Evaluation of Regional Pulmonary Ventilation in Spontaneously Breathing Patients with Idiopathic Pulmonary Fibrosis (IPF) Employing Electrical Impedance Tomography (EIT): A Pilot Study from the European IPF Registry (eurIPFreg)
Abstract
1. Introduction
2. Aim of the Study
3. Materials and Methods
3.1. Patients’ Enrollment and Data Collection
3.2. EurIPFreg
3.3. EIT Measurements
3.4. EIT Data Analysis and Measured Variables
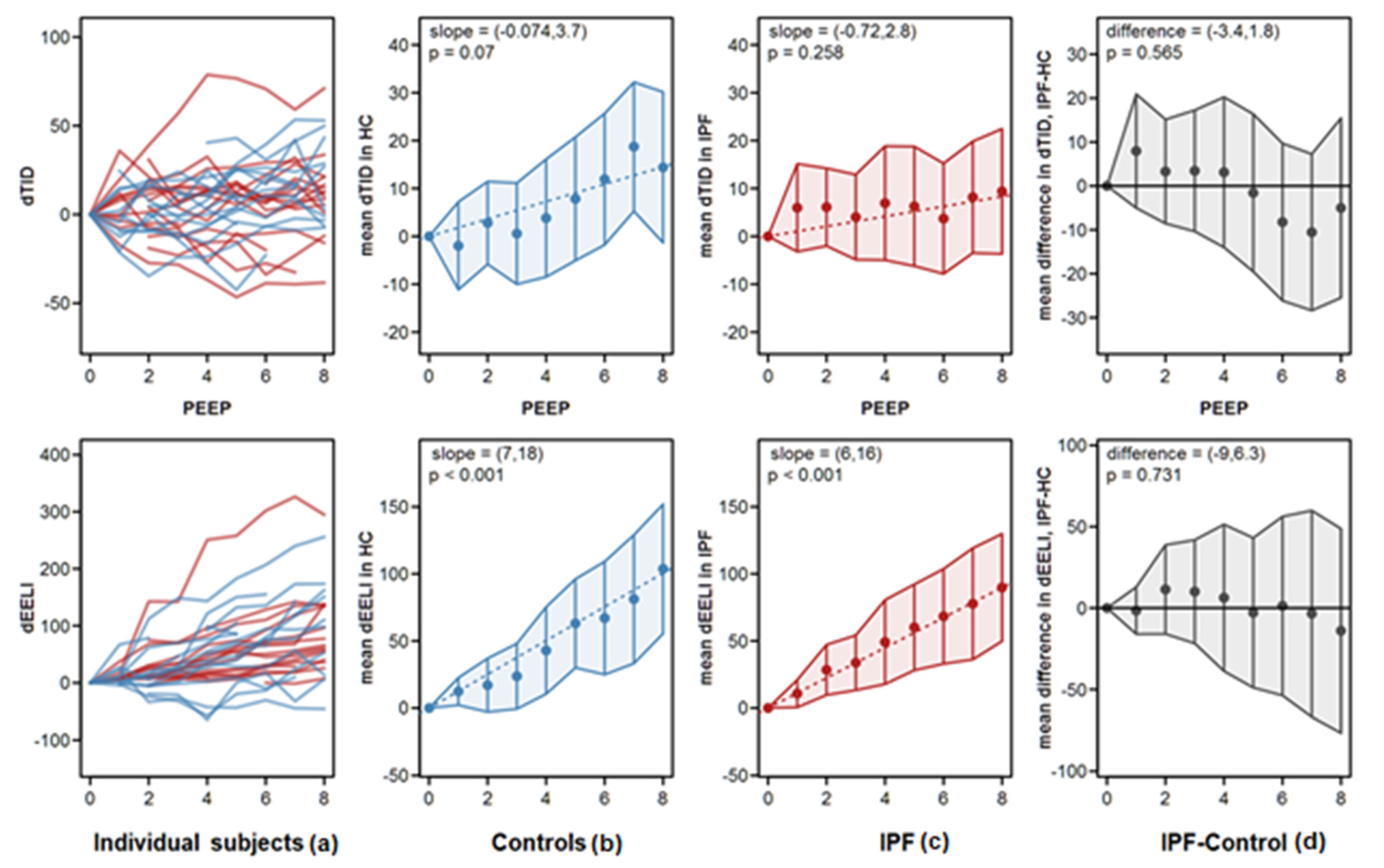
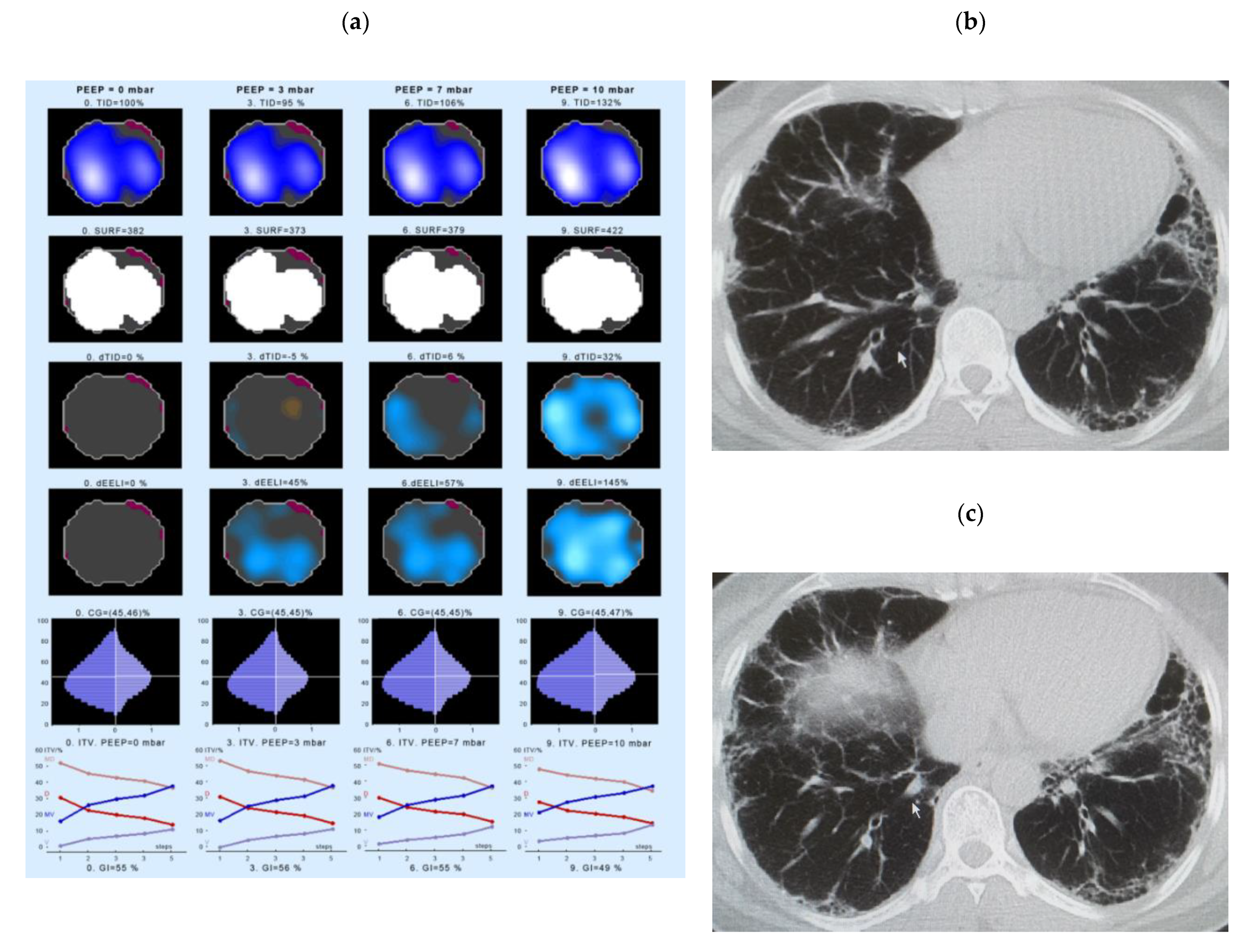
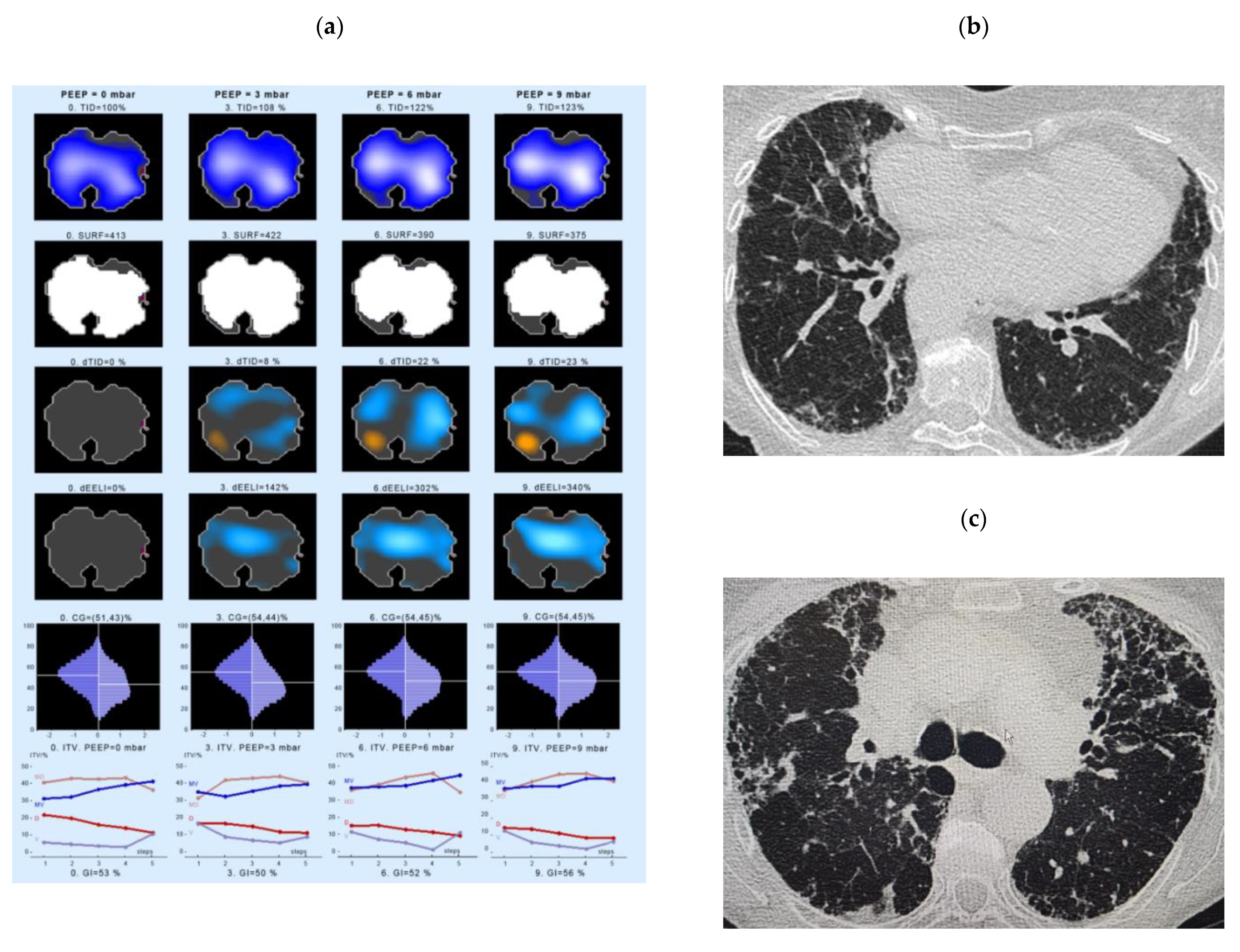
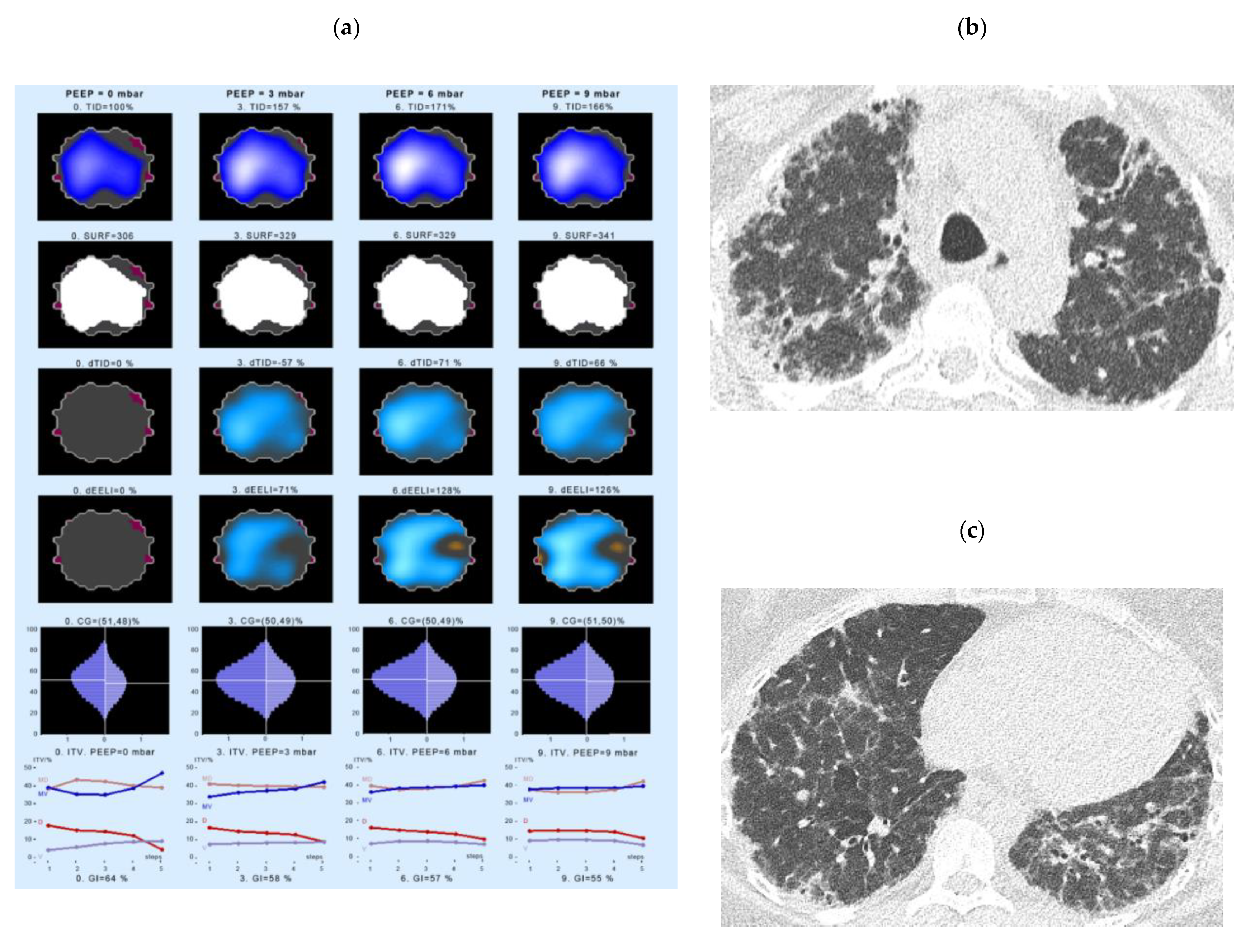
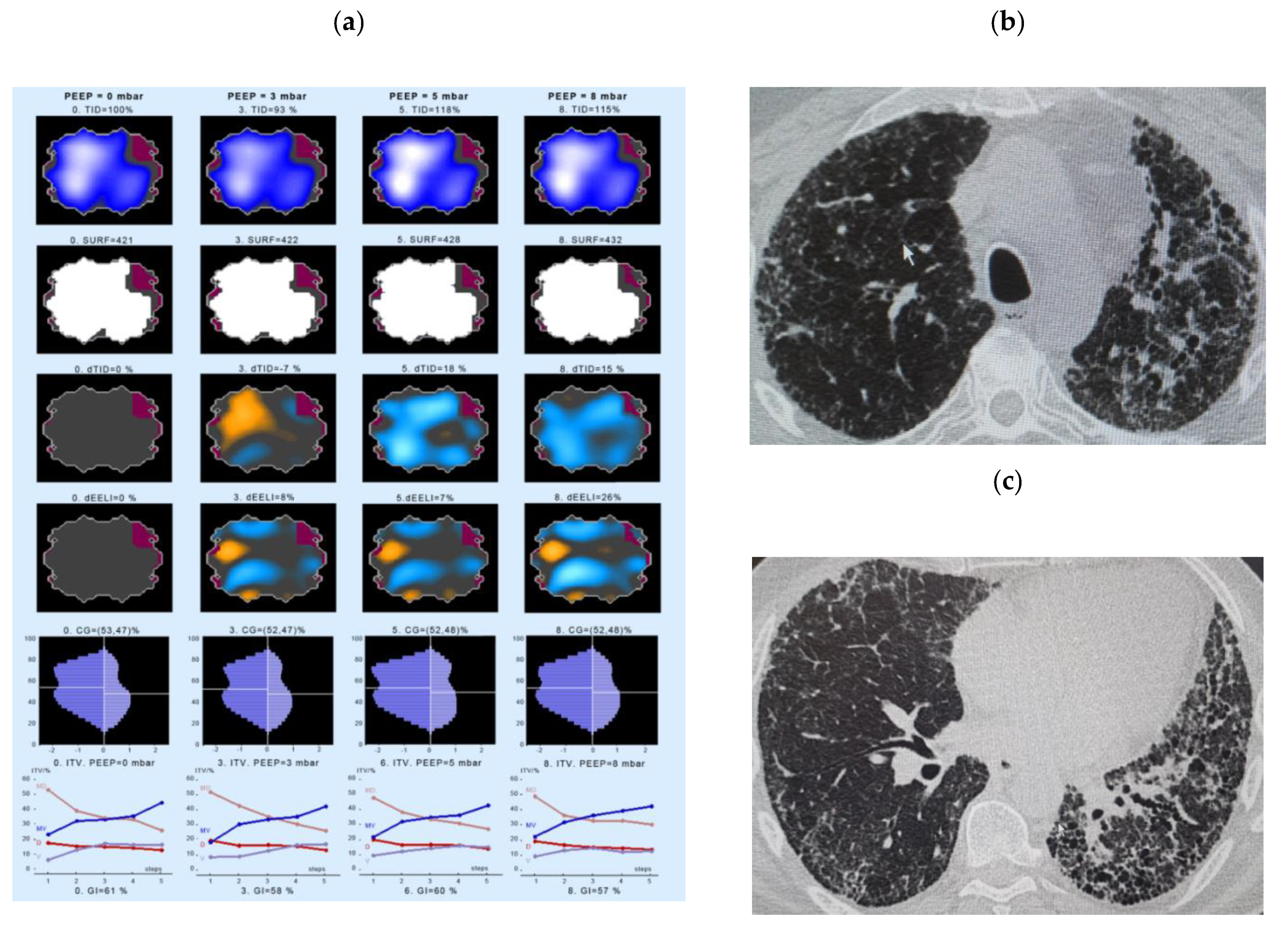
- Intratidal gas distribution (ITV) exhibits the way the gas distributes in the lungs during the tidal breath and delineates the changes in regional ventilation over a time course during one breath in different ROIs.
- Tidal Impedance Distribution (TID) reflects the average ventilation for a defined period of breath, as reflected by the difference in impedance values at the beginning and the end of an inspiration. For illustration of regional changes in TID, impedance changes above 10% of the maximum regional impedance change are displayed in dark blue. As values increase, the dark blue turns into a lighter blue. A white color indicates the regions of maximum regional impedance change.
- Difference of TID over time (dTID) displays a global change of TID between two points of time, i.e., between baseline (PEEP = 0 cm H2O) and the various PEEP increment time points. For illustration of regional changes in dTID, the differential image indicates increases in dTID (vs. the reference) in turquoise and decreases in orange color.
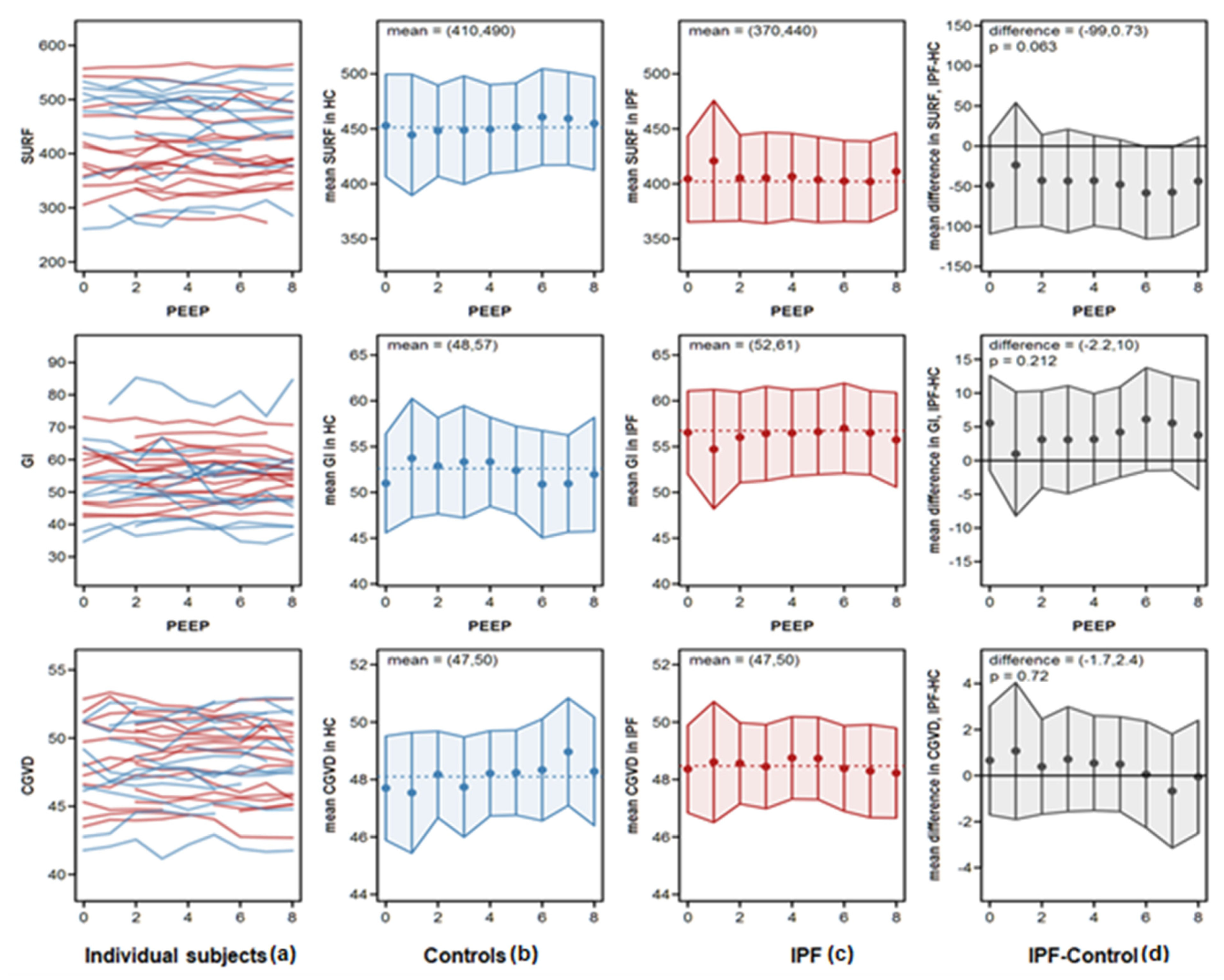
- Surface of ventilated areas (SURF) describes the surface of ventilated areas of the tomogram, as defined by a regional impedance chance between inspiration and expiration. For illustration of regional changes in the tomogram, ventilated pixels are displayed in white color; non-aerated pixels are given in a dark grey color.
- Global inhomogeneity index (GI) represents the spatial extent and dispersion in the distribution of tidal breath, i.e., global inhomogeneity in tidal ventilation [28]. A GI with a value 0 represents a perfectly homogeneous distribution. For illustration of regional changes in the tomogram, the absolute differences between the median impedance value of all pixels and each single pixel value are depicted.
- Center of ventilation (CGVD) represents a vertical shift of the ventilation distribution along a gravitational axis in supine position [17]. CGVD (Center of Gravity of Ventilation Distribution) describes how ventilation is distributed between ventral and dorsal lung regions [29]. For the global analysis, this variable is expressed as a percentage of impedance on the dorsal-ventral scale from 0% (= ventilation completely in the dorsal image line) to 100% (in the ventral image line).
- End-expiratory Lung Impedance (EELI) reflects the impedance at end-expiration. The higher the EELI, the more lung volume is present at end-expiration. As the absolute lung volume is rarely known, EELI cannot be directly related to the end-expiratory lung volume.
- Difference of EELI over time (dEELI) depicts change of EELI between two points of time, i.e., the change in EELI between PEEP = 0 cm H2O and the various PEEP increment time points. For illustration of regional changes, the dEELI value of each pixel is indicated by a blue to white color and the more color the higher the percental increase.
3.5. Quality of Data and Statistical Analysis
4. Results
4.1. Demographics and Clinical Characteristic of the Study Cohort
4.2. Analysis of EIT Variables
4.3. Examples of EIT Measurements in IPF Patients
5. Discussion
Study Limitations
- In general, due to spontaneous breathing, a profound heterogeneity and inconsistent variations were observed with regard to some EIT variables, which somewhat limited the overall conclusiveness. Also, not all IPF patients did manage to complete the protocol up to the highest PEEP level of 10, reporting that they felt uncomfortable and would experience the end-expiratory pressure as too high.
- Our control subjects were younger as compared to the IPF patients.
- Another limitation of our study was our inability to simultaneously assess EIT and lung function. Of our special interest would also be measurements of lung compliance and transpulmonary pressure gradients, which we would have liked to be correlated to the observed changes in regional ventilation.
- The results of this study were partly affected by the fact that subjects may react very differently to a PEEP titration. Here, the true transpulmonary pressure gradients (and hence the compliance) were unknown and, while some patients seemed to tolerate higher PEEP levels easily, others were not. Therefore, an optimal setting would be to combine the EIT measurement with a regular lung function test, including measurement of the transpulmonary pressure gradient via esophagus balloon and we expect this to be studied in the near future.
6. Conclusions
6.1. Key Results
6.2. Summary Statement
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guenther, A.; Krauss, E.; Tello, S.; Wagner, J.; Paul, B.; Kuhn, S.; Maurer, O.; Heinemann, S.; Costabel, U.; Barbero, M.A.N.; et al. The European IPF registry (eurIPFreg): Baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Krauss, E.; Gehrken, G.; Drakopanagiotakis, F.; Tello, S.; Dartsch, R.C.; Maurer, O.; Windhorst, A.; von der Beck, D.; Griese, M.; Seeger, W.; et al. Clinical characteristics of patients with familial idiopathic pulmonary fibrosis (f-IPF). BMC Pulm. Med. 2019, 19, 130. [Google Scholar] [CrossRef] [PubMed]
- Krauss, E.; Froehler, M.; Degen, M.; Mahavadi, P.; Dartsch, R.C.; Korfei, M.; Ruppert, C.; Seeger, W.; Guenther, A. Exhalative Breath Markers Do Not Offer for Diagnosis of Interstitial Lung Diseases: Data from the European IPF Registry (eurIPFreg) and Biobank. J. Clin. Med. 2019, 8, 643. [Google Scholar] [CrossRef] [PubMed]
- Plantier, L.; Cazes, A.; Dinh-Xuan, A.-T.; Bancal, C.; Marchand-Adam, S.; Crestani, B. Physiology of the lung in idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2018, 27. [Google Scholar] [CrossRef]
- Günther, A.; Schmidt, R.; Nix, F.; Yabut-Perez, M.; Guth, C.; Rosseau, S.; Siebert, C.; Grimminger, F.; Morr, H.; Velcovsky, H.G.; et al. Surfactant abnormalities in idiopathic pulmonary fibrosis, hypersensitivity pneumonitis and sarcoidosis. Eur. Respir. J. 1999, 14, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Steffen, L.; Ruppert, C.; Hoymann, H.-G.; Funke, M.; Ebener, S.; Kloth, C.; Mühlfeld, C.; Ochs, M.; Knudsen, L.; Lopez-Rodriguez, E. Surfactant replacement therapy reduces acute lung injury and collapse induration-related lung remodeling in the bleomycin model. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L313–L327. [Google Scholar] [CrossRef]
- Ruppert, C.; Kuchenbuch, T.; Boensch, M.; Schmidt, S.; Mathes, U.; Hillebrand, V.; Henneke, I.; Markart, P.; Reiss, I.; Schermuly, R.T.; et al. Dry powder aerosolization of a recombinant surfactant protein-C-based surfactant for inhalative treatment of the acutely inflamed lung. Crit. Care Med. 2010, 38, 1584–1591. [Google Scholar] [CrossRef]
- Thrall, R.S.; Swendsen, C.L.; Shannon, T.H.; Kennedy, C.A.; Frederick, D.S.; Grunze, M.F.; Sulavik, S.B. Correlation of changes in pulmonary surfactant phospholipids with compliance in bleomycin-induced pulmonary fibrosis in the rat. Am. Rev. Respir. Dis. 1987, 136, 113–118. [Google Scholar] [CrossRef]
- Günther, A.; Korfei, M.; Mahavadi, P.; von der Beck, D.; Ruppert, C.; Markart, P. Unravelling the progressive pathophysiology of idiopathic pulmonary fibrosis. Eur. Respir. Rev. 2012, 21, 152–160. [Google Scholar] [CrossRef]
- Burkhardt, A. Alveolitis and collapse in the pathogenesis of pulmonary fibrosis. Am. Rev. Respir. Dis. 1989, 140, 513–524. [Google Scholar] [CrossRef]
- Walsh, S.L.F.; Humphries, S.M.; Wells, A.U.; Brown, K.K. Imaging research in fibrotic lung disease; applying deep learning to unsolved problems. Lancet Respir. Med. 2020, 8. [Google Scholar] [CrossRef]
- Lynch, D.A.; Sverzellati, N.; Travis, W.D.; Brown, K.K.; Colby, T.V.; Galvin, J.R.; Goldin, J.G.; Hansell, D.M.; Inoue, Y.; Johkoh, T.; et al. Diagnostic criteria for idiopathic pulmonary fibrosis: A Fleischner Society White Paper. Lancet Respir. Med. 2018, 6, 138–153. [Google Scholar] [CrossRef]
- Brown, B.H.; Barber, D.C.; Seagar, A.D. Applied potential tomography: Possible clinical applications. Clin. Phys. Physiol. Meas. 1985, 6, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Frerichs, I.; Becher, T.; Weiler, N. Methodology of electrical impedance tomography-derived measures of regional lung ventilation. Crit. Care 2014, 18, 635. [Google Scholar] [CrossRef][Green Version]
- Heines, S.J.H.; Strauch, U.; van de Poll, M.C.G.; Roekaertes, P.M.H.J.; Bergmans, D.C.J.J. Clinical implementation of electric impedance tomography in the treatment of ARDS: A single centre experience. J. Clin. Monit. Comput. 2019, 33, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Bluth, T.; Kiss, T.; Kircher, M.; Braune, A.; Bozsak, C.; Huhle, R.; Scharffenberg, M.; Herzog, M.; Roegner, J.; Herzog, P.; et al. Measurement of relative lung perfusion with electrical impedance and positron emission tomography: An experimental comparative study. Br. J. Anaesth. 2019, 123. [Google Scholar] [CrossRef]
- Frerichs, I.; Dargaville, P.A.; Rimensberger, P.C. Regional pulmonary effects of bronchoalveolar lavage procedure determined by electrical impedance tomography. Intensive Care Med. Exp. 2019, 7, 11. [Google Scholar] [CrossRef]
- Shono, A.; Kotani, T. Clinical implication of monitoring regional ventilation using electrical impedance tomography. J. Intensive Care 2019, 7, 4. [Google Scholar] [CrossRef]
- Thürk, F.; Boehme, S.; Mudrak, D.; Kampusch, S.; Wielandner, A.; Prosch, H.; Braun, C.; Toemboel, F.P.R.; Hofmanninger, J.; Kaniusas, E. Effects of individualized electrical impedance tomography and image reconstruction settings upon the assessment of regional ventilation distribution: Comparison to 4-dimensional computed tomography in a porcine model. PLoS ONE 2017, 12, e0182215. [Google Scholar] [CrossRef]
- Chiumello, D.; Sferrazza Papa, G.F.; Artigas, A.; Bouhemad, B.; Grgic, A.; Heunks, L.; Markstaller, K.; Pellegrino, G.M.; Pisani, L.; Rigau, D.; et al. ERS statement on chest imaging in acute respiratory failure. Eur. Respir. J. 2019, 56. [Google Scholar] [CrossRef]
- Guenther, A. The European IPF Network: Towards better care for a dreadful disease. Eur. Respir. J. 2011, 37, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An official ATS/ERS/JRS/ALAT statement: Idiopathic pulmonary fibrosis: Evidence-based guidelines for diagnosis and management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef] [PubMed]
- Reinartz, S.D.; Imhoff, M.; Tolba, R.; Fischer, F.; Fischer, E.G.; Teschner, E.; Koch, S.; Gärber, Y.; Isfort, P.; Gremse, F. EIT monitors valid and robust regional ventilation distribution in pathologic ventilation states in porcine study using differential DualEnergy-CT (ΔDECT). Sci. Rep. 2019, 9, 9796. [Google Scholar] [CrossRef]
- Bachmann, M.C.; Morais, C.; Bugedo, G.; Bruhn, A.; Morales, A.; Borges, J.B.; Costa, E.; Retamal, J. Electrical impedance tomography in acute respiratory distress syndrome. Crit. Care 2018, 22, 263. [Google Scholar] [CrossRef]
- Frerichs, I.; Amato, M.B.P.; van Kaam, A.H.; Tingay, D.G.; Zhao, Z.; Grychtol, B.; Bodenstein, M.; Gagnon, H.; Böhm, S.H.; Teschner, E.; et al. Chest electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2016, 72, 83–93. [Google Scholar] [CrossRef]
- Karsten, J.; Stueber, T.; Voigt, N.; Teschner, E.; Heinze, H. Influence of different electrode belt positions on electrical impedance tomography imaging of regional ventilation: A prospective observational study. Crit. Care 2016, 20, 3. [Google Scholar] [CrossRef]
- Zhao, Z.; Möller, K.; Steinmann, D.; Frerichs, I.; Guttmann, J. Evaluation of an electrical impedance tomography-based Global Inhomogeneity Index for pulmonary ventilation distribution. Intensive Care Med. 2009, 35, 1900–1906. [Google Scholar] [CrossRef]
- Putensen, C.; Hentze, B.; Muenster, S.; Muders, T. Electrical Impedance Tomography for Cardio-Pulmonary Monitoring. J. Clin. Med. 2019, 8, 1176. [Google Scholar] [CrossRef]
- Costa, E.L.V.; Borges, J.B.; Melo, A.; Suarez-Sipmann, F.; Toufen, C.; Bohm, S.H.; Amato, M.B.P. Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med. 2009, 35, 1132–1137. [Google Scholar] [CrossRef]
- Zhao, Z.; Chang, M.-Y.; Chang, M.-Y.; Gow, C.-H.; Zhang, J.-H.; Hsu, Y.-L.; Frerichs, I.; Chang, H.-T.; Möller, K. Positive end-expiratory pressure titration with electrical impedance tomography and pressure-volume curve in severe acute respiratory distress syndrome. Ann. Intensive Care 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Bikker, I.G.; Leonhardt, S.; Reis Miranda, D.; Bakker, J.; Gommers, D. Bedside measurement of changes in lung impedance to monitor alveolar ventilation in dependent and non-dependent parts by electrical impedance tomography during a positive end-expiratory pressure trial in mechanically ventilated intensive care unit patients. Crit. Care 2010, 14, R100. [Google Scholar] [CrossRef]
- Luepschen, H.; Meier, T.; Grossherr, M.; Leibecke, T.; Karsten, J.; Leonhardt, S. Protective ventilation using electrical impedance tomography. Physiol. Meas. 2007, 28, S247–S260. [Google Scholar] [CrossRef] [PubMed]
- Lowhagen, K.; Lundin, S.; Stenqvist, O. Regional intratidal gas distribution in acute lung injury and acute respiratory distress syndrome—Assessed by electric impedance tomography. Minerva Anesthesiol. 2010, 76, 1024–1035. [Google Scholar]
- Radke, O.C.; Schneider, T.; Heller, A.R.; Koch, T. Spontaneous breathing during general anesthesia prevents the ventral redistribution of ventilation as detected by electrical impedance tomography: A randomized trial. Anesthesiology 2012, 116, 1227–1234. [Google Scholar] [CrossRef]
| Parameters | IPF (n = 17) | HC (n = 15) |
|---|---|---|
| Age (years) mean value ± SD (range) | 65 ± 12 (44–79) | 31 ± 14 (20–62) |
| Sex (male) n (% of the whole cohort) | 13 (76%) | 8 (53%) |
| Time to diagnosis (years) mean value ± SD (range) | 3.1 ± 1.8 (0–7) | - |
| GAP Stage (I/II/III) n (% of the whole cohort) | 6/9/2 (35%/53%/12%) | - |
| Body weight (kg) mean value ± SD (range) | 80.9 ± 15.8 (46–109) | n = 11; 70.6 ± 17.3 (53–116) |
| BMI (kg/m2) mean value ± SD (range) | 26.7 ± 4.1 (18.9–34.8) | 24.4 ± 6.0 (19.4–39.2) |
| Smoker (active/never/ex) n (% of the whole cohort) | 3/5/9 (18%/29%/53%) | - |
| Pack years mean value ± SD (range) | 34 ± 20 (15–80) | - |
| Medication n (% of the whole cohort) | - | - |
| Prednisolone | 7 (41%) | 0 (0%) |
| Pirfenidone | 8 (47%) | 0 (0%) |
| N-Acetylcysteine | 3 (18%) | 0 (0%) |
| Azathioprine | 3 (18%) | 0 (0%) |
| PFT Variable | IPF (n = 17) Mean Value ± SD (Range: Minimum–Maximum Value) | HC (n = 15) Mean Value ± SD (Range: Minimum–Maximum Value) |
|---|---|---|
| VC (% pred.) | 72 ± 18 (44–114) | 104 ± 10 (82–118) |
| FVC (% pred.) | 71 ± 18 (46–118) | 104 ± 11 (80–117) |
| TLC (% pred.) | 77 ± 21 (50–126) | 112 ± 12 (92–128) |
| FEV1 (% pred.) | 76 ± 19 (47–132) | 104 ± 10 (83–117) |
| FEV1/VC (% pred.) | 106 ± 13 (77–134) | 101.1 ± 5.1 (89–105) |
| ITGV (% pred.) | 80 ± 27 (51–132) | 102 ± 23 (66–140) |
| RV (% pred.) | 92 ± 31 (60–160) | 133 ± 35 (74–195) |
| RV/TLC (% pred.) | 110 ± 12 (88–135) | 116 ± 24 (69–151) |
| DLco (% pred.) | 40 ± 16 (20–83) | 87 ± 14 (71–120) |
| SaO2 (%) | 95.3 ± 1.2 (92–97) | 97.5 ± 0.9 (96–98) |
| pO2 (mmHg) | 73.1 ± 7.6 (62–88) | 89.2 ± 9.1 (77–103) |
| pCO2 (mmHg) | 37.8 ± 3.4 (31–44) | 37.2 ± 4.1 (31–44) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krauss, E.; van der Beck, D.; Schmalz, I.; Wilhelm, J.; Tello, S.; Dartsch, R.C.; Mahavadi, P.; Korfei, M.; Teschner, E.; Seeger, W.; et al. Evaluation of Regional Pulmonary Ventilation in Spontaneously Breathing Patients with Idiopathic Pulmonary Fibrosis (IPF) Employing Electrical Impedance Tomography (EIT): A Pilot Study from the European IPF Registry (eurIPFreg). J. Clin. Med. 2021, 10, 192. https://doi.org/10.3390/jcm10020192
Krauss E, van der Beck D, Schmalz I, Wilhelm J, Tello S, Dartsch RC, Mahavadi P, Korfei M, Teschner E, Seeger W, et al. Evaluation of Regional Pulmonary Ventilation in Spontaneously Breathing Patients with Idiopathic Pulmonary Fibrosis (IPF) Employing Electrical Impedance Tomography (EIT): A Pilot Study from the European IPF Registry (eurIPFreg). Journal of Clinical Medicine. 2021; 10(2):192. https://doi.org/10.3390/jcm10020192
Chicago/Turabian StyleKrauss, Ekaterina, Daniel van der Beck, Isabel Schmalz, Jochen Wilhelm, Silke Tello, Ruth C. Dartsch, Poornima Mahavadi, Martina Korfei, Eckhard Teschner, Werner Seeger, and et al. 2021. "Evaluation of Regional Pulmonary Ventilation in Spontaneously Breathing Patients with Idiopathic Pulmonary Fibrosis (IPF) Employing Electrical Impedance Tomography (EIT): A Pilot Study from the European IPF Registry (eurIPFreg)" Journal of Clinical Medicine 10, no. 2: 192. https://doi.org/10.3390/jcm10020192
APA StyleKrauss, E., van der Beck, D., Schmalz, I., Wilhelm, J., Tello, S., Dartsch, R. C., Mahavadi, P., Korfei, M., Teschner, E., Seeger, W., & Guenther, A. (2021). Evaluation of Regional Pulmonary Ventilation in Spontaneously Breathing Patients with Idiopathic Pulmonary Fibrosis (IPF) Employing Electrical Impedance Tomography (EIT): A Pilot Study from the European IPF Registry (eurIPFreg). Journal of Clinical Medicine, 10(2), 192. https://doi.org/10.3390/jcm10020192





