Effective Orifice Area of Balloon-Expandable and Self-Expandable Transcatheter Aortic Valve Prostheses: An Echo Doppler Comparative Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Study Design
2.3. Echocardiography and Doppler Measurements
2.4. Selection of Prosthesis Size and Preinterventional CT
2.5. Endpoints
2.6. Statistical Analysis
3. Results
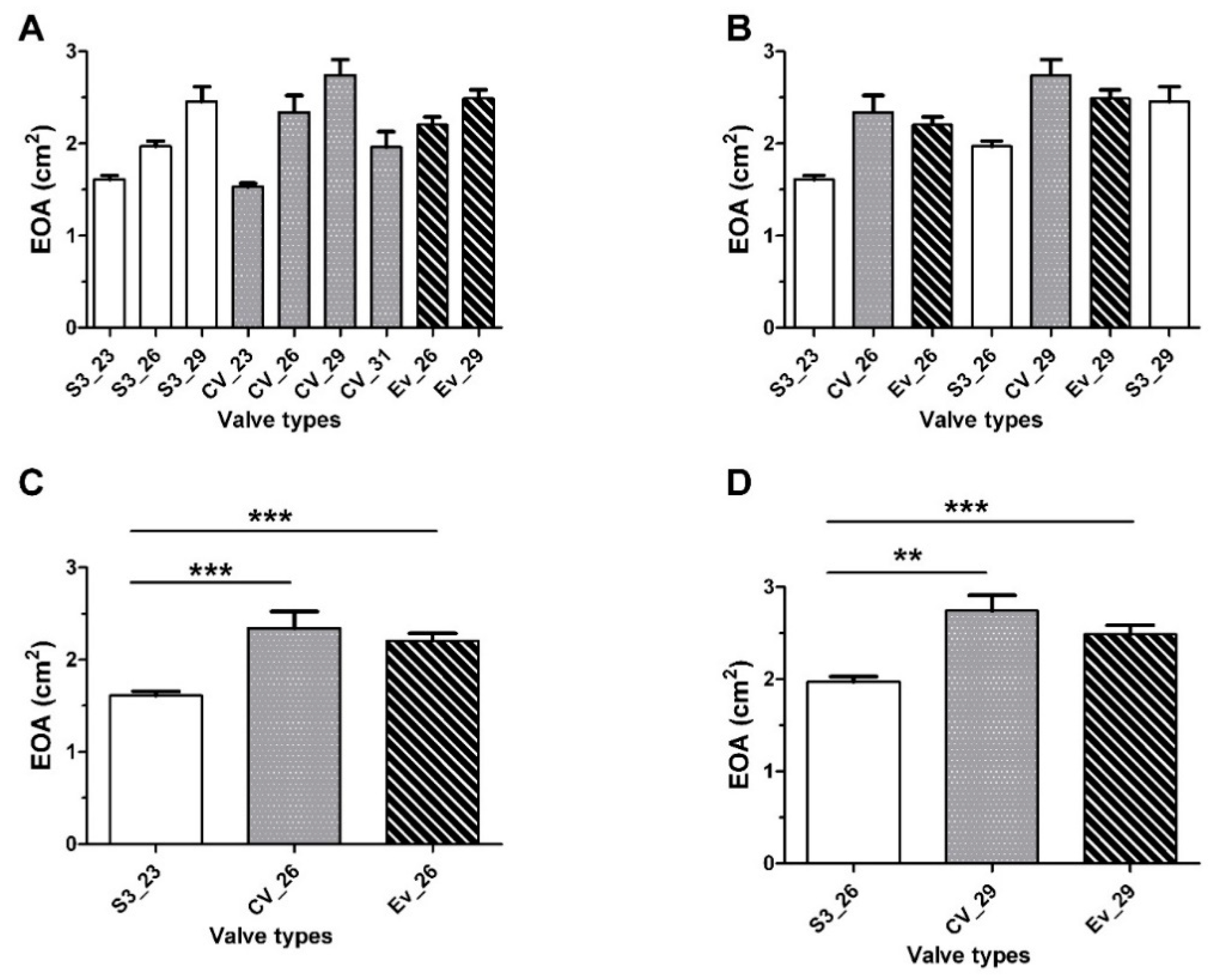
3.1. EOA, Transaortic MG, and LVEF
3.2. Patient–Prosthesis Mismatch
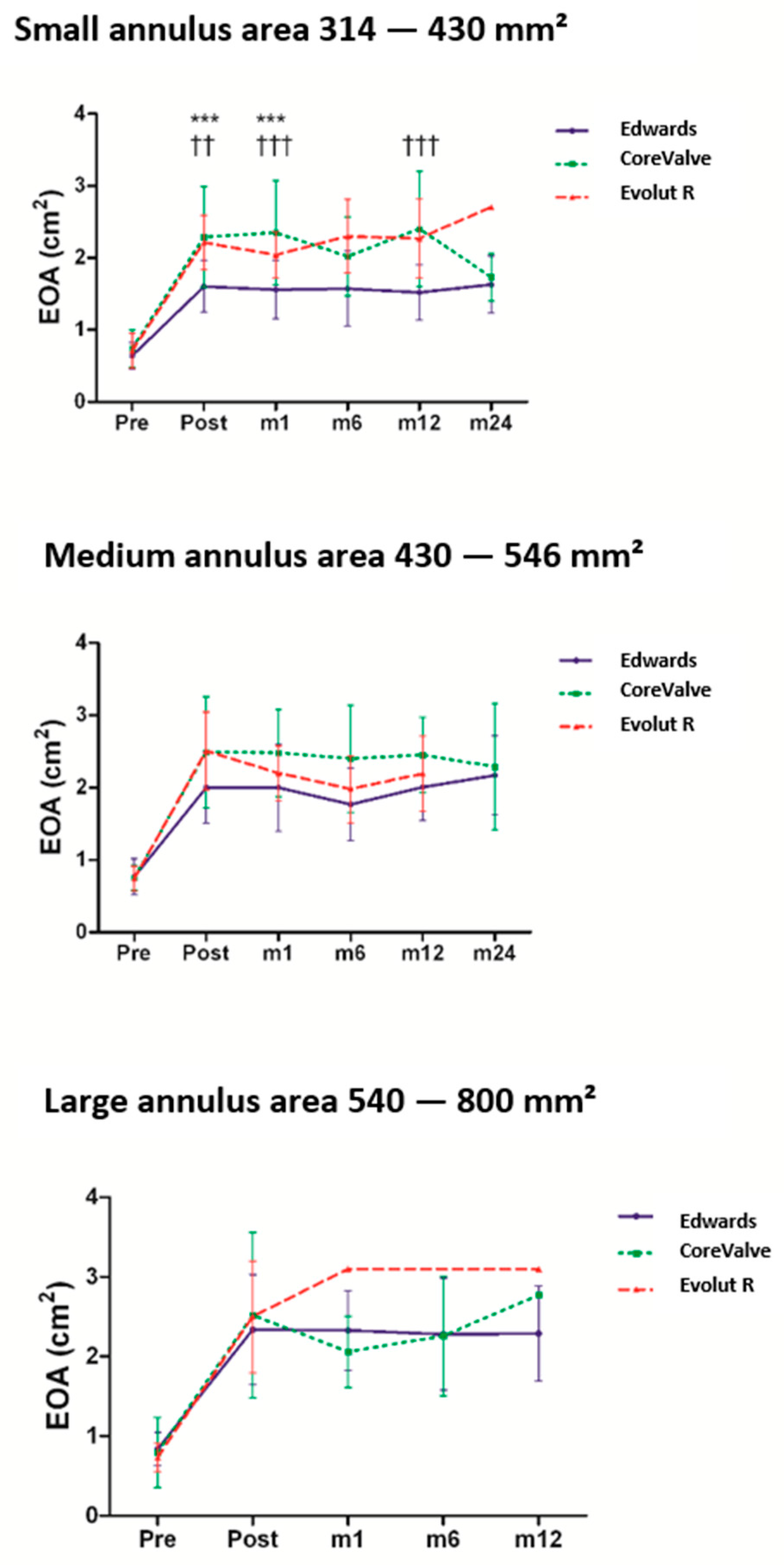
3.3. Impact of Prosthesis Size on the Hemodynamic Characteristics
3.4. Paravalvular Aortic Regurgitation
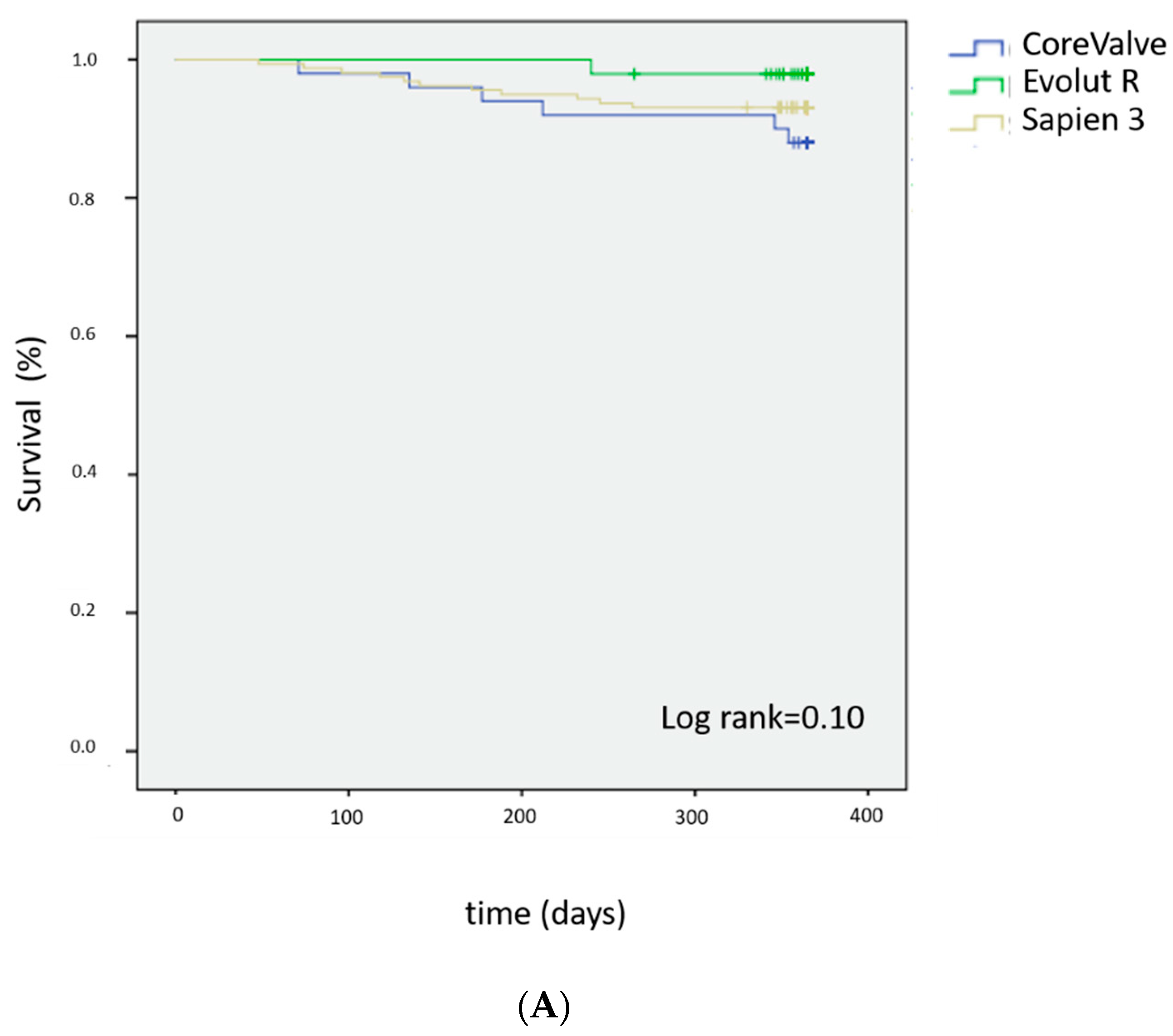
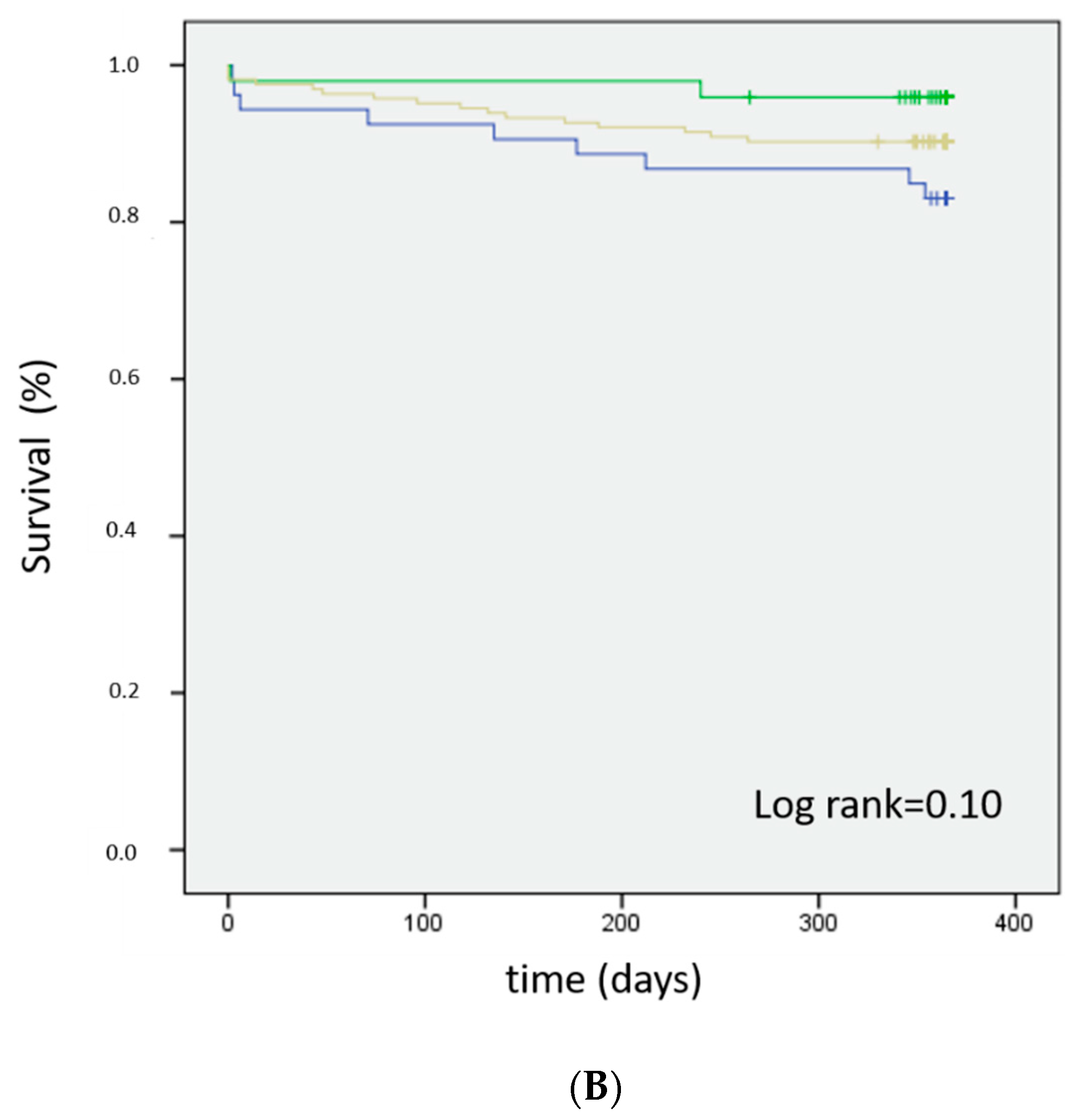
3.5. Survival Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.-L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The euro heart survey on valvular heart disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Muñoz, D.R.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.-A.; Webb, J.G.; Pibarot, P.; Altwegg, L.; Dumont, E.; Thompson, C.; De Larochellière, R.; Doyle, D.; Masson, J.-B.; Bergeron, S.; et al. Comparison of the hemodynamic performance of percutaneous and surgical bioprostheses for the treatment of severe aortic stenosis. J. Am. Coll. Cardiol. 2009, 53, 1883–1891. [Google Scholar] [CrossRef] [PubMed]
- Rosenhek, R.; Binder, T.; Maurer, G.; Baumgartner, H. Normal values for Doppler echocardiographic assessment of heart valve prostheses. J. Am. Soc. Echocardiogr. 2003, 16, 1116–1127. [Google Scholar] [CrossRef]
- Winter, M.-P.; Zbiral, M.; Kietaibl, A.; Sulzgruber, P.; Kastner, J.; Rosenhek, R.; Binder, T.; Lang, I.M.; Goliasch, G. Normal values for Doppler echocardiographic assessment of prosthetic valve function after transcatheter aortic valve replacement: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 361–368. [Google Scholar] [CrossRef]
- Achenbach, S.; Schuhbäck, A.; Min, J.K.; Leipsic, J. Determination of the aortic annulus plane in CT imaging-a step-by-step approach. JACC Cardiovasc. Imaging 2013, 6, 275–278. [Google Scholar] [CrossRef]
- Khalique, O.K.; Hamid, N.B.; Kodali, S.K.; Nazif, T.M.; Marcoff, L.; Paradis, J.-M.; Williams, M.R.; Vahl, T.P.; George, I.; Leon, M.B.; et al. Improving the accuracy of effective orifice area assessment after transcatheter aortic valve replacement: Validation of left ventricular outflow tract diameter and pulsed-wave doppler location and impact of three-dimensional measurements. J. Am. Soc. Echocardiogr. 2015, 28, 1283–1293. [Google Scholar] [CrossRef]
- Clavel, M.-A.; Rodés-Cabau, J.; Dumont, É.; Bagur, R.; Bergeron, S.; De Larochellière, R.; Doyle, D.; Larose, E.; Dumesnil, J.G.; Pibarot, P. Validation and characterization of transcatheter aortic valve effective orifice area measured by doppler echocardiography. JACC Cardiovasc. Imaging 2011, 4, 1053–1062. [Google Scholar] [CrossRef]
- Shames, S.; Koczo, A.; Hahn, R.; Jin, Z.; Picard, M.H.; Gillam, L.D. Flow characteristics of the sapien aortic valve: The importance of recognizing in-stent flow acceleration for the echocardiographic assessment of valve function. J. Am. Soc. Echocardiogr. 2012, 25, 603–609. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M.; et al. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23. [Google Scholar] [CrossRef]
- Little, S.H.; Oh, J.K.; Gillam, L.; Sengupta, P.P.; Orsinelli, D.A.; Cavalcante, J.L.; Chang, J.D.; Adams, D.H.; Zorn, G.L., 3rd; Pollak, A.W.; et al. Self-expanding transcatheter aortic valve replacement versus surgical valve replacement in patients at high risk for surgery: A study of echocardiographic change and risk prediction. Circ. Cardiovasc. Interv. 2016, 9, e003426. [Google Scholar] [CrossRef] [PubMed]
- Külling, M.; Külling, J.; Wyss, C.; Hürlimann, D.; Reho, I.; Salzberg, S.; Bühler, I.; Noll, G.; Grünenfelder, J.; Corti, R.; et al. Effective orifice area and hemodynamic performance of the transcatheter Edwards Sapien 3 prosthesis: Short-term and 1-year follow-up. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Spethmann, S.; Dreger, H.; Baldenhofer, G.; Pflug, E.; Sanad, W.; Stangl, V.; Baumann, G.; Grubitzsch, H.; Sander, M.; Stangl, K.; et al. Long-Term doppler hemodynamics and effective orifice areas of edwards sapien and medtronic corevalve prostheses after TAVI. Echocardiography 2014, 31, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Enríquez-Rodríguez, E.; Amat-Santos, I.J.; Jiménez-Quevedo, P.; Martín-Morquecho, I.; Tirado-Conte, G.; Pérez-Vizcayno, M.J.; de Diego, J.J.G.; Arnold, R.; Aldazábal, A.; Rojas, P.; et al. Comparison of the hemodynamic performance of the balloon-expandable SAPIEN 3 versus self-expandable evolut r transcatheter valve: A case-matched study. Rev. Espanola Cardiol. Engl. Ed. 2018, 71, 735–742. [Google Scholar] [CrossRef]
- Muñoz-García, A.J.; Muñoz-García, M.; Carrasco-Chinchilla, F.; Molina-Mora, M.J.; Rodríguez-Bailón, I.; Domínguez-Franco, A.J.; Jiménez-Navarro, M.F.; Alonso-Briales, J.H.; Gómez-Doblas, J.J.; Hernández-García, J.M.; et al. Incidence and clinical outcome of prosthesis–patient mismatch after transcatheter aortic valve implantation with the CoreValve prosthesis. Int. J. Cardiol. 2013, 167, 1074–1076. [Google Scholar] [CrossRef]
- Herrmann, H.C.; Thourani, V.H.; Kodali, S.K.; Makkar, R.R.; Szeto, W.Y.; Anwaruddin, S.; Desai, N.; Lim, S.; Chris Malaisrie, S.; Kereiakes, D.J.; et al. One-Year clinical outcomes with sapien 3 transcatheter aortic valve replacement in high-risk and inoperable patients with severe aortic stenosisclinical perspective. Circulation 2016, 134, 130–140. [Google Scholar] [CrossRef]
- Wendler, O.; Schymik, G.; Treede, H.; Baumgartner, H.; Dumonteil, N.; Neumann, F.-J.; Tarantini, G.; Zamorano, J.L.; Vahanian, A. SOURCE 3: 1-year outcomes post-transcatheter aortic valve implantation using the latest generation of the balloon-expandable transcatheter heart valve. Eur. Heart J. 2017, 38, 2717–2726. [Google Scholar] [CrossRef]
- Deeb, G.M.; Reardon, M.J.; Chetcuti, S.; Patel, H.J.; Grossman, P.M.; Yakubov, S.J.; Kleiman, N.S.; Coselli, J.S.; Gleason, T.G.; Lee, J.S.; et al. 3-Year Outcomes in High-Risk Patients Who Underwent Surgical or Transcatheter Aortic Valve Replacement. J. Am. Coll. Cardiol. 2016, 67, 2565–2574. [Google Scholar] [CrossRef]
- Popma, J.J.; Reardon, M.J.; Khabbaz, K.; Harrison, J.K.; Hughes, G.C.; Kodali, S.; George, I.; Deeb, G.M.; Chetcuti, S.; Kipperman, R.; et al. Early clinical outcomes after transcatheter aortic valve replacement using a novel self-expanding bioprosthesis in patients with severe aortic stenosis who are suboptimal for surgery: Results of the evolut R U.S. study. JACC Cardiovasc. Interv. 2017, 10, 268–275. [Google Scholar] [CrossRef]
- Hahn, R.T.; Leipsic, J.; Douglas, P.S.; Jaber, W.A.; Weissman, N.J.; Pibarot, P.; Blanke, P.; Oh, J.K. Comprehensive echocardiographic assessment of normal transcatheter valve function. JACC Cardiovasc. Imaging 2019, 12, 25–34. [Google Scholar] [CrossRef]
- Head, S.J.; Mokhles, M.M.; Osnabrugge, R.L.J.; Pibarot, P.; Mack, M.J.; Takkenberg, J.J.M.; Bogers, A.J.J.C.; Kappetein, A.P. The impact of prosthesis–patient mismatch on long-term survival after aortic valve replacement: A systematic review and meta-analysis of 34 observational studies comprising 27 186 patients with 133 141 patient-years. Eur. Heart J. 2012, 33, 1518–1529. [Google Scholar] [CrossRef] [PubMed]
- Takagi, H.; Umemoto, T. Prosthesis–patient mismatch after transcatheter aortic valve implantation. Ann. Thorac. Surg. 2016, 101, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, G.; Van Mieghem, N.M.; Windecker, S.; Bosmans, J.; Bleiziffer, S.; Modine, T.; Linke, A.; Scholtz, W.; Chevalier, B.; Gooley, R.; et al. 1-Year Outcomes with the Evolut R Self-Expanding Transcatheter Aortic Valve: From the International FORWARD Study. JACC Cardiovasc. Interv. 2018, 11, 2326–2334. [Google Scholar] [CrossRef] [PubMed]
| Valve Type | Overall | Sapien 3 (n = 161) | CoreValve (n = 50) | Evolut R (n = 49) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diameter (mm) | (n = 260) | 23 (n = 74) | 26 (n = 67) | 29 (n = 20) | 23 (n = 2) | 26(n = 15) | 29 (n = 24) | 31 (n = 9) | 26 (n = 22) | 29 (n = 27) | |
| Age (years) | 84.1 ± 6.8 | 85.6 ± 6.5 | 84 ± 5.6 | 82.5 ± 6.2 | 88.9 ± 6.5 | 84.1 ± 8.4 | 83.3 ± 8.9 | 80.5 ± 7.6 | 84.4 ± 6.4 | 83.3 ± 7.5 | 0.347 |
| Female sex, n (%) | 146 (56.1) | 65 (87.8) | 21 (31.3) | 1 (5) | 1 (50) | 13 (86.7) | 11 (45.8) | 1 (11.1) | 20 (90.9) | 13 (48.1) | <0.001 |
| Euroscore I, % | 22 ± 14 | 23.2 ± 13.2 | 21.8 ± 14.2 | 18.9 ± 10.4 | 17.5 ± 0.7 | 20.5 ± 10.9 | 23.8 ± 12.1 | 34.9 ± 32.7 | 17.7 ± 10.6 | 19.8± 12.5 | 0.12 |
| Euroscore II, % | 4.9 ± 5.9 | 4.7 ± 3.5 | 5.2 ± 8 | 3.9 ± 2.9 | 2.5 ± 0.6 | 3.3 ± 1.6 | 4.5 ± 2.2 | 14.2 ± 15.8 | 4 ± 2.7 | 4.2 ± 2.7 | 0.001 |
| Body surface (m2) | 1.8 ± 0.2 | 1.7 ± 0.19 | 1.85 ± 0.2 | 1.88 ± 0.19 | 1.59 ± 0.2 | 1.65 ± 0.1 | 1.92 ± 0.21 | 1.90 ± 0.1 | 1.77 ± 0.1 | 1.84 ± 0.1 | <0.001 |
| BMI (kg/m2) | 26.3 ± 5.3 | 25.7 ± 6.2 | 26.9 ± 4.8 | 26.4 ± 3.7 | 22.3 ± 0.1 | 23.9 ± 5.9 | 28.5 ± 5.5 | 24.4 ± 3.4 | 26.6 ± 4.8 | 26.5 ± 3.7 | 0.186 |
| Heart failure, n (%) | 184 (70.8) | 53 (71.6) | 44 (65.7) | 14 (70) | 1 (50) | 10 (66.7) | 15 (62.5) | 6 (66.7) | 17 (77.3) | 24 (88.9) | 0.53 |
| Diabetes, n (%) | 92 (35.4) | 25 (33.8) | 24 (35.8) | 2 (10) | 0 (0) | 3 (20) | 14 (58.3) | 3 (33.3) | 11 (50) | 10 (37) | 0.042 |
| Hypertension, n (%) | 224 (86.2) | 64 (86.5) | 57 (85.1) | 13 (65) | 2 (100) | 11 (73.3) | 22 (91.7) | 8 (88.9) | 22 (100) | 25 (92.6) | 0.057 |
| AFIB, n (%) | 71 (27.3) | 16 (21.6) | 19 (28.4) | 4 (20) | 0 (0) | 4 (26.7) | 4 (16.7) | 3 (33.3) | 8 (36.4) | 13 (48.1) | 0.2 |
| Pacemaker, n (%) | 36 (13.8) | 9 (12.2) | 14 (20.9) | 2 (10) | 0 (0) | 0 (0) | 5 (20.8) | 2 (22.2) | 2 (9.1) | 2 (7.4) | 0.35 |
| CKD, n (%) | 140 (53.8) | 35 (47.3) | 31 (46.3) | 11 (55) | 2 (100) | 7 (46.7) | 16 (66.7) | 7 (77.8) | 14 (63.6) | 17 (63) | 0.24 |
| PCI, n (%) | 97 (37.3) | 29 (39.2) | 25 (37.3) | 7 (35) | 2 (100) | 4 (26.7) | 9 (37.5) | 5 (55.6) | 6 (27.3) | 10 (37) | 0.59 |
| CABG, n (%) | 25 (9.6) | 10 (13.5) | 4 (6) | 1 (5) | 0 (0) | 0 (0) | 2 (8.3) | 2 (22.2) | 1 (4.5) | 5 (18.5) | 0.3 |
| Peripheral artery disease, n (%) | 68 (26.2) | 16 (21.6) | 12 (17.9) | 5 (25) | 0 (0) | 3 (20) | 12 (50) | 5 (55.6) | 5 (22.7) | 10 (37) | 0.03 |
| Stroke, n (%) | 32 (12.3) | 9 (12.2) | 9 (13.4) | 2 (10) | 0 (0) | 0 (0) | 5 (16.7) | 1 (11.1) | 3 (13.6) | 4 (14.8) | 0.92 |
| COPD, n (%) | 23 (8.8) | 6 (8.1) | 8 (11.9) | 2 (10) | 0 (0) | 0 (0) | 1 (4.2) | 1 (11.1) | 2 (9.1) | 3 (11.1) | 0.9 |
| Previous BAV, n (%) | 27 (10.4) | 9 (12.2) | 7 (10.4) | 5 (25) | 0 (0) | 0 (0) | 3 (12.5) | 0 (0) | 2 (9.1) | 1 (3.7) | 0.31 |
| Valve Type | Overall | Sapien 3 (n = 161) | CoreValve (n = 50) | Evolut R (n = 49) | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Diameter (mm) | (n = 260) | 23 (n = 74) | 26 (n = 67) | 29 (n = 20) | 23 (n = 2) | 26(n = 15) | 29 (n = 24) | 31 (n = 9) | 26 (n = 22) | 29 (n = 27) | |
| Echocardiography | |||||||||||
| LVEF, % | 53.8 ± 13.3 | 54.9 ± 12.9 | 49.9 ± 13.1 | 47.7 ± 11.8 | 68.5 ± 13.3 | 58.3 ± 14.6 | 54 ± 12.3 | 45.2 ± 19.4 | 60.4 ± 12.3 | 58.9 ± 8.7 | <0.001 |
| MG, mmHg | 49.5 ± 15.1 | 52.7 ± 17 | 44.1 ± 13.1 | 48.8 ± 19.5 | 53 ± 24 | 55 ± 11.9 | 47.8 ± 12.4 | 41.5 ± 13.2 | 53.8 ± 13.3 | 51.9 ± 12.7 | 0.013 |
| SVi, mL/m2 | 41.6 ± 11.3 | 40.1 ± 9.6 | 39.9 ± 11.2 | 44.9 ± 10.1 | 40 ± 4.2 | 44.3 ± 12.6 | 39.3 ± 9.6 | 39.3 ± 14 | 44.9 ± 13.4 | 46.4 ± 13.1 | 0.093 |
| AVA, cm2 | 0.73 ± 0.22 | 0.62 ± 0.17 | 0.79 ± 0.22 | 0.87 ± 0.23 | 0.9 ± 0.67 | 0.66 ± 0.19 | 0.78 ± 0.14 | 0.81 ± 0.32 | 0.71 ± 0.24 | 0.76 ± 0.18 | <0.001 |
| AR > 2, n (%) | 27 (10.4) | 6 (8.1) | 5 (7.4) | 5 (25) | 0 (0) | 0 (0) | 2 (8.3) | 2 (22.2) | 2 (9.1) | 2 (7.4) | 0.009 |
| MR > 2, n (%) | 33 (12.7) | 14 (18.9) | 5 (7.4) | 5 (25) | 0 (0) | 4 (26.7) | 2 (8.3) | 1 (11.1) | 0 (0) | 2 (7.4) | 0.049 |
| LVOT, mm | 21.3 ± 1.9 | 20.2 ± 1.4 | 21.9 ± 1.6 | 24 ± 1.8 | 21 ± 2.8 | 20 ± 1.7 | 22 ± 1.3 | 22.8 ± 1.6 | 20 ± 1.5 | 21.9 ± 1.4 | <0.001 |
| CT | |||||||||||
| Area-derived diam | 24 ± 2.1 | 22.4 ± 1.5 | 24.8 ± 1.5 | 27.4 ± 0.96 | 23 | 21.7 ± 1.3 | 25.2 ± 1.1 | 26.8 ± 1.2 | 22 ± 1.3 | 24 ± 1.2 | <0.001 |
| Ann area, mm2 | 453.3 ± 86.5 | 388.6 ± 44.7 | 488 ± 60.5 | 608.4 ± 53.5 | 420 | 391 ± 64.4 | 494 ± 45.5 | 545.1 ± 68 | 375.6 ± 46.2 | 460.2 ± 66.6 | <0.001 |
| Ann perim, mm | 75.7 ± 6.7 | 70.2 ± 3.1 | 77.9 ± 8.5 | 90.7 ± 6.08 | - | - | 79.2 ± 1.7 | 83.7 ± 4.9 | 70.1 ± 4.5 | 77.1 ± 2.9 | <0.001 |
| Calcium score, AU | 3383 ± 1682 | 2614 ± 1129 | 3392 ± 1529 | 5790 ± 2596 | - | 2957 ± 1642 | 3016 ± 989 | 4195 ± 1932 | 3384 ± 1503 | 3365 ± 1637 | 0.01 |
| Valve Type | Overall | Sapien S3 | CoreValve | Evolut R | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diameter (mm) | 23 | 26 | 29 | 23 | 26 | 29 | 31 | 26 | 29 | |
| n = 260 | n = 74 | n = 67 | n = 20 | n = 2 | n = 15 | n = 24 | n = 9 | n = 22 | n = 27 | |
| EOA, cm2 (SD) | ||||||||||
| Discharge | 2.0 ± 0.6 | 1.6 ± 0.3 | 1.9 ± 0.4 | 2.4 ± 0.7 | 1.5 | 2.3 ± 0.7 | 2.7 ± 0.8 | 1.9 ± 0.5 | 2.2 ± 0.3 | 2.4 ± 0.4 |
| 1 month | 2.0 ± 0.6 | 1.5 ± 0.4 | 2 ± 0.5 | 2.3 ± 0.5 | 1.7 | 2.3 ± 0.6 | 2.6 ± 0.5 | 1.9 ± 0.4 | 2 ± 0.3 | 2.2 ± 0.4 |
| 6 months | 1.8 ± 0.6 | 1.4 ± 0.3 | 1.9 ± 0.6 | 2.3 ± 0.7 | / | 1.8 ± 0.6 | 2.5 ± 0.6 | 2 ± 0.7 | 2.1 ± 0.5 | 2.2 ± 0.5 |
| 1 year | 2.0 ± 0.6 | 1.4 ± 0.3 | 2.1 ± 0.4 | 2.3 ± 0.6 | / | 2.1 ± 0.5 | 2.5 ± 0.5 | 2.4 ± 0.2 | 2.1 ± 0.4 | 2.5 ± 0.6 |
| EOAi, cm2 (SD) | ||||||||||
| Discharge | 1.1 ± 0.3 | 0.9 ± 0.2 | 1 ± 0.2 | 1.3 ± 0.4 | 0.94 | 1.4 ± 0.4 | 1.51 ± 0.5 | 1.0 ± 0.3 | 1.2 ± 0.2 | 1.4 ± 0.3 |
| 1 month | 1.1 ± 0.3 | 0.9 ± 0.2 | 1.1 ± 0.3 | 1.2 ± 0.3 | 1.25 | 1.3 ± 0.3 | 1.4 ± 0.3 | 1 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 |
| 6 months | 1 ± 0.3 | 0.8 ± 0.2 | 1 ± 0.3 | 1.2 ± 0.3 | / | 1 ± 0.3 | 1.3 ± 0.2 | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.2 ± 0.2 |
| 1 year | 1.1 ± 0.3 | 0.8 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.3 | / | 1.2 ± 0.3 | 1.3 ± 0.2 | 1.2 ± 0.0 | 1.2 ± 0.2 | 1.3 ± 0.4 |
| MG, mmHg (SD) | ||||||||||
| Discharge | 9.4 ± 3.9 | 11.7 ± 3.4 $ | 9.5 ± 3.6 * | 8.9 ± 2.9 | 14.5 ± 6.3 | 6.7 ± 3.9 *** | 6.4 ± 3.2 *** $ | 8.5 ± 4.7 | 8.7 ± 3.2 * | 7.4 ± 3.5 *** |
| 1 month | 10 ± 4.3 | 12.4 ± 4.3 | 10.3 ± 3.7 | 10.5 ± 5.7 | 8 | 6.5 ± 2.6 *** $ | 6.1 ± 2.3 *** $$$ | 10.2 ± 5.1 | 8.4 ± 2.6 * | 8.5 ± 2.8 *** |
| 6 months | 10.9 ± 4.3 | 13.5 ± 3.5 % | 11.1 ± 4.3 | 9.1 ± 3.6 | / | 7.8 ± 3.1 * | 7 ± 3 * $ | 10 ± 2.9 | 8.8 ± 4.2 * | 9.2 ± 3.5 |
| 1 year | 10.2 ± 4.7 | 14 ± 4.5 | 9.6 ± 3.6 *** | 9.7 ± 3.7 * | / | 6.9 ± 3.6 * | 6.8 ± 2.5 *** | 10 ± 3 | 8.3 ± 2.5 ** | 7.3 ± 4.3 *** |
| LVEF, %(SD) | ||||||||||
| Discharge | 56 ± 13 | 59 ± 12 | 52 ± 14 | 47 ± 14 | 69 ± 4 | 58 ± 15 | 58 ± 11 | 45 ± 15 | 65 ± 6 | 58 ± 9 |
| 1 month | 57 ± 11 | 60 ± 11 | 54 ± 10 | 46 ± 12 | 66 | 61 ± 6 | 58 ± 8 | 45 ± 14 | 62 ± 7 | 59 ± 8 |
| 6 months | 58 ± 11 | 60 ± 11 | 56 ± 9.5 | 49 ± 16 | / | 64 ± 6 | 59 ± 13° | 49 ± 12 | 65 ± 7 | 59 ± 13 |
| 1 year | 58 ± 11 | 60 ± 11 | 55 ± 10 | 51 ± 12 | / | 61 ± 11 | 62 ± 10 | 51 ± 16 | 64 ± 7 | 59 ± 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanso, M.; Kibler, M.; Hess, S.; Rischner, J.; Plastaras, P.; Kindo, M.; Hoang, M.; De Poli, F.; Leddet, P.; Petit, H.; et al. Effective Orifice Area of Balloon-Expandable and Self-Expandable Transcatheter Aortic Valve Prostheses: An Echo Doppler Comparative Study. J. Clin. Med. 2021, 10, 186. https://doi.org/10.3390/jcm10020186
Kanso M, Kibler M, Hess S, Rischner J, Plastaras P, Kindo M, Hoang M, De Poli F, Leddet P, Petit H, et al. Effective Orifice Area of Balloon-Expandable and Self-Expandable Transcatheter Aortic Valve Prostheses: An Echo Doppler Comparative Study. Journal of Clinical Medicine. 2021; 10(2):186. https://doi.org/10.3390/jcm10020186
Chicago/Turabian StyleKanso, Mohamad, Marion Kibler, Sebastien Hess, Jérome Rischner, Philoktimon Plastaras, Michel Kindo, Minh Hoang, Fabien De Poli, Pierre Leddet, Hélène Petit, and et al. 2021. "Effective Orifice Area of Balloon-Expandable and Self-Expandable Transcatheter Aortic Valve Prostheses: An Echo Doppler Comparative Study" Journal of Clinical Medicine 10, no. 2: 186. https://doi.org/10.3390/jcm10020186
APA StyleKanso, M., Kibler, M., Hess, S., Rischner, J., Plastaras, P., Kindo, M., Hoang, M., De Poli, F., Leddet, P., Petit, H., Zeyons, F., Trinh, A., Matsushita, K., Morel, O., & Ohlmann, P. (2021). Effective Orifice Area of Balloon-Expandable and Self-Expandable Transcatheter Aortic Valve Prostheses: An Echo Doppler Comparative Study. Journal of Clinical Medicine, 10(2), 186. https://doi.org/10.3390/jcm10020186





