A Meta-Analysis on the Rate of Hepatocellular Carcinoma Recurrence after Liver Transplant and Associations to Etiology, Alpha-Fetoprotein, Income and Ethnicity
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
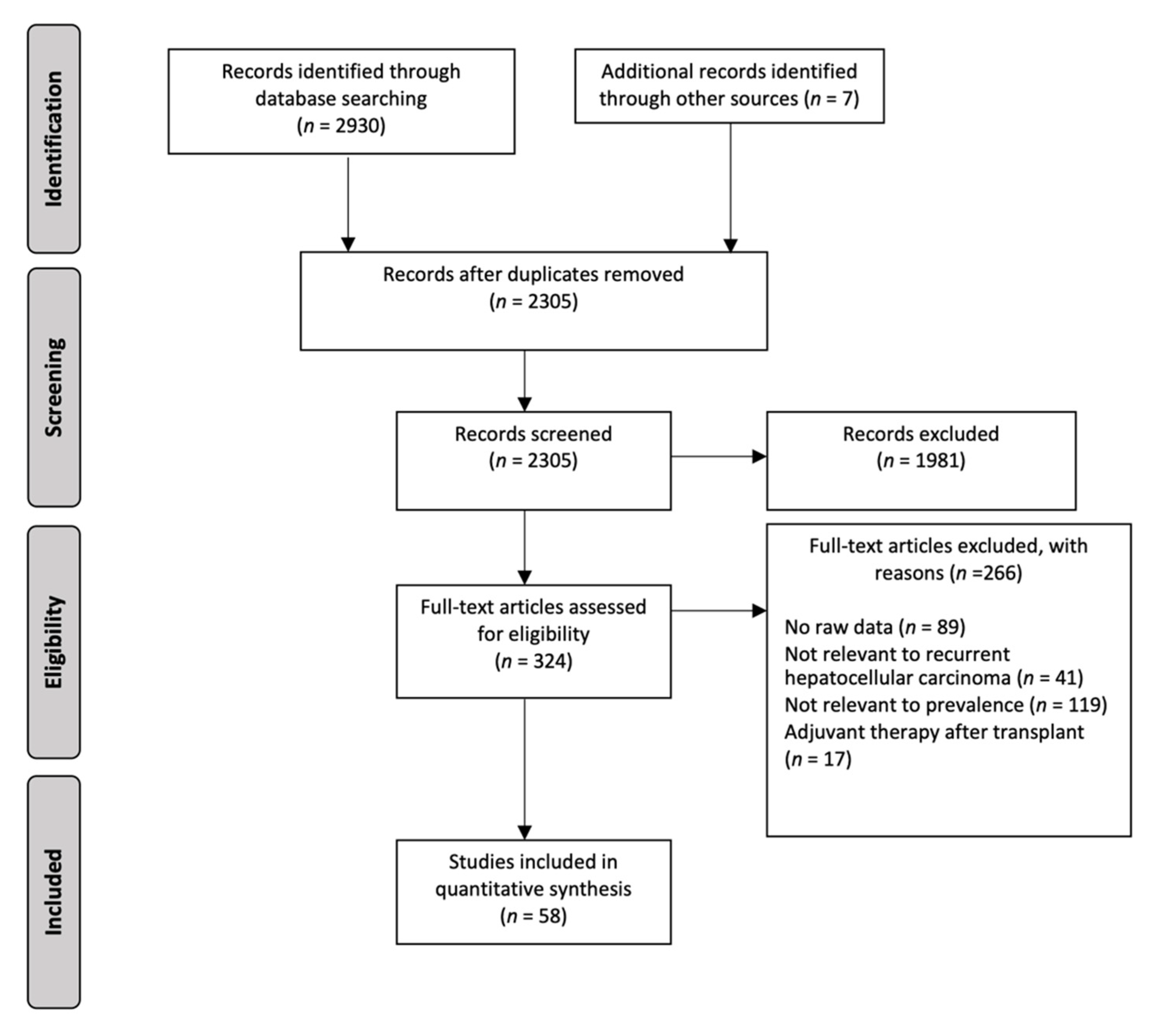
2.2. Study Selection and Eligibility Criteria
2.3. Data Extraction and Outcomes
2.4. Statistical Analysis and Quality Assessment
3. Results
3.1. Overall Prevalence of HCC Recurrence
3.2. Analysis by Pretransplant LRT
3.3. Analysis by Pretransplant AFP
3.4. Analyses by Viral and Nonviral Aetiology
3.5. Analyses by Ethnicity
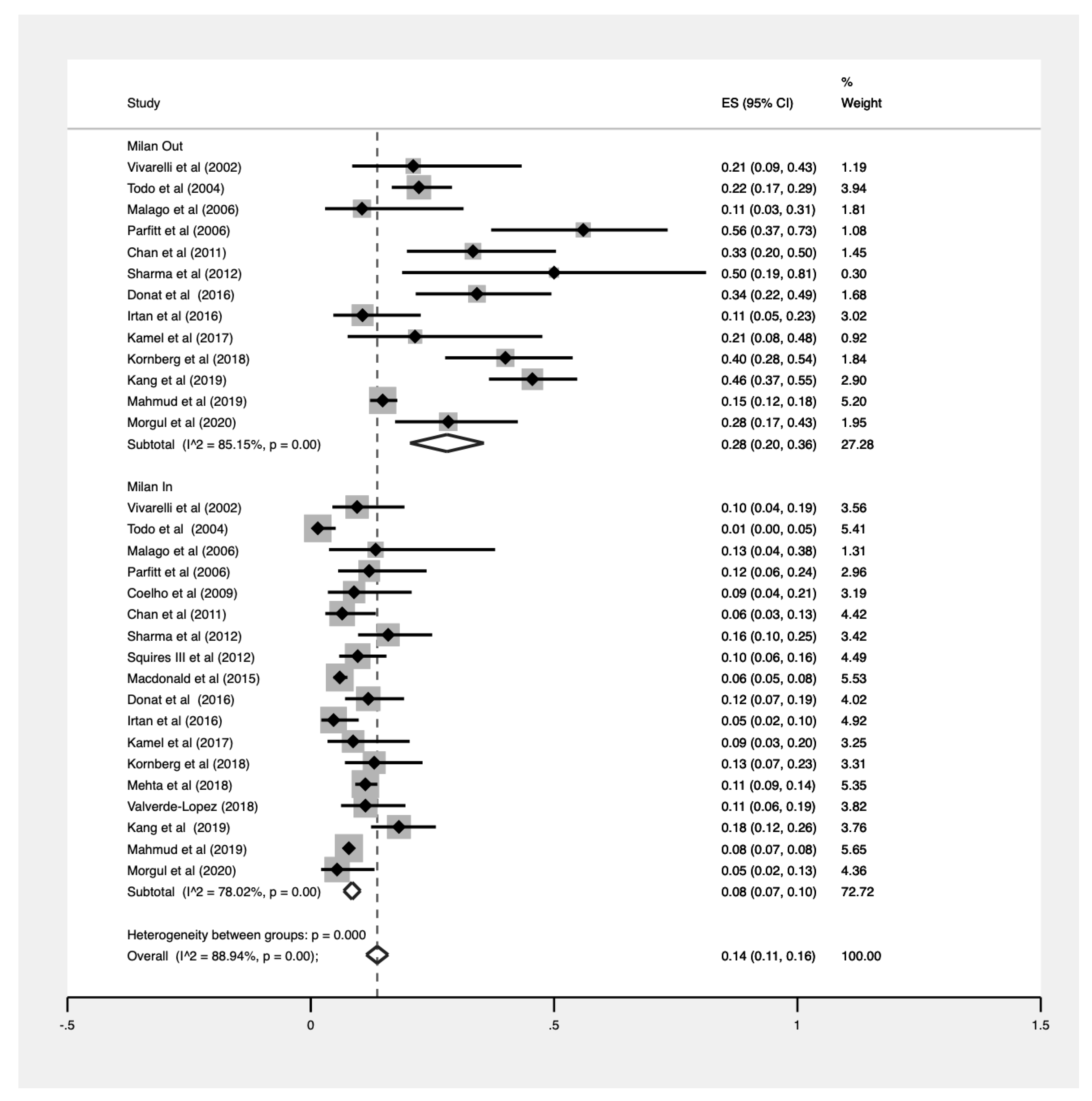
3.6. Analyses by Milan Criteria
3.7. Analysis by Deceased Donor Versus Living Donor Liver Transplant
3.8. Analysis by Income
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mazzaferro, V.; Regalia, E.; Doci, R.; Andreola, S.; Pulvirenti, A.; Bozzetti, F.; Montalto, F.; Ammatuna, M.; Morabito, A.; Gennari, L. Liver Transplantation for the Treatment of Small Hepatocellular Carcinomas in Patients with Cirrhosis. N. Engl. J. Med. 1996, 334, 693–700. [Google Scholar] [CrossRef]
- Raza, A.; Sood, G.K. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. Hepatocellular carcinoma review: Current treatment, and evidence-based medicine. World J. Gastroenterol. 2014, 20, 4115–4127. [Google Scholar] [CrossRef]
- Santopaolo, F.; Lenci, I.; Milana, M.; Manzia, T.M.; Baiocchi, L. Liver transplantation for hepatocellular carcinoma: Where do we stand? World J. Gastroenterol. 2019, 25, 2591–2602. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–775. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Citterio, D.; Bhoori, S.; Bongini, M.; Miceli, R.; De Carlis, L.; Colledan, M.; Salizzoni, M.; Romagnoli, R.; Antonelli, B.; et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): A randomised, controlled, phase 2b/3 trial. Lancet Oncol. 2020, 21, 947–956. [Google Scholar] [CrossRef]
- de’Angelis, N.; Landi, F.; Carra, M.C.; Azoulay, D. Managements of recurrent hepatocellular carcinoma after liver transplantation: A systematic review. World J. Gastroenterol. 2015, 21, 11185–11198. [Google Scholar] [CrossRef]
- Nagai, S.; Mangus, R.S.; Kubal, C.A.; Ekser, B.; Fridell, J.A.; Klingler, K.R.; Maluccio, M.A.; Tector, A.J. Prognosis after recurrence of hepatocellular carcinoma in liver transplantation: Predictors for successful treatment and survival. Clin. Transplant. 2015, 29, 1156–1163. [Google Scholar] [CrossRef]
- Bodzin, A.S.; Lunsford, K.E.; Markovic, D.; Harlander-Locke, M.P.; Busuttil, R.W.; Agopian, V.G. Predicting Mortality in Patients Developing Recurrent Hepatocellular Carcinoma After Liver Transplantation: Impact of Treatment Modality and Recurrence Characteristics. Ann. Surg. 2017, 266, 118–125. [Google Scholar] [CrossRef]
- Clavien, P.; Lesurtel, M.; Bossuyt, P.M.M.; Gores, G.J.; Langer, B.; Perrier, A. Recommendations for liver transplantation for hepatocellular carcinoma: An international consensus conference report. Lancet Oncol. 2012, 13, e11–e22. [Google Scholar] [CrossRef]
- Mazzaferro, V.; Sposito, C.; Zhou, J.; Pinna, A.D.; De Carlis, L.; Fan, J.; Cescon, M.; Di Sandro, S.; Yi-Feng, H.; Lauterio, A.; et al. Metroticket 2.0 Model for Analysis of Competing Risks of Death After Liver Transplantation for Hepatocellular Carcinoma. Gastroenterology 2018, 154, 128–139. [Google Scholar] [CrossRef]
- DiNorcia, J.; Florman, S.S.; Haydel, B.; Tabrizian, P.; Ruiz, R.M.; Klintmalm, G.B.; Senguttuvan, S.; Lee, D.D.; Taner, C.B.; Verna, E.C.; et al. Pathologic Response to Pretransplant Locoregional Therapy is Predictive of Patient Outcome After Liver Transplantation for Hepatocellular Carcinoma. Ann. Surg. 2020, 271, 616–624. [Google Scholar] [CrossRef]
- Filgueira, N.A. Hepatocellular carcinoma recurrence after liver transplantation: Risk factors, screening and clinical presentation. World J. Hepatol. 2019, 11, 261–272. [Google Scholar] [CrossRef]
- Moini, M.; Schilsky, M.L.; Tichy, E.M. Review on immunosuppression in liver transplantation. World J. Hepatol. 2015, 7, 1355–1368. [Google Scholar] [CrossRef]
- Cescon, M.; Bertuzzo, V.R.; Ercolani, G.; Ravaioli, M.; Odaldi, F.; Pinna, A.D. Liver transplantation for hepatocellular carcinoma: Role of inflammatory and immunological state on recurrence and prognosis. World J. Gastroenterol. 2013, 19. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- The World Bank. GNI per Capita, PPP (Current International $). Available online: https://data.worldbank.org/indicator/NY.GNP.PCAP.PP.CD (accessed on 9 August 2020).
- Mazzaferro, V.; Bhoori, S.; Sposito, C.; Bongini, M.; Langer, M.; Miceli, R.; Mariani, L. Milan criteria in liver transplantation for hepatocellular carcinoma: An evidence-based analysis of 15 years of experience. Liver Transplant. 2011, 17, S44–S57. [Google Scholar] [CrossRef]
- Germani, G.; Gurusamy, K.S.; Garcovich, M.; Toso, C.; Fede, G.; Hemming, A.; Suh, K.-S.; Weber, A.; Burroughs, A.K. Which matters most: Number of tumors, size of the largest tumor, or total tumor volume? Liver Transplant. 2011, 17, S58–S66. [Google Scholar] [CrossRef]
- Davis, E.; Wiesner, R.; Valdecasas, J.; Kita, Y.; Rossi, M.; E Schwartz, M. Treatment of recurrent hepatocellular carcinoma after liver transplantation. Liver Transplant. 2011, 17, S162–S166. [Google Scholar] [CrossRef]
- Chin, Y.H.; Ng, C.H.; Lee, M.H.; Koh, J.W.H.; Kiew, J.; Yang, S.P.; Sundar, G.; Khoo, C.M. Prevalence of thyroid eye disease in Graves’ disease: A meta-analysis and systematic review. Clin. Endocrinol. 2020, 93, 363–374. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res. Methodol. 2014, 14, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nyaga, V.N.; Arbyn, M.; Aerts, M. Metaprop: A Stata command to perform meta-analysis of binomial data. Arch. Public Health 2014, 72, 1–10. [Google Scholar] [CrossRef]
- Harbord, R.M.; Higgins, J.P.T. Meta-Regression in Stata. Stata J. Promot. Commun. Stat. Stata 2008, 8, 493–519. [Google Scholar] [CrossRef]
- Ng, C.H.; Chin, Y.H.; Tan, M.H.Q.; Ng, J.X.; Yang, S.P.; Kiew, J.J.; Khoo, C.M. Cinacalcet and primary hyperparathyroidism: Systematic review and meta regression. Endocr. Connect. 2020, 9, 724–735. [Google Scholar] [CrossRef] [PubMed]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Hoy, D.G.; Brooks, P.; Woolf, A.; Blyth, F.; March, L.; Bain, C.; Baker, P.; Smith, E.; Buchbinder, R. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J. Clin. Epidemiol. 2012, 65, 934–939. [Google Scholar] [CrossRef]
- Finn, R.S. Current and Future Treatment Strategies for Patients with Advanced Hepatocellular Carcinoma: Role of mTOR Inhibition. Liver Cancer 2012, 1, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Duvoux, C.; Toso, C. mTOR inhibitor therapy: Does it prevent HCC recurrence after liver transplantation? Transplant. Rev. 2015, 29, 168–174. [Google Scholar] [CrossRef]
- Ju, M.R.; Yopp, A.C. Evolving thresholds for liver transplantation in hepatocellular carcinoma: A Western experience. Ann. Gastroenterol. Surg. 2020, 4, 208–215. [Google Scholar] [CrossRef]
- Sotiropoulos, G.C.; Molmenti, E.P.; Lösch, C.; Beckebaum, S.; Broelsch, C.E.; Lang, H. Meta-analysis of tumor recurrence after liver transplantation for hepatocellular carcinoma based on 1,198 cases. Eur. J. Med. Res. 2007, 12. [Google Scholar]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.S.; Muthiah, M.D.; Koh, T.; Teoh, Y.L.; Chan, A.; Kow, A.; Zheng, Q.; Kwon, C.H.D.; Lee, G.H.; Lesmana, C.R.A.; et al. Asian Liver Transplant Network Clinical Guidelines on Immunosuppression in Liver Transplantation. Transplantation 2019, 103, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Omata, M.; Cheng, A.-L.; Kokudo, N.; Kudo, M.; Lee, J.M.; Jia, J.; Tateishi, R.; Han, K.-H.; Chawla, Y.K.; Shiina, S.; et al. Asia–Pacific clinical practice guidelines on the management of hepatocellular carcinoma: A 2017 update. Hepatol. Int. 2017, 11, 317–370. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-L.; Kabiling, C.S.; Concejero, A.M. Why does living donor liver transplantation flourish in Asia? Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 746–751. [Google Scholar] [CrossRef]
- Akamatsu, N.; Sugawara, Y.; Kokudo, N. Living Donor Liver Transplantation for Patients with Hepatocellular Carcinoma. Liver Cancer 2014, 3, 108–118. [Google Scholar] [CrossRef]
- Ashtari, S.; Pourhoseingholi, M.A.; Sharifian, A.; Zali, M.R. Hepatocellular carcinoma in Asia: Prevention strategy and planning. World J. Hepatol. 2015, 7, 1708–1717. [Google Scholar] [CrossRef]
- Muthiah, M.; Chong, C.H.; Lim, S.G. Liver Disease in Singapore. Euroasian J. Hepatogastroenterol. 2018, 8, 66–68. [Google Scholar] [CrossRef]
- Yang, J.D.; Roberts, L.R. Hepatocellular carcinoma: A global view. Nat. Rev. Gastroenterol. Hepatol. 2010, 7, 448–458. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Tanaka, Y.; Mizokami, M.; Yuen, J.C.-H.; Wong, D.K.-H.; Yuan, H.-J.; Sum, S.-M.; Chan, A.O.-O.; Wong, B.C.-Y.; Lai, C.L. Role of hepatitis B virus genotypes Ba and C, core promoter and precore mutations on hepatocellular carcinoma: A case control study. Carcinogenesis 2004, 25, 1593–1598. [Google Scholar] [CrossRef][Green Version]
- Norder, H.; Hammas, B.; Lee, S.D.; Bile, K.; Couroucé, A.M.; Mushahwar, I.K.; Magnius, L.O. Genetic relatedness of hepatitis B viral strains of diverse geographical origin and natural variations in the primary structure of the surface antigen. J. Gen. Virol. 1993, 74, 1341–1348. [Google Scholar] [CrossRef]
- Muthiah, M.D.; Tan, E.Y.; Chua, S.H.M.; Huang, D.Q.Y.; Bonney, G.K.; Kow, A.W.C.; Lim, S.G.; Dan, Y.Y.; Tan, P.S.; Lee, G.-H.; et al. Nucleoside analog monotherapy for prophylaxis in Hepatitis B liver transplant patients is safe and efficacious. Hepatol. Int. 2020, 14, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Akateh, C.; Pawlik, T.M.; Cloyd, J.M. Adjuvant antiviral therapy for the prevention of hepatocellular carcinoma recurrence after liver resection: Indicated for all patients with chronic hepatitis B? Ann. Transl. Med. 2018, 6, 397. [Google Scholar] [CrossRef] [PubMed]
- Pazgan-Simon, M.; Simon, K.A.; Jarowicz, E.; Rotter, K.; Szymanek-Pasternak, A.; Zuwala-Jagiello, J. Hepatitis B virus treatment in hepatocellular carcinoma patients prolongs survival and reduces the risk of cancer recurrence. Clin. Exp. Hepatol. 2018, 4, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Stepanova, M.; Ong, J.; Jacobson, I.M.; Bugianesi, E.; Duseja, A.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol. 2019, 17, 748–755.e3. [Google Scholar] [CrossRef] [PubMed]
- Lewin, S.M.; Mehta, N.; Kelley, R.K.; Roberts, J.P.; Yao, F.Y.; Brandman, D. Liver transplantation recipients with nonalcoholic steatohepatitis have lower risk hepatocellular carcinoma. Liver Transplant. 2017, 23, 1015–1022. [Google Scholar] [CrossRef]
- Sadler, E.M.; Mehta, N.J.; Bhat, M.; Ghanekar, A.; Greig, P.D.; Grant, D.R.; Yao, F.; Sapisochin, G. Liver Transplantation for NASH-Related Hepatocellular Carcinoma Versus Non-NASH Etiologies of Hepatocellular Carcinoma. Transplantation 2018, 102, 640–647. [Google Scholar] [CrossRef]
- Shen, Y.; Guo, H.; Wu, T.; Lu, Q.; Nan, K.-J.; Lv, Y.; Zhang, X. Lower Education and Household Income Contribute to Advanced Disease, Less Treatment Received and Poorer Prognosis in Patients with Hepatocellular Carcinoma. J. Cancer 2017, 8, 3070–3077. [Google Scholar] [CrossRef]
- Kew, M.C. Hepatocellular carcinoma in developing countries: Prevention, diagnosis and treatment. World J. Hepatol. 2012, 4, 99–104. [Google Scholar] [CrossRef]
- Caldwell, S.; Marchesini, G. Cryptogenic vs. NASH-cirrhosis: The rose exists well before its name. J. Hepatol. 2018, 68, 391–392. [Google Scholar] [CrossRef]
- Brunt, E.M. Nonalcoholic Fatty Liver Disease: Pros and Cons of Histologic Systems of Evaluation. Int. J. Mol. Sci. 2016, 17, 97. [Google Scholar] [CrossRef]




| No. of Papers | Count | Total Sample Size | Pooled Prevalence (CI) | |
|---|---|---|---|---|
| Overall Prevalence | 58 | 3888 | 40,495 | 13% (0.12–0.15) |
| Alpha-Fetoprotein | ||||
| <50 ng/mL | 17 | 3012 | 34,488 | 11% (0.10–0.13) |
| ≥50 ng/mL | 7 | 225 | 1491 | 15% (0.10–0.21) |
| Transplant Type a | ||||
| Living donor | 8 | 246 | 1380 | 17% (0.12–0.21) |
| Deceased donor | 7 | 127 | 922 | 14% (0.10–0.18) |
| Milan Criteria a | ||||
| Within Milan Criteria | 18 | 1663 | 20,884 | 8% (0.07–0.10) |
| Beyond Milan Criteria | 18 | 269 | 1199 | 28% (0.20–0.36) |
| Income | ||||
| Middle income | 13 | 439 | 2908 | 15% (0.12–0.19) |
| High income | 45 | 3449 | 37,587 | 13% (0.11–0.14) |
| Ethnicity | ||||
| Predominantly Asian | 13 | 365 | 1887 | 19% (0.15–0.24) |
| Predominantly Western | 36 | 3327 | 37,142 | 12% (0.11–0.13) |
| Predominantly Middle Eastern | 3 | 66 | 412 | 16% (0.12–0.20) |
| Predominantly Latin American | 6 | 130 | 1054 | 11% (0.09–0.14) |
| Aetiology a | ||||
| HBV | 13 | 277 | 1947 | 18% (0.11–0.27) |
| HCV | 12 | 1037 | 12,331 | 11% (0.08–0.15) |
| NASH | 7 | 125 | 1791 | 8% (0.01–0.20) |
| ALD | 7 | 134 | 1868 | 10% (0.05–0.17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, D.J.H.; Wong, C.; Ng, C.H.; Poh, C.W.; Jain, S.R.; Huang, D.Q.; Muthiah, M.D. A Meta-Analysis on the Rate of Hepatocellular Carcinoma Recurrence after Liver Transplant and Associations to Etiology, Alpha-Fetoprotein, Income and Ethnicity. J. Clin. Med. 2021, 10, 238. https://doi.org/10.3390/jcm10020238
Tan DJH, Wong C, Ng CH, Poh CW, Jain SR, Huang DQ, Muthiah MD. A Meta-Analysis on the Rate of Hepatocellular Carcinoma Recurrence after Liver Transplant and Associations to Etiology, Alpha-Fetoprotein, Income and Ethnicity. Journal of Clinical Medicine. 2021; 10(2):238. https://doi.org/10.3390/jcm10020238
Chicago/Turabian StyleTan, Darren J. H., Chloe Wong, Cheng Han Ng, Chen Wei Poh, Sneha Rajiv Jain, Daniel Q. Huang, and Mark D. Muthiah. 2021. "A Meta-Analysis on the Rate of Hepatocellular Carcinoma Recurrence after Liver Transplant and Associations to Etiology, Alpha-Fetoprotein, Income and Ethnicity" Journal of Clinical Medicine 10, no. 2: 238. https://doi.org/10.3390/jcm10020238
APA StyleTan, D. J. H., Wong, C., Ng, C. H., Poh, C. W., Jain, S. R., Huang, D. Q., & Muthiah, M. D. (2021). A Meta-Analysis on the Rate of Hepatocellular Carcinoma Recurrence after Liver Transplant and Associations to Etiology, Alpha-Fetoprotein, Income and Ethnicity. Journal of Clinical Medicine, 10(2), 238. https://doi.org/10.3390/jcm10020238





