Three-Dimensional Surface Texture Analysis of Fluoride’s Effect on Enamel Erosion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sample
2.2. Sample Preparation and Study Workflow
2.3. Enamel Erosion Evaluation
2.4. Statistical Analysis
3. Results
3.1. Surface Texture Analysis
3.2. Irreversible Erosion Evaluation
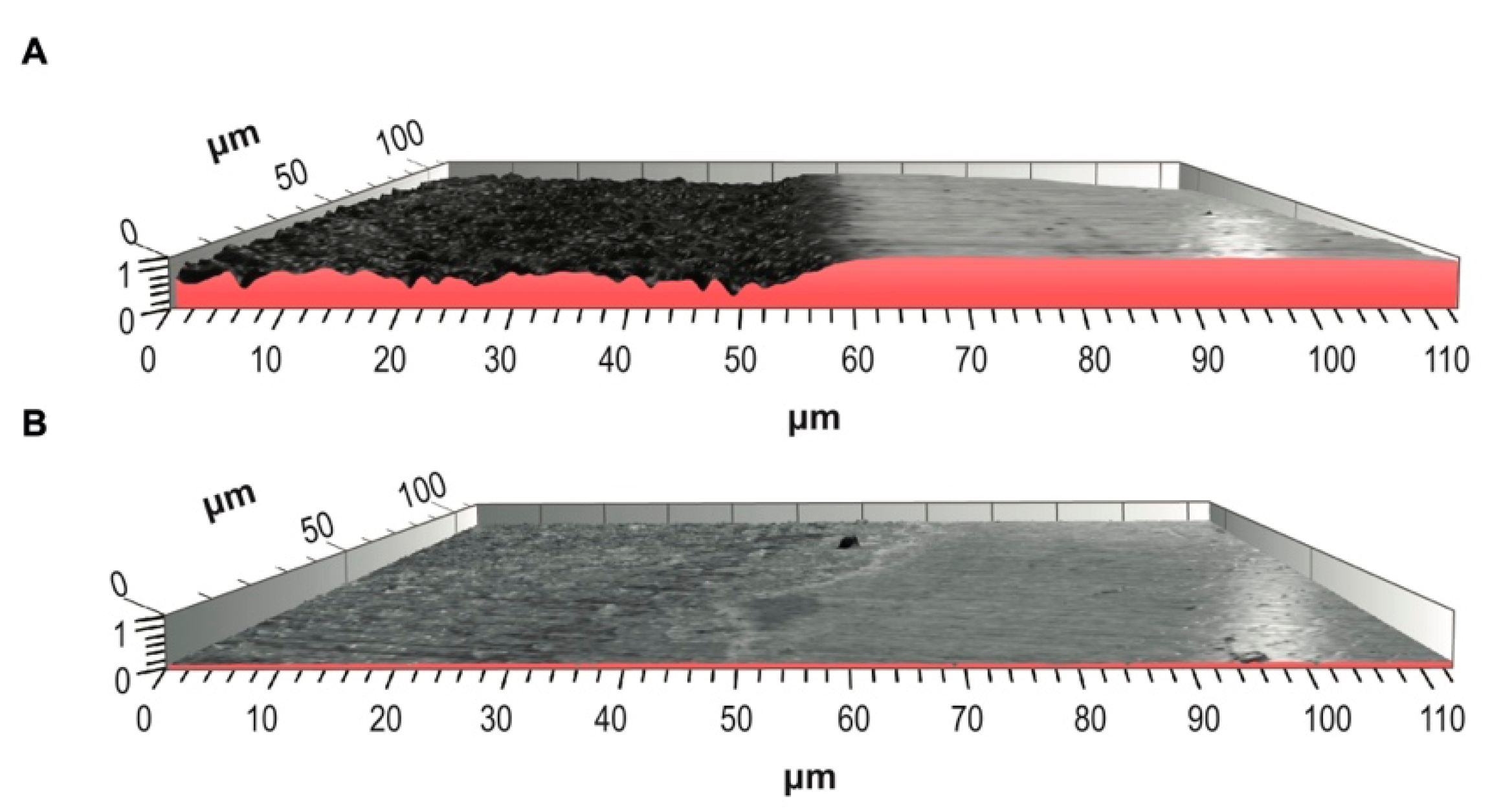
3.3. Descriptive Images
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peter Holbrook, W. Dental Erosion: From Diagnosis to Therapy; Lussi, A., Ed.; S. Karger AG: Basel, Switzerland, 2006; Volume 34. [Google Scholar]
- Lussi, A.; Schlueter, N.; Rakhmatullina, E.; Ganss, C. Dental erosion—An overview with emphasis on chemical and histopathological aspects. Caries Res. 2011, 45, 2–12. [Google Scholar] [CrossRef]
- Steiger-Ronay, V.; Kuster, I.M.; Wiedemeier, D.B.; Attin, T.; Wegehaupt, F.J. Erosive loss of tooth substance is dependent on enamel surface structure and presence of pellicle—An in vitro study. Arch. Oral Biol. 2020, 112, 104686. [Google Scholar] [CrossRef]
- Imfeld, T. Prevention of progression of dental erosion by professional and individual prophylactic measures. Eur. J. Oral Sci. 1996, 104, 215–220. [Google Scholar] [CrossRef]
- Austin, R.S.; Rodriguez, J.M.; Dunne, S.; Moazzez, R.; Bartlett, D.W. The effect of increasing sodium fluoride concentrations on erosion and attrition of enamel and dentine in vitro. J. Dent. 2010, 38, 782–787. [Google Scholar] [CrossRef]
- Alexandria, A.K.; Vieira, T.I.; Pithon, M.M.; da Silva Fidalgo, T.K.; Fonseca-Gonçalves, A.; Valença, A.M.G.; Cabral, L.M.; Maia, L.C. In vitro enamel erosion and abrasion-inhibiting effect of different fluoride varnishes. Arch. Oral Biol. 2017, 77, 39–43. [Google Scholar] [CrossRef]
- Ren, Y.F.; Zhao, Q.; Malmstrom, H.; Barnes, V.; Xu, T. Assessing fluoride treatment and resistance of dental enamel to soft drink erosion in vitro: Applications of focus variation 3D scanning microscopy and stylus profilometry. J. Dent. 2009, 37, 167–176. [Google Scholar] [CrossRef]
- Mylonas, P.; Austin, R.S.; Moazzez, R.; Joiner, A.; Bartlett, D.W. In vitro evaluation of the early erosive lesion in polished and natural human enamel. Dent. Mater. 2018, 34, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, R.F.; Caneppele, T.M.F.; Scaramucci, T.; El Dib, R.; Maia, L.C.; Ferreira, D.M.T.P.; Borges, A.B. Protective effect of fluorides on erosion and erosion/abrasion in enamel: A systematic review and meta-analysis of randomized in situ trials. Arch. Oral Biol. 2020, 120, 104945. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D. How to qualify and validate wear simulation devices and methods. Dent. Mater. 2006, 22, 712–734. [Google Scholar] [CrossRef]
- Azzopardi, A.; Bartlett, D.W.; Watson, T.F.; Smith, B.G. A literature review of the techniques to measure tooth wear and erosion. Eur. J. Prosthodont. Restor. Dent. 2000, 8, 93–97. [Google Scholar] [PubMed]
- Adamczak, S.; Zmarzły, P. Research of the influence of the 2D and 3D surface roughness parameters of bearing raceways on the vibration level. J. Phys. Conf. Ser. 2019, 1183, 012001. [Google Scholar] [CrossRef]
- Townsend, A.; Senin, N.; Blunt, L.; Leach, R.K.; Taylor, J.S. Surface texture metrology for metal additive manufacturing: A review. Precis. Eng. 2016, 46, 34–47. [Google Scholar] [CrossRef] [Green Version]
- Mathia, T.G.; Pawlus, P.; Wieczorowski, M. Recent trends in surface metrology. Wear 2011, 271, 494–508. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Turan, A.; Mann, C.; Gully, G.A.; Marsman, M.; Edwards, S.; Kaidonis, J.A.; Hall, C.; Lekkas, D.; Wetselaar, P. Surface-sensitive microwear texture analysis of attrition and erosion. J. Dent. Res. 2017, 96, 300–307. [Google Scholar] [CrossRef]
- Ungar, P.S.; Merceron, G.; Scott, R.S. Dental microwear texture analysis of Varswater bovids and early Pliocene paleoenvironments of Langebaanweg, Western Cape Province, South Africa. J. Mamm. Evol. 2007, 14, 163–181. [Google Scholar] [CrossRef]
- Pokhojaev, A.; Habashi, W.; May, H.; Schulz-Kornas, E.; Shvalb, N.; Sarig, R. Examination of the interproximal wear mechanism: Facet morphology and surface texture analysis. J. Dent. Res. 2018, 97, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Elbahary, S.; Haj-Yahya, S.; Khawalid, M.; Tsesis, I.; Rosen, E.; Habashi, W.; Pokhojaev, A.; Sarig, R. Effects of different irrigation protocols on dentin surfaces as revealed through quantitative 3D surface texture analysis. Sci. Rep. 2020, 10, 22073. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 25178-2: Geometrical Product Specifications (GPS)—Surface Texture: Areal Part—2: Terms, Definitions and Surface Texture Parameters; ISO: Geneva, Switzerland, 2007. [Google Scholar]
- Schlüter, N.; Hara, A.; Shellis, R.P.; Ganss, C. Methods for the measurement and characterization of erosion in enamel and dentine. Caries Res. 2011, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Moura, J.S.; Rodrigues, L.K.A.; Del Bel Cury, A.A.; Lima, E.M.C.X.; Garcia, R.M.C.R. Influence of storage solution on enamel demineralization submitted to pH cycling. J. Appl. Oral Sci. 2004, 12, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Mullan, F.; Austin, R.S.; Parkinson, C.R.; Hasan, A.; Bartlett, D.W. Measurement of surface roughness changes of unpolished and polished enamel following erosion. PLoS ONE 2017, 12, e0182406. [Google Scholar] [CrossRef] [Green Version]
- Eisenburger, M.; Addy, M.; Hughes, J.A.; Shellis, R.P. Effect of time on the remineralisation of enamel by synthetic saliva after citric acid erosion. Caries Res. 2001, 35, 211–215. [Google Scholar] [CrossRef]
- Sundaram, G.; Wilson, R.; Watson, T.F.; Bartlett, D. Clinical measurement of palatal tooth wear following coating by a resin sealing system. Oper. Dent. 2007, 32, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.M.; Bartlett, D.W. A comparison of two-dimensional and three-dimensional measurements of wear in a laboratory investigation. Dent. Mater. 2010, 26, e221–e225. [Google Scholar] [CrossRef]
- Motulsky, H.J.; Brown, R.E. Detecting outliers when fitting data with nonlinear regression—A new method based on robust nonlinear regression and the false discovery rate. BMC Bioinform. 2006, 7, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Paleontological statistics software: Package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Meurman, J.H.; ten Cate, J.M. Pathogenesis and modifying factors of dental erosion. Eur. J. Oral Sci. 1996, 104, 199–206. [Google Scholar] [CrossRef]
- Ganss, C.; Schlueter, N.; Klimek, J. Retention of KOH-soluble fluoride on enamel and dentine under erosive conditions—A comparison of in vitro and in situ results. Arch. Oral Biol. 2007, 52, 9–14. [Google Scholar] [CrossRef]
- Ten Cate, J.M.; Featherstone, J.D.B. Mechanistic aspects of the interactions between fluoride and dental enamel. Crit. Rev. Oral Biol. Med. 1991, 2, 283–296. [Google Scholar] [CrossRef]
- Carvalho, T.S.; Colon, P.; Ganss, C.; Huysmans, M.C.; Lussi, A.; Schlueter, N.; Schmalz, G.; Shellis, R.P.; Tveit, A.B.; Wiegand, A. Consensus report of the European Federation of Conservative Dentistry: Erosive tooth wear—diagnosis and management. Clin. Oral Investig. 2015, 19, 1557–1561. [Google Scholar] [CrossRef] [Green Version]
- Lussi, A.; Buzalaf, M.A.R.; Duangthip, D.; Anttonen, V.; Ganss, C.; João-Souza, S.H.; Baumann, T.; Carvalho, T.S. The use of fluoride for the prevention of dental erosion and erosive tooth wear in children and adolescents. Eur. Arch. Paediatr. Dent. 2019, 20, 517–527. [Google Scholar] [CrossRef]
- Wiegand, A.; Schlueter, N. The role of oral hygiene: Does toothbrushing harm? Monogr. Oral Sci. 2014, 25, 215–219. [Google Scholar] [CrossRef]
- Tahmassebi, J.F.; Duggal, M.S.; Malik-Kotru, G.; Curzon, M.E.J. Soft drinks and dental health: A review of the current literature. J. Dent. 2006, 34, 2–11. [Google Scholar] [CrossRef]
- Meurman, J.H.; Härkönen, M.; Näveri, H.; Koskinen, J.; Torkko, H.; Rytömaa, I.; Järvinen, V.T.R. Experimental sports drinks with minimal dental erosion effect. Eur. J. Oral Sci. 1990, 98, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Scaramucci, T.; Borges, A.B.; Lippert, F.; Frank, N.E.; Hara, A.T. Sodium fluoride effect on erosion-abrasion under hyposalivatory simulating conditions. Arch. Oral Biol. 2013, 58, 1457–1463. [Google Scholar] [CrossRef] [PubMed]
- Field, J.; Waterhouse, P.; German, M. Quantifying and qualifying surface changes on dental hard tissues in vitro. J. Dent. 2010, 38, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Fita, K.; Kaczmarek, U. The impact of selected fluoridated toothpastes on dental erosion in profilometric measurement. Adv. Clin. Exp. Med. 2016, 25, 327–333. [Google Scholar] [CrossRef] [Green Version]
- Fujii, M.; Kitasako, Y.; Sadr, A.; Tagami, J. Roughness and pH changes of enamel surface induced by soft drinks in vitroapplications of stylus profilometry, focus variation 3D scanning microscopy and micro pH sensor. Dent. Mater. J. 2011, 30, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Moazzez, R.V.; Austin, R.S.; Rojas-Serrano, M.; Carpenter, G.; Cotroneo, E.; Proctor, G.; Zaidel, L.; Bartlett, D.W. Comparison of the possible protective effect of the salivary pellicle of individuals with and without erosion. Caries Res. 2014, 48, 57–62. [Google Scholar] [CrossRef]
- Leach, R. (Ed.) Characterisation of Areal Surface Texture; Springer: Berlin/Heidelberg, Germany, 2013; Volume 9783642364, ISBN 9783642364587. [Google Scholar]
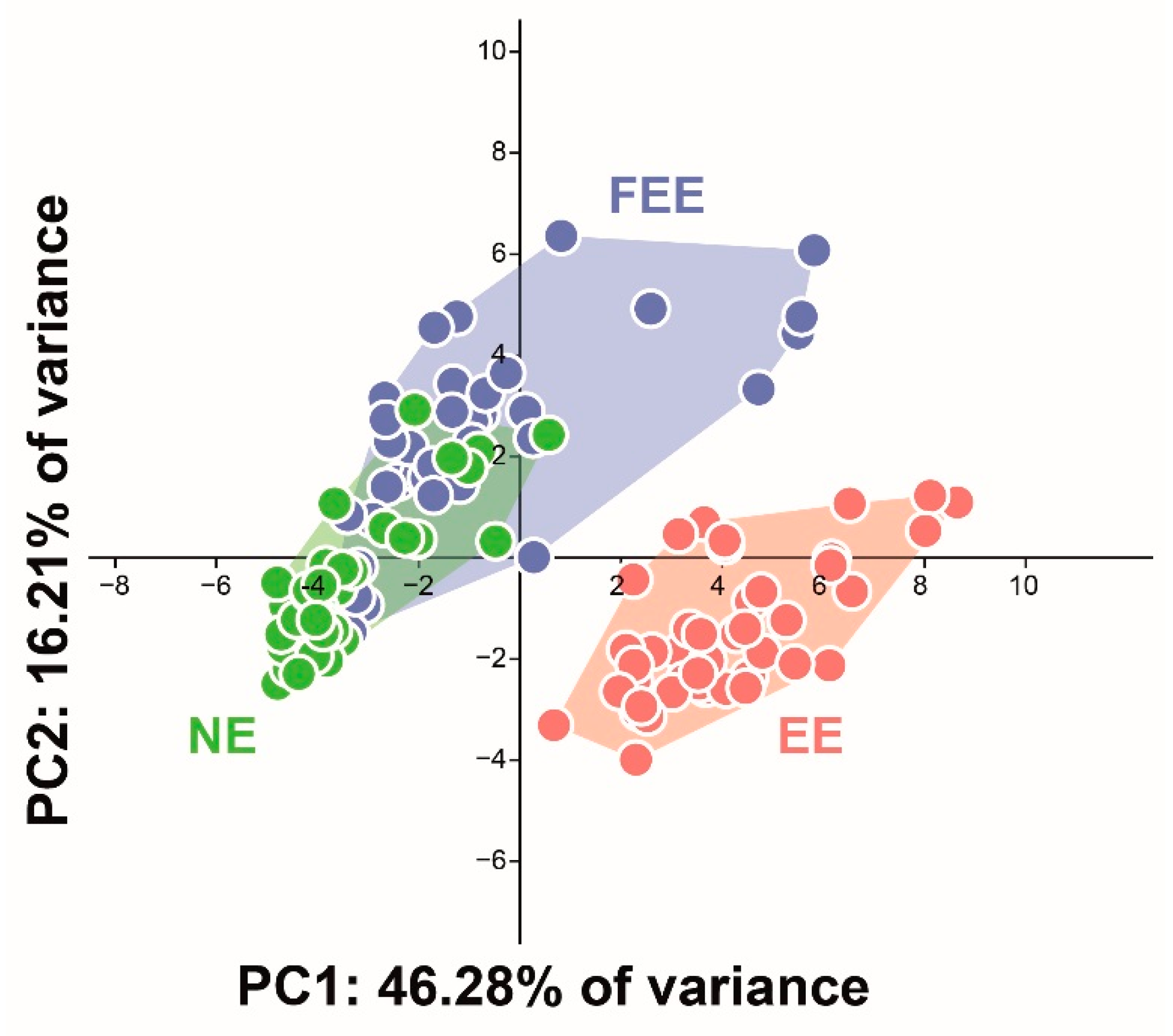

| Surface Texture Parameter | Polished Enamel (NE) (1) | Erosion Enamel (EE) (2) | Fluoridated/Erosion Enamel (FEE) (3) | Sig | Post Hoc Analysis: | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Units | Mean | SD | Mean | SD | Mean | SD | |||||
| Height | Root-mean-square height | Sq | µm | 0.037 | 0.014 | 0.168 | 0.036 | 0.066 | 0.022 | <0.001 | 1 ≠ 2 ≠ 3 |
| Skewness | Ssk | −0.841 | 1.607 | −0.381 | 0.3656 | 0.040 | 2.198 | <0.001 | 1 ≠ 3,2 | ||
| Kurtosis | Sku | 15.67 | 11.5 | 4.39 | 1.15 | 22.38 | 19.13 | 0.003 | 1,3 ≠ 2 | ||
| Maximum peak height | Sp | µm | 0.428 | 0.278 | 0.919 | 0.460 | 0.918 | 0.585 | <0.001 | 2,3 ≠ 1 | |
| Maximum pit height | Sv | µm | 0.371 | 0.194 | 1.066 | 0.398 | 0.751 | 0.445 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Maximum height | Sz | µm | 0.889 | 0.514 | 2.041 | 0.753 | 1.813 | 0.992 | <0.001 | 2,3 ≠ 1 | |
| Arithmetic mean height | Sa | µm | 0.024 | 0.007 | 0.126 | 0.025 | 0.041 | 0.010 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Functional (plane) | Areal material ratio | Smr | % | 99.99 | 0.026 | 60.43 | 43.95 | 51.3 | 49.08 | <0.001 | 2,3 ≠ 1 |
| Inverse areal material ratio | Smc | µm | 0.031 | 0.008 | 0.195 | 0.039 | 0.058 | 0.013 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Extreme peak height | Sxp | µm | 0.082 | 0.029 | 0.362 | 0.101 | 0.130 | 0.036 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Spatial | Autocorrelation length | Sal | µm | 3.684 | 1.196 | 3.825 | 1.194 | 5.827 | 1.741 | <0.001 | 1,2 ≠ 3 |
| Texture-aspect ratio | Str | 0.136 | 0.098 | 0.325 | 0.180 | 0.514 | 0.202 | <0.001 | 1,2 ≠ 3 | ||
| Texture direction | Std | ° | 99.22 | 70.48 | 164.5 | 7.951 | 71.32 | 72.34 | <0.001 | 1,3 ≠ 2 | |
| Hybrid | Root-mean-square gradient | Sdq | 0.038 | 0.010 | 0.152 | 0.015 | 0.056 | 0.015 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Developed interfacial area ratio | Sdr | % | 0.067 | 0.029 | 1.15 | 0.219 | 0.148 | 0.062 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Functional (volume) | Material volume | Vm | µm3/µm2 | 0.002 | 0.001 | 0.007 | 0.001 | 0.004 | 0.001 | <0.001 | 1 ≠ 2 ≠ 3 |
| Void volume | Vv | µm3/µm2 | 0.034 | 0.009 | 0.202 | 0.038 | 0.063 | 0.014 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Peak material volume | Vmp | µm3/µm2 | 0.002 | 0.001 | 0.007 | 0.001 | 0.004 | 0.001 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Core material volume | Vmc | µm3/µm2 | 0.022 | 0.005 | 0.136 | 0.028 | 0.041 | 0.008 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Core void volume | Vvc | µm3/µm2 | 0.028 | 0.007 | 0.181 | 0.036 | 0.053 | 0.010 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Pit void volume | Vvv | µm3/µm2 | 0.006 | 0.002 | 0.022 | 0.006 | 0.009 | 0.003 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Feature | Density of peaks | Spd | 1/µm2 | 0.003 | 0.003 | 0.016 | 0.009 | 0.003 | 0.003 | <0.001 | 1,3 ≠ 2 |
| Arithmetic mean peak curvature | Spc | 1/µm | 0.183 | 0.066 | 0.372 | 0.035 | 0.279 | 0.130 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Ten point height | S10z | µm | 0.353 | 0.129 | 1.258 | 0.335 | 0.743 | 0.320 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Five point peak height | S5p | µm | 0.174 | 0.088 | 0.490 | 0.138 | 0.418 | 0.238 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Five point pit height | S5v | µm | 0.201 | 0.096 | 0.742 | 0.240 | 0.298 | 0.107 | <0.001 | 1 ≠ 2 ≠ 3 | |
| Mean dale area | Sda | µm2 | 164.1 | 117 | 56.95 | 26.6 | 336 | 317.9 | <0.001 | 1,3 ≠ 2 | |
| Mean hill area | Sha | µm2 | 157.4 | 112.5 | 66.65 | 39.81 | 248.2 | 198.3 | 0.02 | 1 ≠ 2 ≠ 3 | |
| Mean dale volume | Sdv | µm3 | 0.573 | 0.527 | 0.814 | 0.490 | 3.401 | 3.991 | 0.01 | 1 ≠ 2 ≠ 3 | |
| Mean hill volume | Shv | µm3 | 0.693 | 0.606 | 1.046 | 0.710 | 2.118 | 1.990 | 0.02 | 1 ≠ 2 ≠ 3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Habashi, W.; Bader-Farraj, A.; Shpack, N.; Beitlitum, I.; May, H.; Sarig, R. Three-Dimensional Surface Texture Analysis of Fluoride’s Effect on Enamel Erosion. J. Clin. Med. 2021, 10, 4528. https://doi.org/10.3390/jcm10194528
Habashi W, Bader-Farraj A, Shpack N, Beitlitum I, May H, Sarig R. Three-Dimensional Surface Texture Analysis of Fluoride’s Effect on Enamel Erosion. Journal of Clinical Medicine. 2021; 10(19):4528. https://doi.org/10.3390/jcm10194528
Chicago/Turabian StyleHabashi, Waseem, Amal Bader-Farraj, Nir Shpack, Ilan Beitlitum, Hila May, and Rachel Sarig. 2021. "Three-Dimensional Surface Texture Analysis of Fluoride’s Effect on Enamel Erosion" Journal of Clinical Medicine 10, no. 19: 4528. https://doi.org/10.3390/jcm10194528
APA StyleHabashi, W., Bader-Farraj, A., Shpack, N., Beitlitum, I., May, H., & Sarig, R. (2021). Three-Dimensional Surface Texture Analysis of Fluoride’s Effect on Enamel Erosion. Journal of Clinical Medicine, 10(19), 4528. https://doi.org/10.3390/jcm10194528






