Preliminary Study on the Diagnostic Performance of a Deep Learning System for Submandibular Gland Inflammation Using Ultrasonography Images
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. USG Protocol
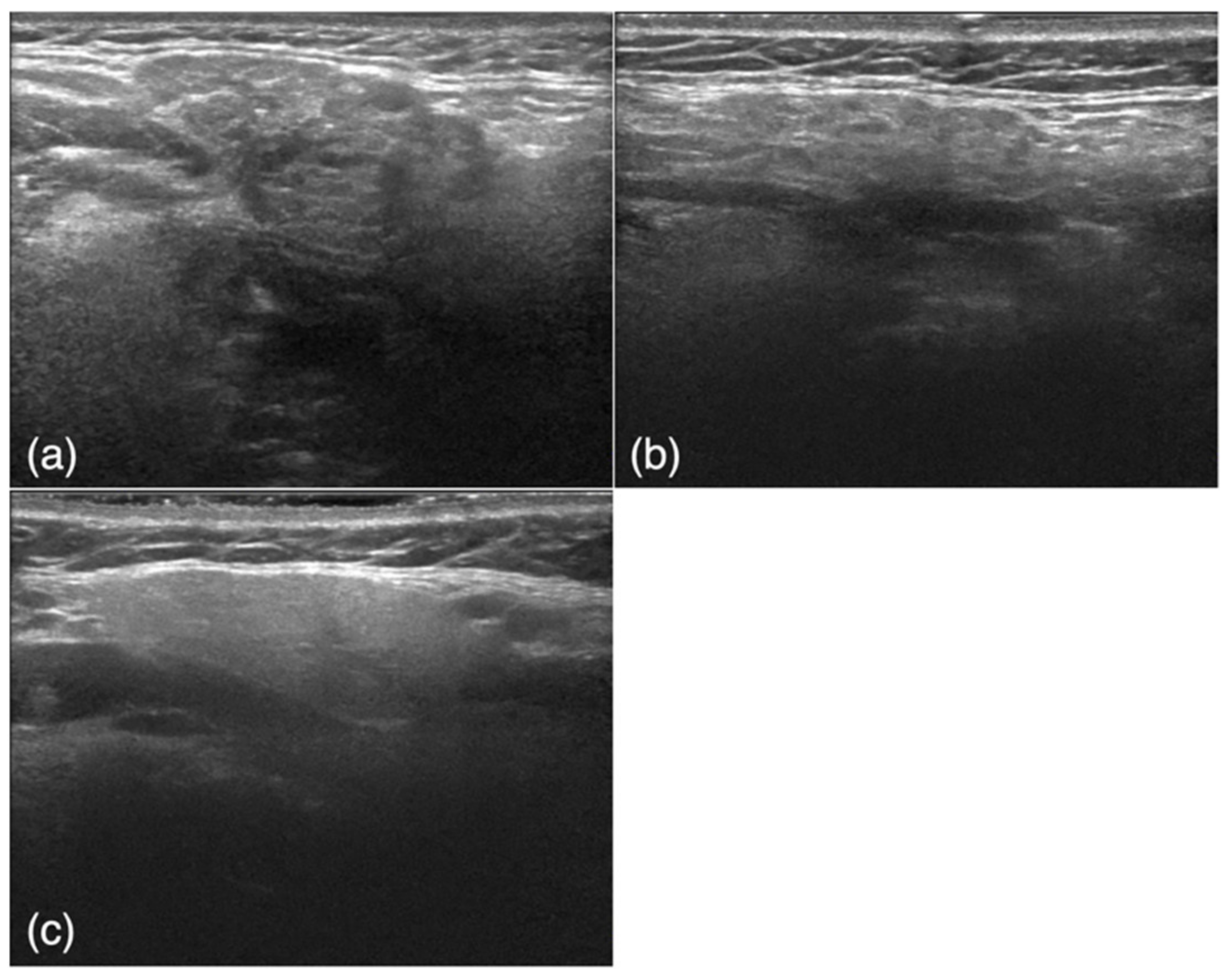
2.3. Imaging Data
2.4. Diagnostic Performance of the Deep Learning System
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krishnamurthy, S.; Vasudeva, S.B.; Vijayasarathy, S. Salivary gland disorders: A comprehensive review. World J. Stomatol. 2015, 4, 56–71. [Google Scholar] [CrossRef]
- Rzymska-Grala, I.; Stopa, Z.; Grala, B.; Gołębiowski, M.; Wanyura, H.; Zuchowska, A.; Sawicka, M.; Zmorzyński, M. Sali-vary gland calculi—Contemporary methods of imaging. Pol. J. Radiol. 2010, 75, 25–37. [Google Scholar]
- Gandage, S.G.; Kachewar, S.G. An Imaging Panorama of Salivary Gland Lesions as seen on High Resolution Ultrasound. J. Clin. Diagn. Res. 2014, 8, RC01–RC13. [Google Scholar] [CrossRef]
- Elbeblawy, Y.M.; Eshaq Amer Mohamed, M. Strain and shear wave ultrasound elastography in evaluation of chronic inflammatory disorders of major salivary glands. Dentomaxillofac. Radiol. 2020, 49, 20190225. [Google Scholar] [CrossRef] [PubMed]
- Szyfter, W.; Wierzbicka, M.; Kałużny, J.; Ruchała, M.; Stajgis, M.; Kopeć, T. Sonoelastography—A Useful Adjunct for Parotid Gland Ultrasound Assessment in Patients Suffering from Chronic Inflammation. Med Sci. Monit. 2014, 20, 2311–2317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badea, A.F.; Lupsor Platon, M.; Crisan, M.; Cattani, C.; Badea, I.; Pierro, G.; Sannino, G.; Baciut, G. Fractal analysis of elasto-graphic images for automatic detection of diffuse diseases of salivary glands: Preliminary results. Comput. Math Methods Med. 2013, 2013, 347238. [Google Scholar] [CrossRef] [Green Version]
- Zajkowski, P.; Ochal-Choińska, A. Standards for the assessment of salivary glands—An update. J. Ultrason. 2016, 16, 175–190. [Google Scholar] [CrossRef]
- Bialek, E.J.; Jakubowski, W.; Zajkowski, P.; Szopinski, K.T.; Osmolski, A. US of the major salivary glands: Anatomy and spa-tial relationships, pathologic conditions, and pitfalls. Radiographics 2006, 26, 745–763. [Google Scholar] [CrossRef] [Green Version]
- Hocevar, A.; Ambrozic, A.; Rozman, B.; Kveder, T.; Tomsic, M. Ultrasonographic changes of major salivary glands in pri-mary Sjögren’s syndrome. Diagnostic value of a novel scoring system. Rheumatology 2005, 44, 768–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariji, Y.; Fukuda, M.; Kise, Y.; Nozawa, M.; Yanashita, Y.; Fujita, H.; Katsumata, A.; Ariji, E. Contrast-enhanced computed tomography image assessment of cervical lymph node metastasis in patients with oral cancer by using a deep learning system of artificial intelligence. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 458–463. [Google Scholar] [CrossRef]
- Ariji, Y.; Sugita, Y.; Nagao, T.; Nakayama, A.; Fukuda, M.; Kise, Y.; Nozawa, M.; Nishiyama, M.; Katumata, A.; Ariji, E. CT evaluation of extranodal extension of cervical lymph node metastases in patients with oral squamous cell carcinoma using deep learning classification. Oral Radiol. 2019, 36, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Kise, Y.; Ikeda, H.; Fujii, T.; Fukuda, M.; Ariji, Y.; Fujita, H.; Katsumata, A.; Ariji, E. Preliminary study on the application of deep learning system to diagnosis of Sjögren’s syndrome on CT images. Dentomaxillofac. Radiol. 2019, 48, 20190019. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.J.; Jang, J.K.; Lee, S.S.; Sung, Y.S.; Shim, W.H.; Kim, H.S.; Yun, J.; Choi, J.-Y.; Lee, Y.; Kang, B.-K.; et al. Development and Validation of a Deep Learning System for Staging Liver Fibrosis by Using Contrast Agent–enhanced CT Images in the Liver. Radiology 2018, 289, 688–697. [Google Scholar] [CrossRef] [PubMed]
- Walsh, S.L.F.; Calandriello, L.; Silva, M.; Sverzellati, N. Deep learning for classifying fibrotic lung disease on high-resolution computed tomography: A case-cohort study. Lancet Respir. Med. 2018, 6, 837–845. [Google Scholar] [CrossRef]
- Song, Q.; Zhao, L.; Luo, X.; Dou, X. Using Deep Learning for Classification of Lung Nodules on Computed Tomography Images. J. Heal. Eng. 2017, 2017, 8314740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.W.; Hui, R.; Tian, Z. Classification of CT brain images based on deep learning networks. Comput. Methods Programs Biomed. 2016, 138, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kise, Y.; Shimizu, M.; Ikeda, H.; Fujii, T.; Kuwada, C.; Nishiyama, M.; Funakoshi, T.; Ariji, Y.; Fujita, H.; Katsumata, A.; et al. Usefulness of a deep learning system for diagnosing Sjögren’s syndrome using ultrasonography images. Dentomaxillofac. Radiol. 2020, 49, 20190348. [Google Scholar] [CrossRef] [PubMed]
- Becker, A.S.; Mueller, M.; Stoffel, E.; Marcon, M.; Ghafoor, S.; Boss, A. Classification of breast cancer from ultrasound imaging using a generic deep learning analysis software: A pilot study. Br. J. Radiol. 2017, 91, 20170576. [Google Scholar] [CrossRef]
- Choi, J.S.; Han, B.-K.; Ko, E.Y.; Bae, J.M.; Ko, E.Y.; Song, S.H.; Kwon, M.-R.; Shin, J.H.; Hahn, S.Y. Effect of a Deep Learning Framework-Based Computer-Aided Diagnosis System on the Diagnostic Performance of Radiologists in Differentiating between Malignant and Benign Masses on Breast Ultrasonography. Korean J. Radiol. 2019, 20, 749–758. [Google Scholar] [CrossRef]
- Fujioka, T.; Kubota, K.; Mori, M.; Kikuchi, Y.; Katsuta, L.; Kasahara, M.; Oda, G.; Ishiba, T.; Nakagawa, T.; Tateishi, U. Distinction between benign and malignant breast masses at breast ultrasound using deep learning method with convolutional neural network. Jpn. J. Radiol. 2019, 37, 466–472. [Google Scholar] [CrossRef]
- Stoffel, E.; Becker, A.S.; Wurnig, M.C.; Marcon, M.; Ghafoor, S.; Berger, N.; Boss, A. Distinction between phyllodes tu-mor and fibroadenoma in breast ultrasound using deep learning image analysis. Eur. J. Radiol. Open 2018, 5, 165–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, X.; Yao, Z.; Huang, Y.; Yu, Y.; Wang, Y.; Liu, Y.; Mao, R.; Li, F.; Xiao, Y.; Wang, Y.; et al. Deep learning radiomics can predict axillary lymph node status in early-stage breast cancer. Nat. Commun. 2020, 11, 1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.T.; Kang, J.K.; Pham, T.D.; Batchuluun, G.; Park, K.R. Ultrasound Image-Based Diagnosis of Malignant Thyroid Nodule Using Artificial Intelligence. Sensors 2020, 20, 1822. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Lin, X.; Zhao, Y.; Li, L.; Yan, K.; Liang, D.; Sun, D.; Li, Z.-C. Deep Learning vs. Radiomics for Predicting Axillary Lymph Node Metastasis of Breast Cancer Using Ultrasound Images: Don’t Forget the Peritumoral Region. Front. Oncol. 2020, 10, 53. [Google Scholar] [CrossRef] [Green Version]
- Hiraiwa, T.; Ariji, Y.; Fukuda, M.; Kise, Y.; Nakata, K.; Katsumata, A.; Fujita, H.; Ariji, E. A deep-learning artificial intelli-gence system for assessment of root morphology of the mandibular first molar on panoramic radiography. Dentomaxillofac. Radiol. 2019, 48, 20180218. [Google Scholar] [CrossRef]
- Murata, M.; Ariji, Y.; Ohashi, Y.; Kawai, T.; Fukuda, M.; Funakoshi, T.; Kise, Y.; Nozawa, M.; Katsumata, A.; Fujita, H.; et al. Deep-learning classification using convolutional neural network for evaluation of maxillary sinusitis on panoramic radiography. Oral Radiol. 2019, 35, 301–307. [Google Scholar] [CrossRef]
- Ariji, Y.; Yanashita, Y.; Kutsuna, S.; Muramatsu, C.; Fukuda, M.; Kise, Y.; Nozawa, M.; Kuwada, C.; Fujita, H.; Katsumata, A.; et al. Automatic detection and classification of radiolucent lesions in the mandible on panoramic radiographs using a deep learning object detection technique. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 128, 424–430. [Google Scholar] [CrossRef]
- Fujibayashi, T.; Sugai, S.; Miyasaka, N.; Hayashi, Y.; Tsubota, K. Revised Japanese criteria for Sjögren’s syndrome (1999): Availability and validity. Mod. Rheumatol. 2004, 14, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Vitali, C.; Bombardieri, S.; Jonsson, R.; Moutsopoulos, H.M.; Alexander, E.L.; Carsons, S.E.; Daniels, T.E.; Fox, P.C.; Fox, R.I.; Kassan, S.S.; et al. Classification criteria for Sjogren’s syndrome: A revised version of the European criteria proposed by the American-European Consensus Group. Ann. Rheum. Dis. 2002, 61, 554–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Obstructive Sialoadenitis | SjS | Control | PPV (%) | ||
|---|---|---|---|---|---|
| DL | Obstructive sialoadenitis | 55 | 12 | 20 | 63.2 |
| SjS | 29 | 83 | 7 | 69.7 | |
| Control | 16 | 5 | 73 | 77.7 | |
| Sensitivity (%) | 55.0 | 83.0 | 73.0 | 70.3 (accuracy) | |
| Radiologists | Obstructive sialoadenitis | 64 | 22 | 14 | 64.0 |
| SjS | 26 | 72 | 1 | 72.7 | |
| Control | 10 | 6 | 86 | 84.3 | |
| Sensitivity (%) | 64.0 | 72.0 | 86.0 | 74.0 (accuracy) |
| Intraobserver Agreement | ||
|---|---|---|
| Radiologist A | 0.64 | |
| Radiologist B | 0.69 | |
| Mean | 0.66 | Good |
| Interobserver/model agreement | ||
| Radiologist A vs. B (first) | 0.60 | |
| Radiologist A vs. B (second) | 0.53 | |
| Mean | 0.57 | Moderate |
| DL (first) vs. DL (second) | 0.72 | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kise, Y.; Kuwada, C.; Ariji, Y.; Naitoh, M.; Ariji, E. Preliminary Study on the Diagnostic Performance of a Deep Learning System for Submandibular Gland Inflammation Using Ultrasonography Images. J. Clin. Med. 2021, 10, 4508. https://doi.org/10.3390/jcm10194508
Kise Y, Kuwada C, Ariji Y, Naitoh M, Ariji E. Preliminary Study on the Diagnostic Performance of a Deep Learning System for Submandibular Gland Inflammation Using Ultrasonography Images. Journal of Clinical Medicine. 2021; 10(19):4508. https://doi.org/10.3390/jcm10194508
Chicago/Turabian StyleKise, Yoshitaka, Chiaki Kuwada, Yoshiko Ariji, Munetaka Naitoh, and Eiichiro Ariji. 2021. "Preliminary Study on the Diagnostic Performance of a Deep Learning System for Submandibular Gland Inflammation Using Ultrasonography Images" Journal of Clinical Medicine 10, no. 19: 4508. https://doi.org/10.3390/jcm10194508
APA StyleKise, Y., Kuwada, C., Ariji, Y., Naitoh, M., & Ariji, E. (2021). Preliminary Study on the Diagnostic Performance of a Deep Learning System for Submandibular Gland Inflammation Using Ultrasonography Images. Journal of Clinical Medicine, 10(19), 4508. https://doi.org/10.3390/jcm10194508







