell, G.; Lee, C.Z.; Yuen, H.; Tanwandee, T.; Tao, Q.M.; Shue, K.; Keene, O.N.; et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N. Engl. J. Med. 2004, 351, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Voulgaris, T.; Papatheodoridi, M.; Kim, W.R. Risk Scores for Hepatocellular Carcinoma in Chronic Hepatitis B: A Promise for Precision Medicine. Hepatology 2020, 72, 2197–2205. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Chan, H.L.; Hansen, B.E.; Janssen, H.L.; Lampertico, P. Risk of hepatocellular carcinoma in chronic hepatitis B: Assessment and modification with current antiviral therapy. J. Hepatol. 2015, 62, 956–967. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.; Chan, H.L.; Mak, C.H.; Lee, S.K.; Ip, Z.M.; Lam, A.T.; Iu, H.W.; Leung, J.M.; Lai, J.W.; Lo, A.O.; et al. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology 2013, 58, 1537–1547. [Google Scholar] [CrossRef]
- Su, T.H.; Hu, T.H.; Chen, C.Y.; Huang, Y.H.; Chuang, W.L.; Lin, C.C.; Wang, C.C.; Su, W.W.; Chen, M.Y.; Peng, C.Y.; et al. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016, 36, 1755–1764. [Google Scholar] [CrossRef]
- Papatheodoridis, G.V.; Dalekos, G.N.; Yurdaydin, C.; Buti, M.; Goulis, J.; Arends, P.; Sypsa, V.; Manolakopoulos, S.; Mangia, G.; Gatselis, N.; et al. Incidence and predictors of hepatocellular carcinoma in Caucasian chronic hepatitis B patients receiving entecavir or tenofovir. J. Hepatol. 2015, 62, 363–370. [Google Scholar] [CrossRef]
- Cho, J.Y.; Paik, Y.H.; Sohn, W.; Cho, H.C.; Gwak, G.Y.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W.; Yoo, B.C. Patients with chronic hepatitis B treated with oral antiviral therapy retain a higher risk for HCC compared with patients with inactive stage disease. Gut 2014, 63, 1943–1950. [Google Scholar] [CrossRef]
- Hosaka, T.; Suzuki, F.; Kobayashi, M.; Seko, Y.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013, 58, 98–107. [Google Scholar] [CrossRef]
- Lim, Y.S.; Han, S.; Heo, N.Y.; Shim, J.H.; Lee, H.C.; Suh, D.J. Mortality, liver transplantation, and hepatocellular carcinoma among patients with chronic hepatitis B treated with entecavir vs lamivudine. Gastroenterology 2014, 147, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, M.H.; Yang, H.I.; Le, A.; Henry, L.; Nguyen, N.; Lee, M.H.; Zhang, J.; Wong, C.; Wong, C.; Trinh, H. Reduced Incidence of Hepatocellular Carcinoma in Cirrhotic and Noncirrhotic Patients With Chronic Hepatitis B Treated With Tenofovir-A Propensity Score-Matched Study. J. Infect. Dis. 2019, 219, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Papatheodoridis, G.V.; Idilman, R.; Dalekos, G.N.; Buti, M.; Chi, H.; van Boemmel, F.; Calleja, J.L.; Sypsa, V.; Goulis, J.; Manolakopoulos, S.; et al. The risk of hepatocellular carcinoma decreases after the first 5 years of entecavir or tenofovir in Caucasians with chronic hepatitis B. Hepatology 2017, 66, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Liaw, Y.F.; Chu, C.M. Hepatitis B virus infection. Lancet 2009, 373, 582–592. [Google Scholar] [CrossRef]
- Wu, C.Y.; Lin, J.T.; Ho, H.J.; Su, C.W.; Lee, T.Y.; Wang, S.Y.; Wu, C.; Wu, J.C. Association of nucleos(t)ide analogue therapy with reduced risk of hepatocellular carcinoma in patients with chronic hepatitis B: A nationwide cohort study. Gastroenterology 2014, 147, 143–151.e145. [Google Scholar] [CrossRef]
- Choi, J.; Kim, H.J.; Lee, J.; Cho, S.; Ko, M.J.; Lim, Y.S. Risk of Hepatocellular Carcinoma in Patients Treated With Entecavir vs Tenofovir for Chronic Hepatitis B: A Korean Nationwide Cohort Study. JAMA Oncol. 2019, 5, 30–36. [Google Scholar] [CrossRef]
- Yip, T.C.; Wong, V.W.; Chan, H.L.; Tse, Y.K.; Lui, G.C.; Wong, G.L. Tenofovir Is Associated With Lower Risk of Hepatocellular Carcinoma Than Entecavir in Patients With Chronic HBV Infection in China. Gastroenterology 2020, 158, 215–225.e216. [Google Scholar] [CrossRef]
- Tseng, C.H.; Hsu, Y.C.; Chen, T.H.; Ji, F.; Chen, I.S.; Tsai, Y.N.; Hai, H.; Thuy, L.T.T.; Hosaka, T.; Sezaki, H.; et al. Hepatocellular carcinoma incidence with tenofovir versus entecavir in chronic hepatitis B: A systematic review and meta-analysis. Lancet. Gastroenterol. Hepatol. 2020, 5, 1039–1052. [Google Scholar] [CrossRef]
- Dave, S.; Park, S.; Murad, M.H.; Barnard, A.; Prokop, L.; Adams, L.A.; Singh, S.; Loomba, R. Comparative Effectiveness of Entecavir Versus Tenofovir for Preventing Hepatocellular Carcinoma in Patients with Chronic Hepatitis B: A Systematic Review and Meta-Analysis. Hepatology 2020, 5, 1039–1052. [Google Scholar]
- Yip, T.C.; Wong, G.L.; Wong, V.W.; Tse, Y.K.; Liang, L.Y.; Hui, V.W.; Lee, H.W.; Lui, G.C.; Chan, H.L. Reassessing the accuracy of PAGE-B-related scores to predict hepatocellular carcinoma development in patients with chronic hepatitis B. J. Hepatol. 2020, 72, 847–854. [Google Scholar] [CrossRef]
- Kaneko, S.; Kurosaki, M.; Joko, K.; Marusawa, H.; Kondo, M.; Kojima, Y.; Uchida, Y.; Kimura, H.; Tsuji, K.; Yagisawa, H.; et al. Detectable HBV DNA during nucleos(t)ide analogues stratifies predictive hepatocellular carcinoma risk score. Sci. Rep. 2020, 10, 13021. [Google Scholar] [CrossRef] [PubMed]
- Ioannou, G.N.; Green, P.; Kerr, K.F.; Berry, K. Models estimating risk of hepatocellular carcinoma in patients with alcohol or NAFLD-related cirrhosis for risk stratification. J. Hepatol. 2019, 71, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Ganne-Carrie, N.; Nahon, P. Hepatocellular carcinoma in the setting of alcohol-related liver disease. J. Hepatol. 2019, 70, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, K.; Sawai, H.; Ikeo, K.; Ogawa, S.; Iio, E.; Isogawa, M.; Shimada, N.; Komori, A.; Toyoda, H.; Kumada, T.; et al. Genome-Wide Association Study Identifies TLL1 Variant Associated With Development of Hepatocellular Carcinoma After Eradication of Hepatitis C Virus Infection. Gastroenterology 2017, 152, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Degasperi, E.; Galmozzi, E.; Pelusi, S.; D’Ambrosio, R.; Soffredini, R.; Borghi, M.; Perbellini, R.; Facchetti, F.; Iavarone, M.; Sangiovanni, A.; et al. Hepatic Fat-Genetic Risk Score Predicts Hepatocellular Carcinoma in Patients With Cirrhotic HCV Treated With DAAs. Hepatology 2020, 72, 1912–1923. [Google Scholar] [CrossRef] [PubMed]
- Hamdane, N.; Juhling, F.; Crouchet, E.; El Saghire, H.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Roca Suarez, A.A.; et al. HCV-Induced Epigenetic Changes Associated With Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology 2019, 156, 2313–2329.e2317. [Google Scholar] [CrossRef]
- Lupberger, J.; Croonenborghs, T.; Roca Suarez, A.A.; Van Renne, N.; Juhling, F.; Oudot, M.A.; Virzi, A.; Bandiera, S.; Jamey, C.; Meszaros, G.; et al. Combined Analysis of Metabolomes, Proteomes, and Transcriptomes of Hepatitis C Virus-Infected Cells and Liver to Identify Pathways Associated With Disease Development. Gastroenterology 2019, 157, 537–551.e539. [Google Scholar] [CrossRef]

| Variables Included in HCC Risk Scores for Virologically Controlled Patients Infected with HBV | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Risk Score/Reference | Country or Area | Treatment | Host Factors | Liver Disease Activity | Cirrhosis/Fibrosis Parameters | |||||||
| Age | Gender | Others | AFP | AST or ALT | PT | PLT | LSM | Albumin | Bilirubin | |||
| REACH-Bm [37] | Korea | Entecavir | X | X | NA | X | ||||||
| PAGE-B [38] | Europe | Entecavir/Tenofovir | X | X | X | NA | ||||||
| HCC-RESCUE [39] | Korea | Entecavir | X | X | NA | |||||||
| APA-B [40] | Taiwan | Entecavir | X | X | X | NA | ||||||
| CAMD [41] | Taiwan/Hong Kong | Entecavir/Tenofovir | X | X | Diabetes | NA | ||||||
| mPAGE-B [42] | Korea | Entecavir/Tenofovir | X | X | X | NA | X | |||||
| AASL [43] | Korea | Entecavir/Tenofovir | X | X | X | X | ||||||
| Variables Included in HCC Risk Scores for Patients Infected with HCV with Advanced Chronic Liver Disease Who Achieved Sustained Virological Response (SVR) | ||||||||||||
| Host factors | Liver disease activity | Cirrhosis/Fibrosis parameters | ||||||||||
| Age | Gender | Others | AFP | AST or ALT | PT | PLT | LSM | Albumin | Bilirubin | |||
| van der Meer 2017 [19] | Europe | INF | X | Diabetes | X | NA | ||||||
| Calvaruso 2018 [29] | Italy | DAAs | X | NA | X | |||||||
| Ioannou 2018 [33] | USA | INF/DAAs | X | X | X | NA | X | |||||
| Pons 2020 [34] | Spain | DAAs | X | X | ||||||||
| Alonso Lopez 2020 [36] | Spain | DAAs | X | X | ||||||||
| Audureau 2020 [44] | France | INF/DAAs | X | X | X | NA | ||||||
| Variables included in HCC risk scores following HCV eradication or HBV control regardless of the cause of liver disease | ||||||||||||
| Host factors | Liver disease activity | Cirrhosis/Fibrosis parameters | ||||||||||
| Age | Gender | Others | AFP | AST or ALT | PT | PLT | LSM | Albumin | Bilirubin | |||
| aMAP [45] | Worldwide | All regimens | X | X | X | NA | X | X | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahon, P.; Vo Quang, E.; Ganne-Carrié, N. Stratification of Hepatocellular Carcinoma Risk Following HCV Eradication or HBV Control. J. Clin. Med. 2021, 10, 353. https://doi.org/10.3390/jcm10020353
Nahon P, Vo Quang E, Ganne-Carrié N. Stratification of Hepatocellular Carcinoma Risk Following HCV Eradication or HBV Control. Journal of Clinical Medicine. 2021; 10(2):353. https://doi.org/10.3390/jcm10020353
Chicago/Turabian StyleNahon, Pierre, Erwan Vo Quang, and Nathalie Ganne-Carrié. 2021. "Stratification of Hepatocellular Carcinoma Risk Following HCV Eradication or HBV Control" Journal of Clinical Medicine 10, no. 2: 353. https://doi.org/10.3390/jcm10020353
APA StyleNahon, P., Vo Quang, E., & Ganne-Carrié, N. (2021). Stratification of Hepatocellular Carcinoma Risk Following HCV Eradication or HBV Control. Journal of Clinical Medicine, 10(2), 353. https://doi.org/10.3390/jcm10020353





