Circulating Tumor Cells and TWIST Expression in Patients with Metastatic Gastric Cancer: A Preliminary Study
Abstract
:1. Introduction
2. Methods
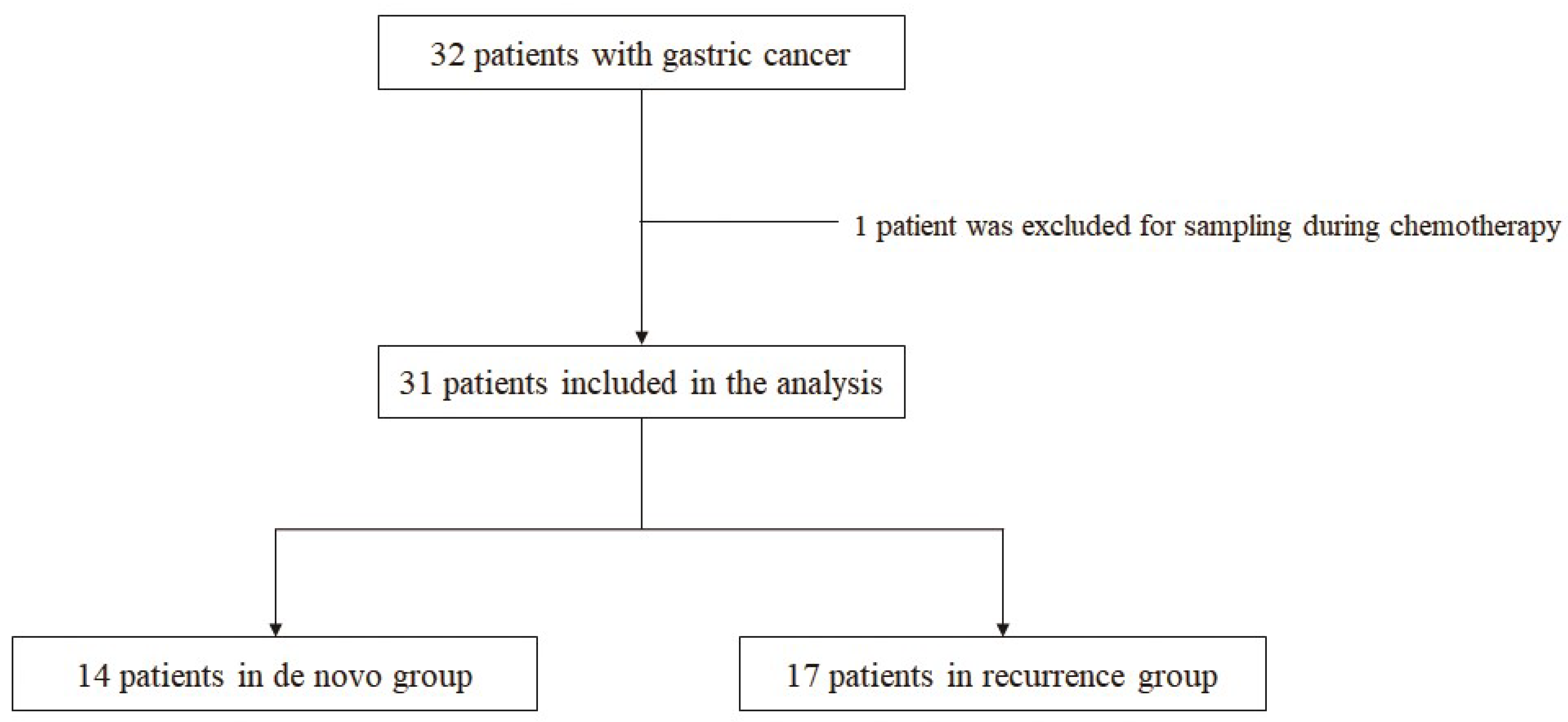
2.1. Study Design (Patients and Sample)
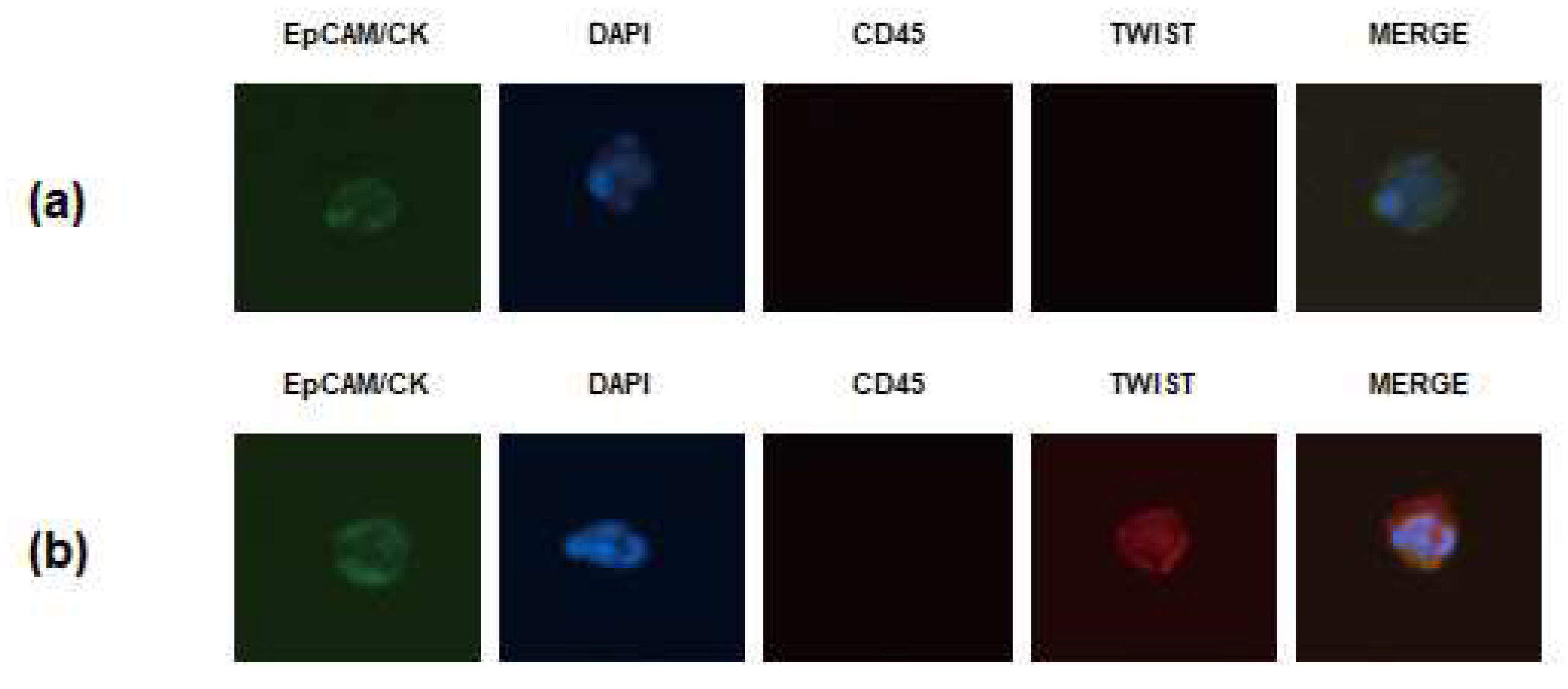
2.2. Isolation and Enumeration of CTCs
2.3. Statistical Analysis
3. Results
3.1. Baseline Clinicopathologic Characteristics of Patients with Metastatic GC
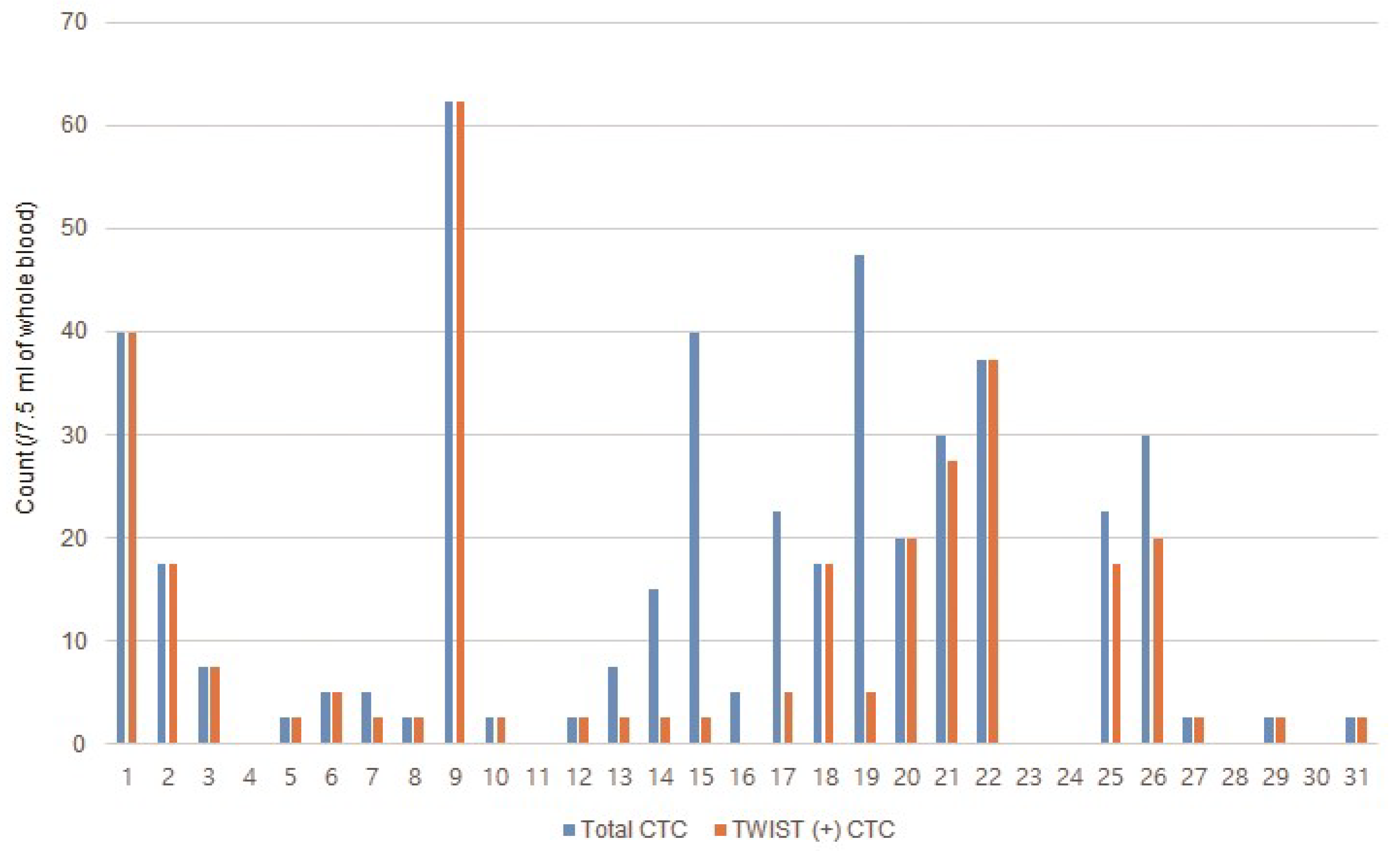
3.2. Incidence of CTCs in Patients with Metastatic GC
3.3. Association between CTCs and Clinicopathologic Characteristics
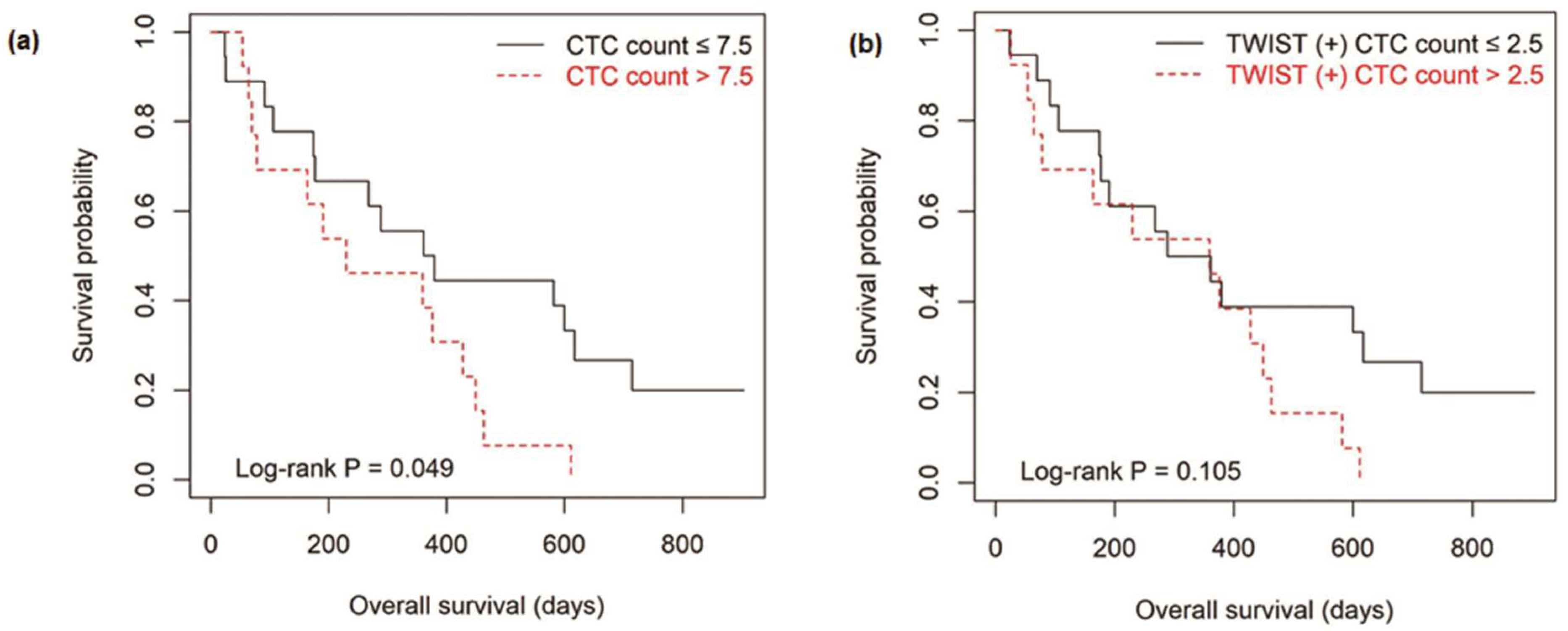
3.4. Survival Outcomes According to the Level of CTCs
3.5. Analysis of Other Prognostic Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [Green Version]
- Digklia, A.; Wagner, A.D. Advanced gastric cancer: Current treatment landscape and future perspectives. World J. Gastroenterol. 2016, 22, 2403–2414. [Google Scholar] [CrossRef]
- Wu, T.; Cheng, B.; Fu, L. Clinical applications of circulating tumor cells in pharmacotherapy: Challenges and perspectives. Mol. Pharmacol. 2017, 92, 232–239. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.W.; Kim, G.H.; Jeon, H.K.; Park, S.J. Clinical application of circulating tumor cells in gastric cancer. Gut Liver 2019, 13, 394–401. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.K.; Kim, G.H. Clinical significance of circulating tumor cells in gastric cancer. Korean J. Helicobacter Gastrointest. Res. 2018, 18, 162–167. [Google Scholar] [CrossRef]
- Uenosono, Y.; Arigami, T.; Kozono, T.; Yanagita, S.; Hagihara, T.; Haraguchi, N.; Matsushita, D.; Hirata, M.; Arima, H.; Funasako, Y.; et al. Clinical significance of circulating tumor cells in peripheral blood from patients with gastric cancer. Cancer 2013, 119, 3984–3991. [Google Scholar] [CrossRef] [PubMed]
- Bertazza, L.; Mocellin, S.; Marchet, A.; Pilati, P.; Gabrieli, J.; Scalerta, R.; Nitti, D. Survivin gene levels in the peripheral blood of patients with gastric cancer independently predict survival. J. Transl. Med. 2009, 7, 111. [Google Scholar] [CrossRef] [Green Version]
- Mimori, K.; Fukagawa, T.; Kosaka, Y.; Ishikawa, K.; Iwatsuki, M.; Yokobori, T.; Hirasaki, S.; Takatsuno, Y.; Sakashita, H.; Ishii, H.; et al. A large-scale study of MT1-MMP as a marker for isolated tumor cells in peripheral blood and bone marrow in gastric cancer cases. Ann. Surg. Oncol. 2008, 15, 2934–2942. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Lim, M.; Park, J.; Oh, J.M.; Kim, H.; Jeong, H.; Lee, S.J.; Park, H.C.; Jung, S.; Kim, B.C.; et al. FAST: Size-selective, clog-free isolation of rare cancer cells from whole blood at a liquid-liquid interface. Anal. Chem. 2017, 89, 1155–1162. [Google Scholar] [CrossRef]
- Lee, A.; Park, J.; Lim, M.; Sunkara, V.; Kim, S.Y.; Kim, G.H.; Kim, M.H.; Cho, Y.K. All-in-one centrifugal microfluidic device for size-selective circulating tumor cell isolation with high purity. Anal. Chem. 2014, 86, 11349–11356. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Kim, G.H.; Jeon, H.K.; Kim, D.H.; Jeon, T.Y.; Park, D.Y.; Jeong, H.; Chun, W.J.; Kim, M.H.; Park, J.; et al. Circulating tumor cells detected by lab-on-a-disc: Role in early diagnosis of gastric cancer. PLoS ONE 2017, 12, e0180251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.; Chen, K.; Che, J.; Hang, J.; Li, H. Detection of epithelial-mesenchymal transition status of circulating tumor cells in patients with esophageal squamous carcinoma. Biomed Res. Int. 2018, 2018, 7610154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Mani, S.A.; Donaher, J.L.; Ramaswamy, S.; Itzykson, R.A.; Come, C.; Savagner, P.; Gitelman, I.; Richardson, A.; Weinberg, R.A. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 2004, 117, 927–939. [Google Scholar] [CrossRef] [Green Version]
- Sun, S.; Gong, Q. The expressions and prognostic implications of Twist and E-cadherin in adenocarcinomas of the gastroesophageal junction and proximal gastric carcinoma. Medicine 2019, 98, e18449. [Google Scholar] [CrossRef] [PubMed]
- Li, T.T.; Liu, H.; Li, F.P.; Hu, Y.F.; Mou, T.Y.; Lin, T.; Yu, J.; Zheng, L.; Li, G.X. Evaluation of epithelial-mesenchymal transitioned circulating tumor cells in patients with resectable gastric cancer: Relevance to therapy response. World J. Gastroenterol. 2015, 21, 13259–13267. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.Y.; Guo, Q.S.; Li, Y.L.; Cui, B.; Guo, J.; Liu, J.X.; Li, P. Twist1 correlates with poor differentiation and progression in gastric adenocarcinoma via elevation of FGFR2 expression. World J. Gastroenterol. 2014, 20, 18306–18315. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.; et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leversha, M.A.; Han, J.; Asgari, Z.; Danila, D.C.; Lin, O.; Gonzalez-Espinoza, R.; Anand, A.; Lilja, H.; Heller, G.; Fleisher, M.; et al. Fluorescence in situ hybridization analysis of circulating tumor cells in metastatic prostate cancer. Clin. Cancer Res. 2009, 15, 2091–2097. [Google Scholar] [CrossRef] [Green Version]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. (Eds.) AJCC Cancer Staging Manual, 8th ed.; Springer: New York, NY, USA, 2017. [Google Scholar]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Choi, M.K.; Kim, G.H.; I, H.; Park, S.J.; Lee, M.W.; Lee, B.E.; Park, D.Y.; Cho, Y.K. Circulating tumor cells detected using fluid-assisted separation technique in esophageal squamous cell carcinoma. J. Gastroenterol. Hepatol. 2019, 34, 552–560. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, N.; Wang, S.; Shi, D.; Zhang, C.; Liu, K.; Xiong, B. Wedge-shaped microfluidic chip for circulating tumor cells isolation and its clinical significance in gastric cancer. J. Transl. Med. 2018, 16, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, X.; Gao, P.; Sun, J.; Chen, X.; Song, Y.; Zhao, J.; Xu, H.; Wang, Z. Clinicopathological and prognostic significance of circulating tumor cells in patients with gastric cancer: A meta-analysis. Int. J. Cancer 2015, 136, 21–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, H.; Sato, J.; Tsujino, Y.; Yamaguchi, N.; Kimura, S.; Gohda, K.; Murakami, K.; Onimaru, M.; Ohmori, T.; Ishikawa, F.; et al. Long-term prognostic impact of circulating tumour cells in gastric cancer patients. World J. Gastroenterol. 2016, 22, 10232–10241. [Google Scholar] [CrossRef] [PubMed]
- Gorges, T.M.; Tinhofer, I.; Drosch, M.; Rose, L.; Zollner, T.M.; Krahn, T.; von Ahsen, O. Circulating tumour cells escape from EpCAM-based detection due to epithelial-to-mesenchymal transition. BMC Cancer 2012, 12, 178. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Gong, J.; Zhang, Q.; Lu, Z.; Gao, J.; Li, Y.; Cao, Y.; Shen, L. Dynamic monitoring of circulating tumour cells to evaluate therapeutic efficacy in advanced gastric cancer. Br. J. Cancer 2016, 114, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Strati, A.; Nikolaou, M.; Georgoulias, V.; Lianidou, E.S. Prognostic significance of TWIST1, CD24, CD44, and ALDH1 transcript quantification in EpCAM-positive circulating tumor cells from early stage breast cancer patients. Cells 2019, 8, 652. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.C.; Luo, Z.C.; Gao, Y.X.; Li, Y.; Peng, Q.; Gao, Y. Twist expression in circulating hepatocellular carcinoma cells predicts metastasis and prognoses. Biomed Res. Int. 2018, 2018, 3789613. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Y.; Lu, M.; Shen, L. Predictive value of serum CEA, CA19-9 and CA72.4 in early diagnosis of recurrence after radical resection of gastric cancer. Hepatogastroenterology 2011, 58, 2166–2170. [Google Scholar] [CrossRef]
- Matsusaka, S.; Chin, K.; Ogura, M.; Suenaga, M.; Shinozaki, E.; Mishima, Y.; Terui, Y.; Mizunuma, N.; Hatake, K. Circulating tumor cells as a surrogate marker for determining response to chemotherapy in patients with advanced gastric cancer. Cancer Sci. 2010, 101, 1067–1071. [Google Scholar] [CrossRef]
- Wu, S.; Liu, S.; Liu, Z.; Huang, J.; Pu, X.; Li, J.; Yang, D.; Deng, H.; Yang, N.; Xu, J. Classification of circulating tumor cells by epithelial-mesenchymal transition markers. PLoS ONE 2015, 10, e0123976. [Google Scholar] [CrossRef]
| Median age, years (range) | 63 (42–87) |
| Sex | |
| Male | 22 |
| Female | 9 |
| Disease status | |
| De novo | 14 |
| Recurrence | 17 |
| Histopathologic type | |
| Intestinal type | 13 |
| Diffuse type | 18 |
| Peritoneal dissemination | |
| Absent | 12 |
| Present | 19 |
| Hematogenous metastasis | |
| Absent | 14 |
| Present | 17 |
| Serum CEA | |
| ≤5 ng/mL | 18 |
| >5 ng/mL | 13 |
| Serum CA19-9 | |
| ≤37 U/mL | 13 |
| >37 U/mL | 18 |
| Chemotherapy | |
| Not administered | 5 |
| Administered | 26 |
| CTC Count (/7.5 mL of Whole Blood) | p Value * | TWIST (+) CTC Count (/7.5 mL of Whole Blood) | p Value * | |
|---|---|---|---|---|
| Age | 0.627 | 0.398 | ||
| ≤65 years | 11.3 (0–62.4) | 3.8 (0–62.4) | ||
| >65 years | 5.0 (0–47.5) | 2.5 (0–20.0) | ||
| Sex | 0.965 | 0.946 | ||
| Male | 6.3 (0–40.0) | 2.5 (0–40.0) | ||
| Female | 5.0 (0–62.4) | 2.5 (0–62.4) | ||
| Disease status | 0.113 | 0.368 | ||
| De novo | 20.0 (0–47.5) | 5.0 (0–40.0) | ||
| Recurrence | 2.5 (0–62.4) | 2.5 (0–62.4) | ||
| Histopathologic type | 0.284 | 0.216 | ||
| Intestinal type | 2.5 (0–40.0) | 2.5 (0–20.0) | ||
| Diffuse type | 11.3 (0–62.4) | 3.8 (0–62.4) | ||
| Peritoneal dissemination | 0.566 | 0.337 | ||
| Absent | 7.5 (0–47.5) | 3.8 (9–40.0) | ||
| Present | 5.0 (0–62.4) | 2.5 (0–62.4) | ||
| Hematogenous metastasis | 0.703 | 0.790 | ||
| Absent | 5.0 (0–62.4) | 2.5 (0–62.4) | ||
| Present | 7.5 (0–47.5) | 2.5 (0–40.0) | ||
| Serum CEA | 0.558 | 0.564 | ||
| ≤5 ng/mL | 6.3 (0–62.4) | 3.8 (0–62.4) | ||
| >5 ng/mL | 2.5 (0–40.0) | 2.5 (0–37.3) | ||
| Serum CA19-9 | 0.175 | 0.155 | ||
| ≤37 U/mL | 2.5 (0–47.5) | 2.5 (0–27.5) | ||
| >37 U/mL | 16.3 (0–62.4) | 3.8 (0–62.4) | ||
| Response to chemotherapy † | 0.717 | 0.832 | ||
| Partial remission/stable disease | 5.0 (0–62.4) | 2.5 (0–62.4) | ||
| Progressive disease | 5.0 (0–40.0) | 2.5 (0–40.0) |
| CTC Count (/7.5 mL of Whole Blood) | p Value * | TWIST (+) CTC Count (/7.5 mL of Whole Blood) | p Value * | |||
|---|---|---|---|---|---|---|
| ≤7.5 (n = 18) | >7.5 (n = 13) | ≤2.5 (n = 18) | >2.5 (n = 13) | |||
| Age | 0.284 | 0.284 | ||||
| ≤65 years | 9 | 9 | 9 | 9 | ||
| >65 years | 9 | 4 | 9 | 4 | ||
| Sex | 1.000 | 1.000 | ||||
| Male | 13 | 9 | 13 | 9 | ||
| Female | 5 | 4 | 5 | 4 | ||
| Disease status | 0.055 | 0.119 | ||||
| De novo | 5 | 9 | 6 | 8 | ||
| Recurrence | 13 | 4 | 12 | 5 | ||
| Histopathologic type | 0.284 | 0.284 | ||||
| Intestinal type | 9 | 4 | 9 | 4 | ||
| Diffuse type | 9 | 9 | 9 | 9 | ||
| Peritoneal dissemination | 0.981 | 0.470 | ||||
| Absent | 7 | 5 | 6 | 6 | ||
| Present | 11 | 8 | 12 | 7 | ||
| Hematogenous metastasis | 0.524 | 0.524 | ||||
| Absent | 9 | 5 | 9 | 5 | ||
| Present | 9 | 8 | 9 | 8 | ||
| Serum CEA | 0.739 | 0.284 | ||||
| ≤5 ng/mL | 10 | 8 | 9 | 9 | ||
| >5 ng/mL | 8 | 5 | 9 | 4 | ||
| Serum CA19-9 | 0.071 | 0.284 | ||||
| ≤37 U/mL | 10 | 3 | 9 | 4 | ||
| >37 U/mL | 8 | 10 | 9 | 9 | ||
| Response to chemotherapy † | 0.420 | 1.000 | ||||
| Partial remission/stable disease | 9 | 4 | 8 | 5 | ||
| Progressive disease | 7 | 6 | 8 | 5 | ||
| Clinicopathologic Factors | Univariate Analysis | Multivariate Analysis | |
|---|---|---|---|
| p Value | HR (95% CI) | p Value | |
| Sex (M vs. F) | 0.956 | 0.860 (0.279−2.651) | 0.793 |
| Age (≤65 years vs. >65 years) | 0.033 | 0.428 (0.127−1.436) | 0.169 |
| Disease status (de novo vs. recurrence) | 0.008 | 2.458 (0.561−10.777) | 0.233 |
| Histopathologic type (intestinal vs. diffuse) | 0.051 | 2.756 (0.976−7.781) | 0.056 |
| Peritoneal dissemination | 0.331 | 4.293 (1.155−15.962) | 0.030 |
| Hematogenous metastasis | 0.183 | 1.281 (0.440−3.732) | 0.650 |
| Serum CEA (≤5 ng/mL vs. >5 ng/mL) | 0.711 | 0.997 (0.328−3.029) | 0.996 |
| Serum CA19-9 (≤37 U/mL vs. >37 U/mL) | 0.005 | 7.531 (2.301−24.645) | 0.001 |
| Chemotherapy | 0.144 | 0.154 (0.028−0.853) | 0.032 |
| CTC count/7.5 mL of whole blood (≤7.5 vs. >7.5) | 0.054 | 0.848 (0.222−3.245) | 0.810 |
| TWIST (+) CTC count/7.5 mL of whole blood (≤2.5 vs. >2.5) | 0.111 | 0.723 (0.167−3.127) | 0.664 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhi, J.H.; Kim, G.H.; Park, S.J.; Kim, D.U.; Lee, M.W.; Lee, B.E.; Kwon, C.H.; Cho, Y.-K. Circulating Tumor Cells and TWIST Expression in Patients with Metastatic Gastric Cancer: A Preliminary Study. J. Clin. Med. 2021, 10, 4481. https://doi.org/10.3390/jcm10194481
Jhi JH, Kim GH, Park SJ, Kim DU, Lee MW, Lee BE, Kwon CH, Cho Y-K. Circulating Tumor Cells and TWIST Expression in Patients with Metastatic Gastric Cancer: A Preliminary Study. Journal of Clinical Medicine. 2021; 10(19):4481. https://doi.org/10.3390/jcm10194481
Chicago/Turabian StyleJhi, Joon Hyung, Gwang Ha Kim, Su Jin Park, Dong Uk Kim, Moon Won Lee, Bong Eun Lee, Chae Hwa Kwon, and Yoon-Kyoung Cho. 2021. "Circulating Tumor Cells and TWIST Expression in Patients with Metastatic Gastric Cancer: A Preliminary Study" Journal of Clinical Medicine 10, no. 19: 4481. https://doi.org/10.3390/jcm10194481
APA StyleJhi, J. H., Kim, G. H., Park, S. J., Kim, D. U., Lee, M. W., Lee, B. E., Kwon, C. H., & Cho, Y.-K. (2021). Circulating Tumor Cells and TWIST Expression in Patients with Metastatic Gastric Cancer: A Preliminary Study. Journal of Clinical Medicine, 10(19), 4481. https://doi.org/10.3390/jcm10194481







