Two-Dimensional Visualization of the Three-Dimensional Planned Sacroiliac Screw Corridor with the Slice Fusion Method
Abstract
:1. Introduction
2. Methods
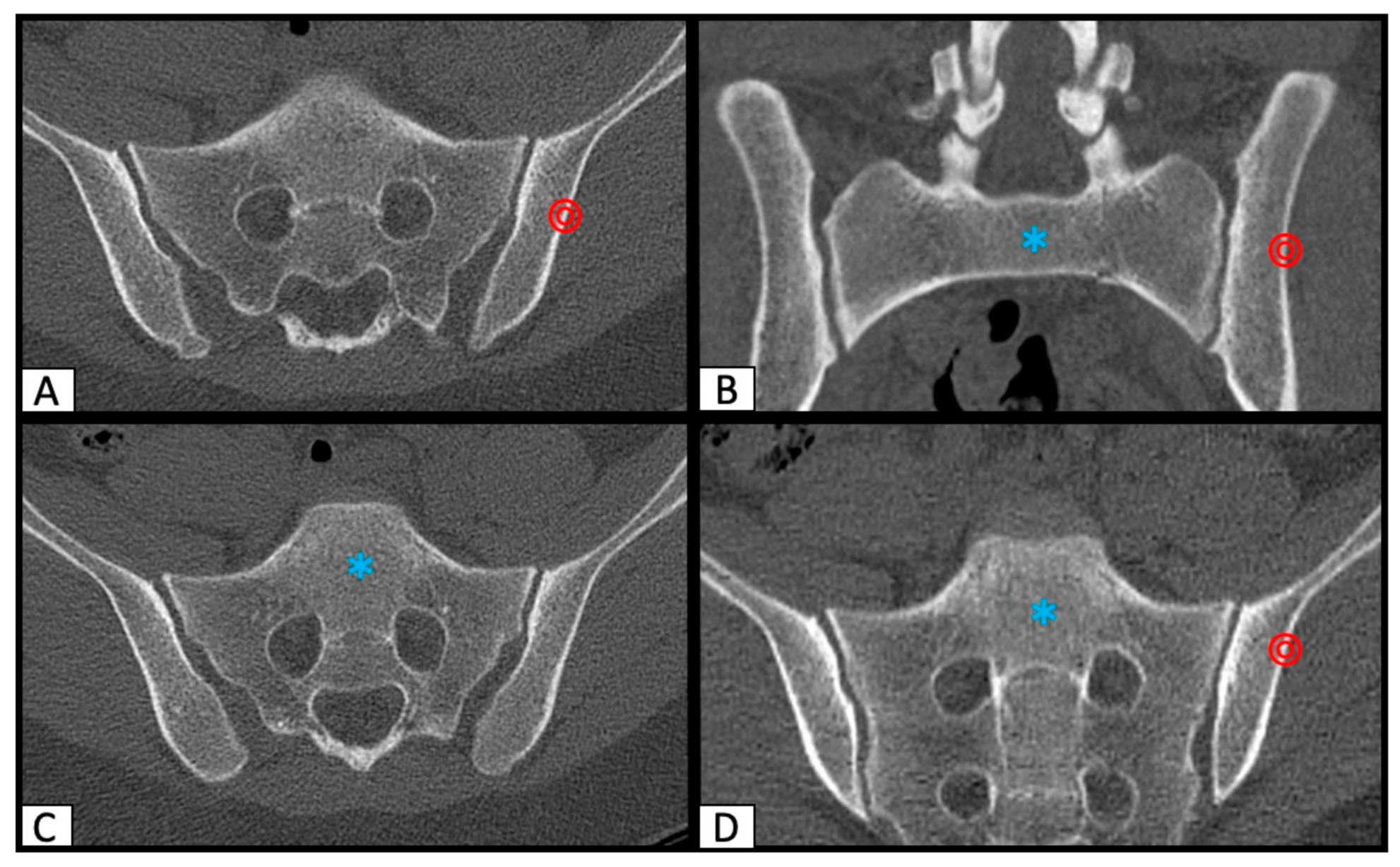
2.1. Determination of SI Screw Corridor
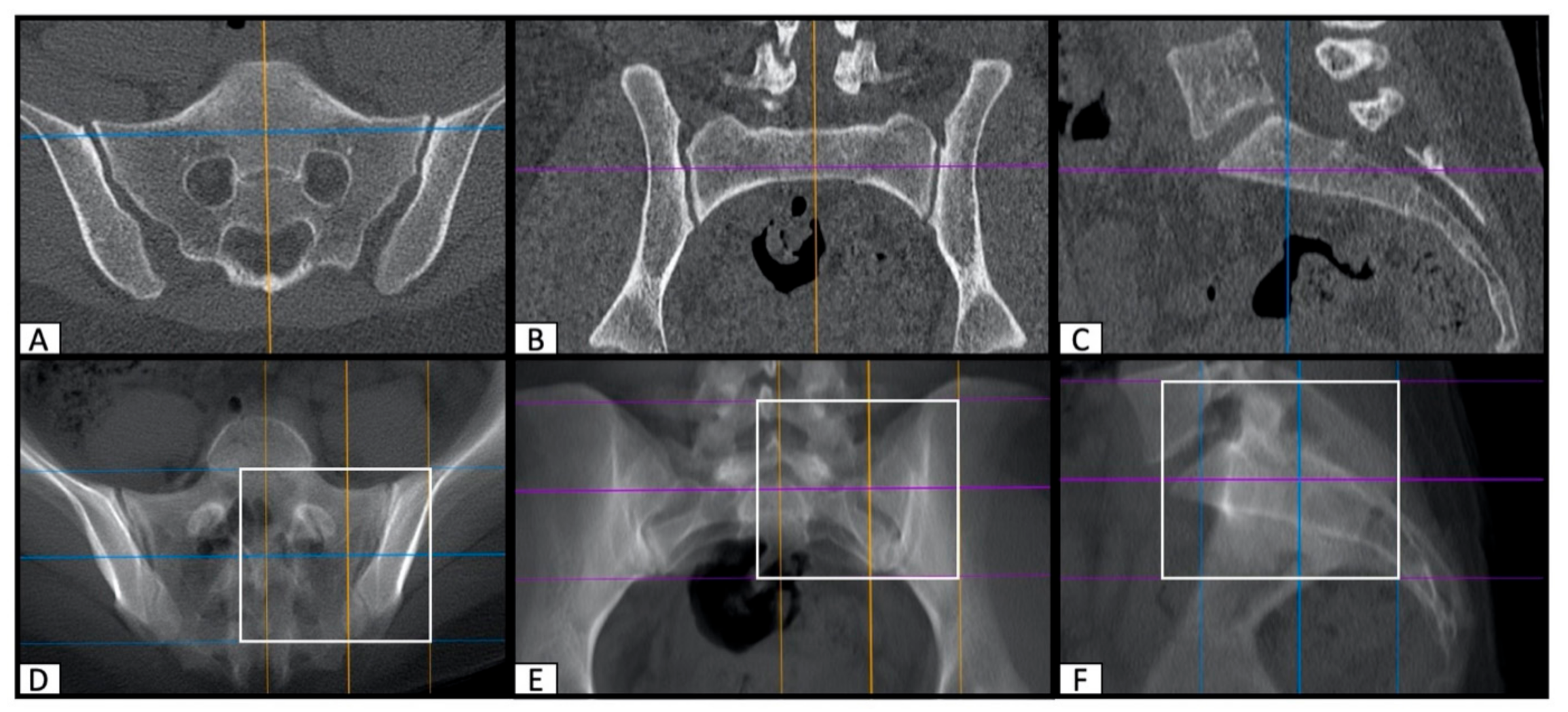
2.2. Image Processing with the Slice Fusion Method
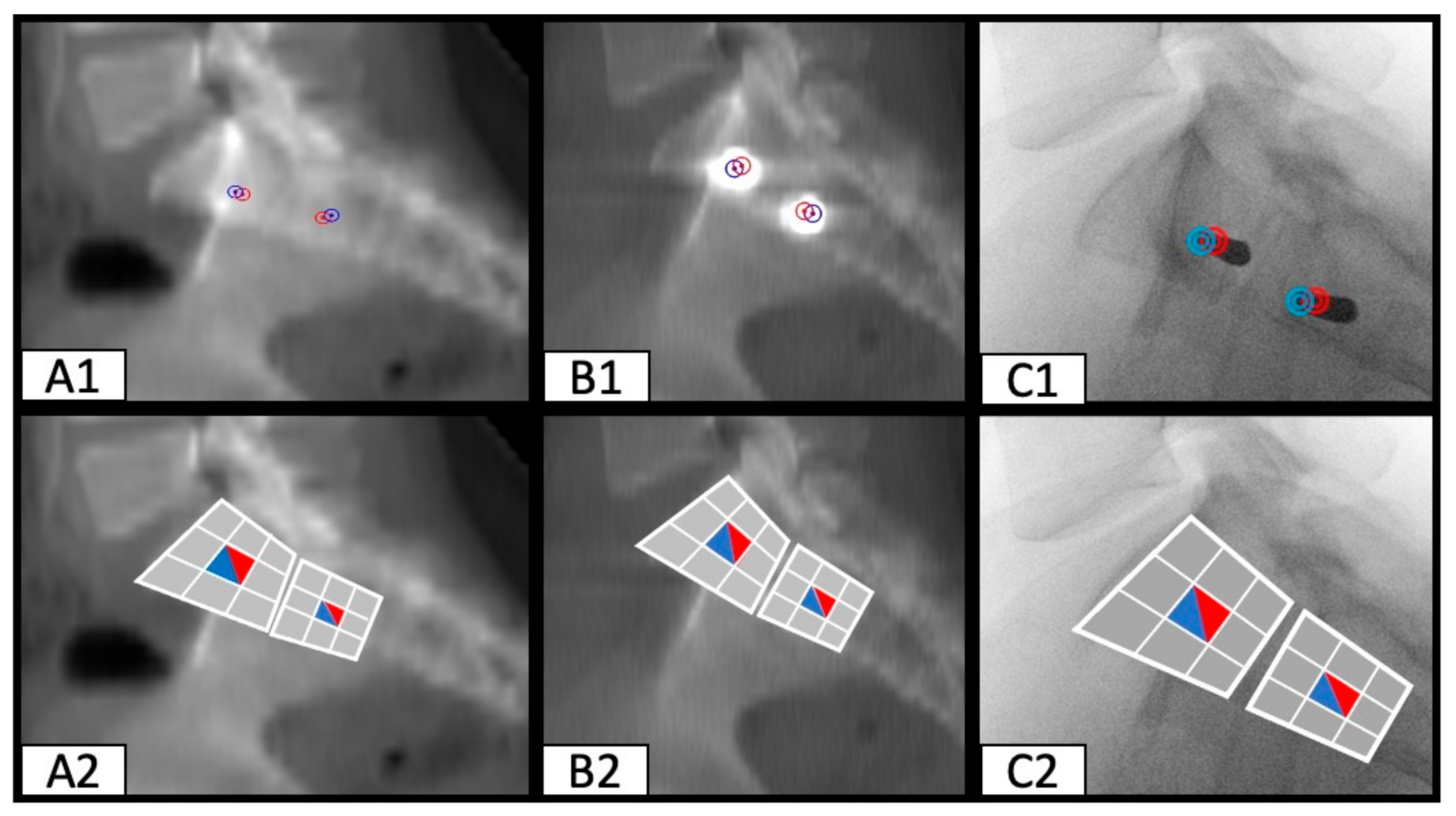
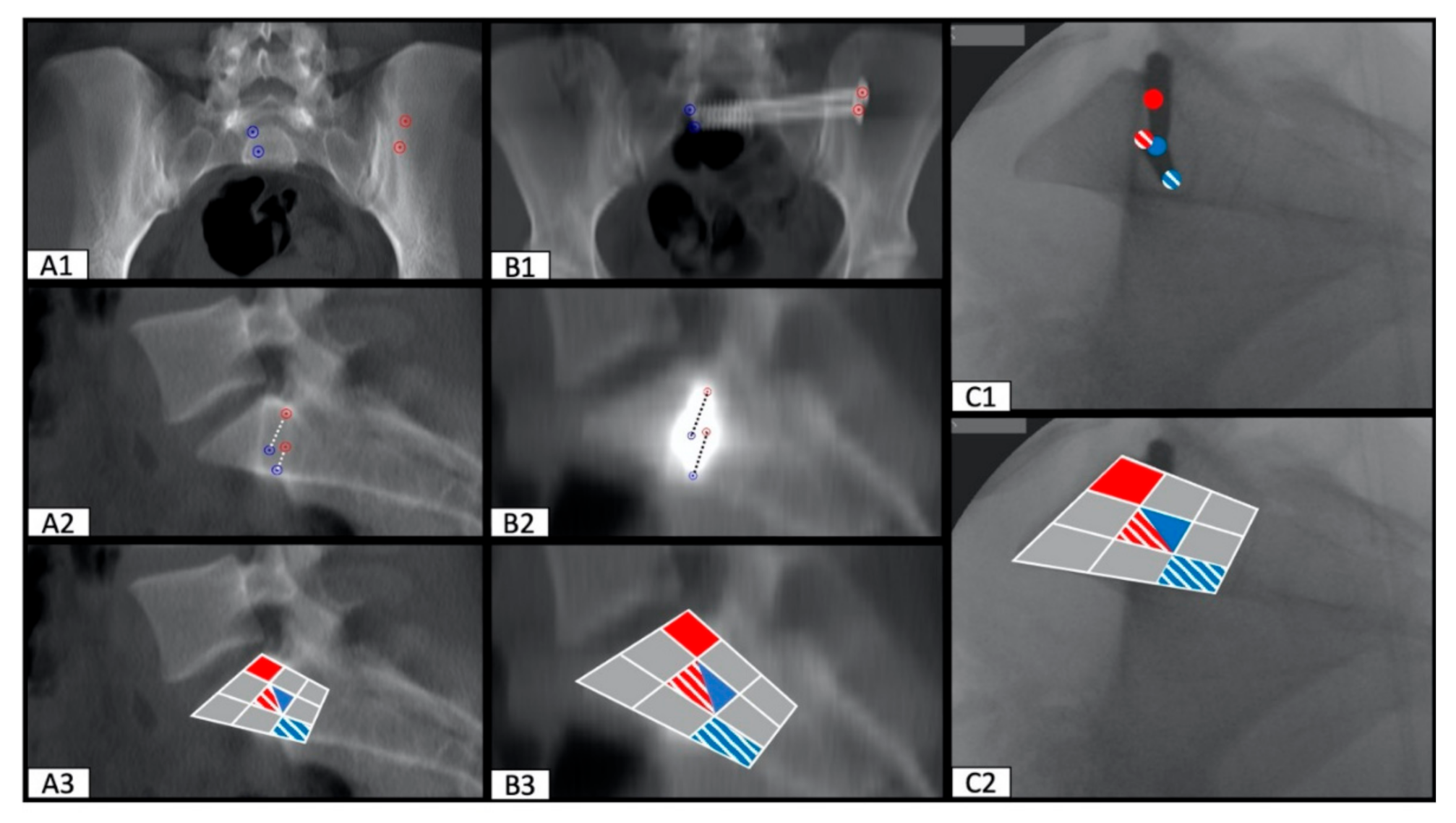
2.3. Determination of SI Screw Corridor Entry- and Endpoint and Direction
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tosounidis, G.; Holstein, J.H.; Culemann, U.; Holmenschlager, F.; Stuby, F.; Pohlemann, T. Changes in epidemiology and treatment of pelvic ring fractures in Germany: An analysis on data of German Pelvic Multicenter Study Groups I and III (DGU/AO). Acta Chir. Orthop. Traumatol. Cechoslov. 2010, 77, 450–456. [Google Scholar]
- Grechenig, S.; Gänsslen, A.; Gueorguiev, B.; Berner, A.; Müller, M.; Nerlich, M. PMMA-augmented SI screw: A biomechanical analysis of stiffness and pull-out force in a matched paired human cadaveric model. Injury 2015, 46, S125–S128. [Google Scholar] [CrossRef]
- Moshirfar, A.; Rand, F.F.; Sponseller, P.D.; Parazin, S.J.; Khanna, A.J.; Kebaish, K.M.; Stinson, J.T.; Iii, L.H.R. Pelvic fixation in spine surgery. Historical overview, indications, biomechanical relevance, and current techniques. J. Bone Jt. Surg. Am. 2005, 87, 89–106. [Google Scholar]
- Abou-Khalil, S.; Steinmetz, S.; Mustaki, L.; Leger, B.; Thein, E.; Borens, O. Results of open reduction internal fixation versus percutaneous iliosacral screw fixation for unstable pelvic ring injuries: Retrospective study of 36 patients. Eur. J. Orthop. Surg. Traumatol. 2020, 30, 877–884. [Google Scholar] [CrossRef]
- Routt, M.L., Jr.; Kregor, P.J.; Simonian, P.T.; Mayo, K.A. Early results of percutaneous iliosacral screws placed with the patient in the supine position. J. Orthop. Trauma 1995, 9, 207–214. [Google Scholar] [CrossRef]
- Giannoudis, P.V.; Tzioupis, C.C.; Pape, H.C.; Roberts, C.S. Percutaneous fixation of the pelvic ring: An update. J. Bone Jt. Surg. Br. 2007, 89, 145–154. [Google Scholar] [CrossRef]
- Schweitzer, D.; Zylberberg, A.; Cordova, M.; Gonzalez, J. Closed reduction and iliosacral percutaneous fixation of unstable pelvic ring fractures. Injury 2008, 39, 869–874. [Google Scholar] [CrossRef]
- Rommens, P.M.; Hofmann, A. Comprehensive classification of fragility fractures of the pelvic ring: Recommendations for surgical treatment. Injury 2013, 44, 1733–1744. [Google Scholar] [CrossRef]
- König, M.A.; Sundaram, R.O.; Saville, P.; Jehan, S.; Boszczyk, B.M. Anatomical considerations for percutaneous trans ilio-sacroiliac S1 and S2 screw placement. Eur. Spine J. 2016, 25, 1800–1805. [Google Scholar] [CrossRef]
- Wagner, D.; Kamer, L.; Sawaguchi, T.; Geoff Richards, R.; Noser, H.; Uesugi, M.; Ossendorf, C.; Rommens, P.M. Critical dimensions of trans-sacral corridors assessed by 3D CT models: Relevance for implant positioning in fractures of the sacrum. J. Orthop. Res. 2017, 35, 2577–2584. [Google Scholar] [CrossRef] [Green Version]
- Shuler, T.E.; Boone, D.C.; Gruen, G.S.; Peitzman, A.B. Percutaneous iliosacral screw fixation: Early treatment for unstable posterior pelvic ring disruptions. J. Trauma 1995, 38, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Keating, J.F.; Werier, J.; Blachut, P.; Broekhuyse, H.; Meek, R.N.; O’Brien, P.J. Early fixation of the vertically unstable pelvis: The role of iliosacral screw fixation of the posterior lesion. J. Orthop. Trauma 1999, 13, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.R.; Starr, A.J.; Reinert, C.M.; Jones, A.L.; Whitlock, S. Vertically unstable pelvic fractures fixed with percutaneous iliosacral screws: Does posterior injury pattern predict fixation failure? J. Orthop. Trauma 2006, 20, S30–S36. [Google Scholar] [CrossRef] [PubMed]
- Sagi, H.C.; Lindvall, E.M. Inadvertent intraforaminal iliosacral screw placement despite apparent appropriate positioning on intraoperative fluoroscopy. J. Orthop. Trauma 2005, 19, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Altman, D.T.; Jones, C.B.; Routt, M.L. Superior gluteal artery injury during iliosacral screw placement. J. Orthop. Trauma 1999, 13, 220–227. [Google Scholar] [CrossRef]
- Ko, P.S.; Kou, S.K. A rare complication of percutaneous iliosacral screw in a vertically unstable pelvic disruption in a child. Injury 2001, 32, 159–161. [Google Scholar] [CrossRef]
- Peeters, G.; Geert, P.; Govaers, K.; Kris, G.; Himpens, J.; Jacques, H. Successful laparoscopic exploration and screw extraction for intractable pain after anterior iliosacral arthrodesis. J. Orthop. Trauma 2010, 24, e83–e85. [Google Scholar]
- Weil, Y.A.; Nousiainen, M.T.; Helfet, D.L. Removal of an iliosacral screw entrapping the L5 nerve root after failed posterior pelvic ring fixation: A case report. J. Orthop. Trauma 2007, 21, 414–417. [Google Scholar] [CrossRef]
- Tonetti, J.; van Overschelde, J.; Sadok, B.; Vouaillat, H.; Eid, A. Percutaneous ilio-sacral screw insertion. fluoroscopic techniques. Orthop. Traumatol. Surg. Res. 2013, 99, 965–972. [Google Scholar] [CrossRef] [Green Version]
- Conflitti, J.M.; Graves, M.L.; Chip Routt, M.L. Radiographic quantification and analysis of dysmorphic upper sacral osseous anatomy and associated iliosacral screw insertions. J. Orthop. Trauma 2010, 24, 630–636. [Google Scholar] [CrossRef]
- Gardner, M.J.; Morshed, S.; Nork, S.E.; Ricci, W.M.; Chip Routt, M.L. Quantification of the upper and second sacral segment safe zones in normal and dysmorphic sacra. J. Orthop. Trauma 2010, 24, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Karachalios, T.; Zibis, A.H.; Zintzaras, E.; Bargiotas, K.; Karantanas, A.H.; Malizos, K.N. An anatomical update on the morphologic variations of S1 and S2. Orthopedics 2010, 33, 733. [Google Scholar] [CrossRef] [PubMed]
- Matta, J.M.; Tornetta, P. Internal fixation of unstable pelvic ring injuries. Clin. Orthop. Relat. Res. 1996, 329, 129–140. [Google Scholar] [CrossRef]
- Routt, M.L.; Simonian, P.T.; Agnew, S.G.; Mann, F.A. Radiographic recognition of the sacral alar slope for optimal placement of iliosacral screws: A cadaveric and clinical study. J. Orthop. Trauma 1996, 10, 171–177. [Google Scholar] [PubMed]
- Routt, M.L.; Nork, S.E.; Mills, W.J. Percutaneous fixation of pelvic ring disruptions. Clin. Orthop. Relat. Res. 2000, 375, 15–29. [Google Scholar] [CrossRef]
- Pohlemann, T.; Bosch, U.; Gänsslen, A.; Tscherne, H. The Hannover experience in management of pelvic fractures. Clin. Orthop. Relat. Res. 1994, 305, 69–80. [Google Scholar] [CrossRef]
- Tile, M. Acute Pelvic Fractures: I. Causation and Classification. J. Am. Acad Orthop. Surg. 1996, 4, 143–151. [Google Scholar] [CrossRef]
- Kannus, P.; Palvanen, M.; Niemi, S.; Parkkari, J.; Järvinen, M. Epidemiology of osteoporotic pelvic fractures in elderly people in Finland: Sharp increase in 1970–1997 and alarming projections for the new millennium. Osteoporos. Int. 2000, 11, 443–448. [Google Scholar] [CrossRef]
- Boufous, S.; Finch, C.; Lord, S.; Close, J. The increasing burden of pelvic fractures in older people, New South Wales, Australia. Injury 2005, 36, 1323–1329. [Google Scholar] [CrossRef]
- Tonetti, J.; Carrat, L.; Blendea, S.; Merloz, P.; Troccaz, J.; Lavallée, S. Clinical results of percutaneous pelvic surgery. Computer assisted surgery using ultrasound compared to standard fluoroscopy. Comput. Aided Surg. 2001, 6, 204–211. [Google Scholar] [CrossRef]
- Routt, M.L.; Simonian, P.T.; Mills, W.J. Iliosacral screw fixation: Early complications of the percutaneous technique. J. Orthop. Trauma 1997, 11, 584–589. [Google Scholar] [CrossRef]
- Kaiser, S.P.; Gardner, M.J.; Liu, J.; Routt, M.L.C.; Morshed, S. Anatomic Determinants of Sacral Dysmorphism and Implications for Safe Iliosacral Screw Placement. J. Bone Jt. Surg. Am. 2014, 96, 1222–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zwingmann, J.; Konrad, G.; Mehlhorn, A.T.; Südkamp, N.P.; Oberst, M. Percutaneous iliosacral screw insertion: Malpositioning and revision rate of screws with regards to application technique (navigated vs. Conventional). J. Trauma 2010, 69, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Ringel, F.; Stüer, C.; Reinke, A.; Preuss, A.; Behr, M.; Auer, F. Accuracy of robot-assisted placement of lumbar and sacral pedicle screws: A prospective randomized comparison to conventional freehand screw implantation. Spine 2012, 37, E496–E501. [Google Scholar] [CrossRef] [PubMed]
- Briem, D.; Windolf, J.; Rueger, J.M. Percutaneous, 2D-fluoroscopic navigated iliosacral screw placement in the supine position: Technique, possibilities, and limits. Der Unf. 2007, 110, 393–401. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kerschbaum, M.; Lang, S.; Baumann, F.; Alt, V.; Worlicek, M. Two-Dimensional Visualization of the Three-Dimensional Planned Sacroiliac Screw Corridor with the Slice Fusion Method. J. Clin. Med. 2021, 10, 184. https://doi.org/10.3390/jcm10020184
Kerschbaum M, Lang S, Baumann F, Alt V, Worlicek M. Two-Dimensional Visualization of the Three-Dimensional Planned Sacroiliac Screw Corridor with the Slice Fusion Method. Journal of Clinical Medicine. 2021; 10(2):184. https://doi.org/10.3390/jcm10020184
Chicago/Turabian StyleKerschbaum, Maximilian, Siegmund Lang, Florian Baumann, Volker Alt, and Michael Worlicek. 2021. "Two-Dimensional Visualization of the Three-Dimensional Planned Sacroiliac Screw Corridor with the Slice Fusion Method" Journal of Clinical Medicine 10, no. 2: 184. https://doi.org/10.3390/jcm10020184
APA StyleKerschbaum, M., Lang, S., Baumann, F., Alt, V., & Worlicek, M. (2021). Two-Dimensional Visualization of the Three-Dimensional Planned Sacroiliac Screw Corridor with the Slice Fusion Method. Journal of Clinical Medicine, 10(2), 184. https://doi.org/10.3390/jcm10020184







