Association of Nonalcoholic Fatty Liver Disease (NAFLD) with Peripheral Diabetic Polyneuropathy: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Endpoints
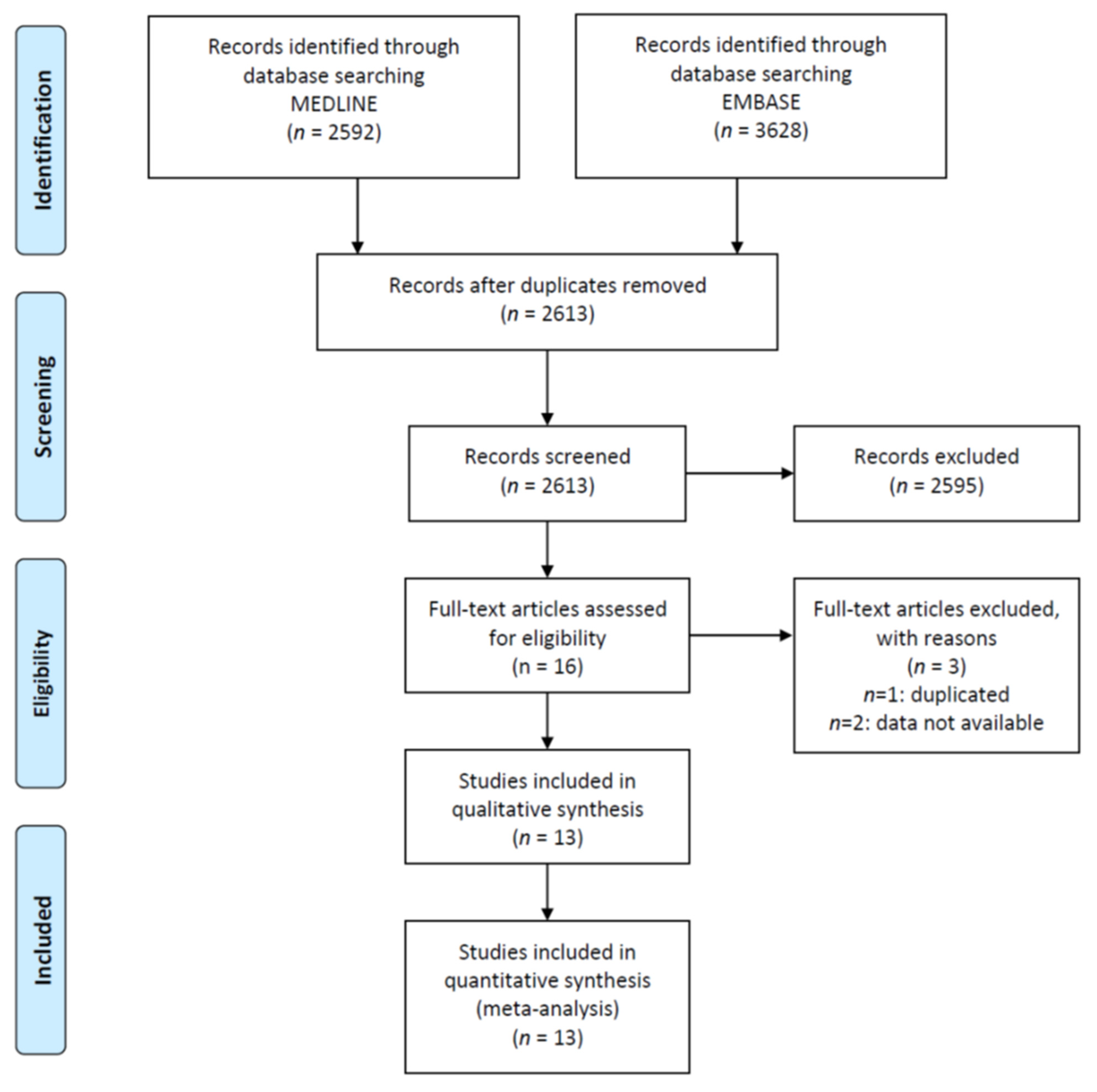
2.2. Study Selection and Inclusion Criteria
2.3. Data Collection Process and Quality
2.4. Data Synthesis and Analysis
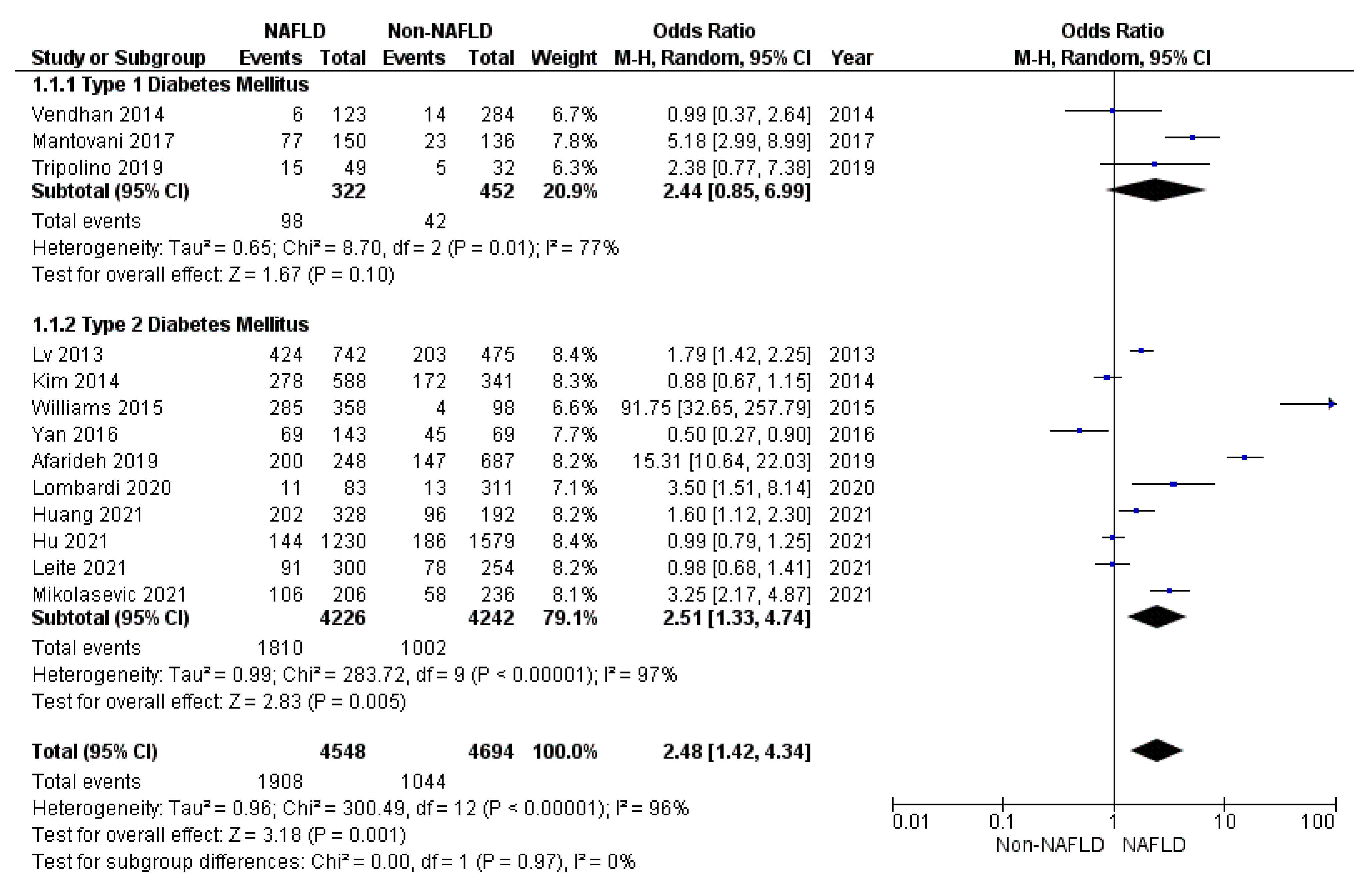
3. Results
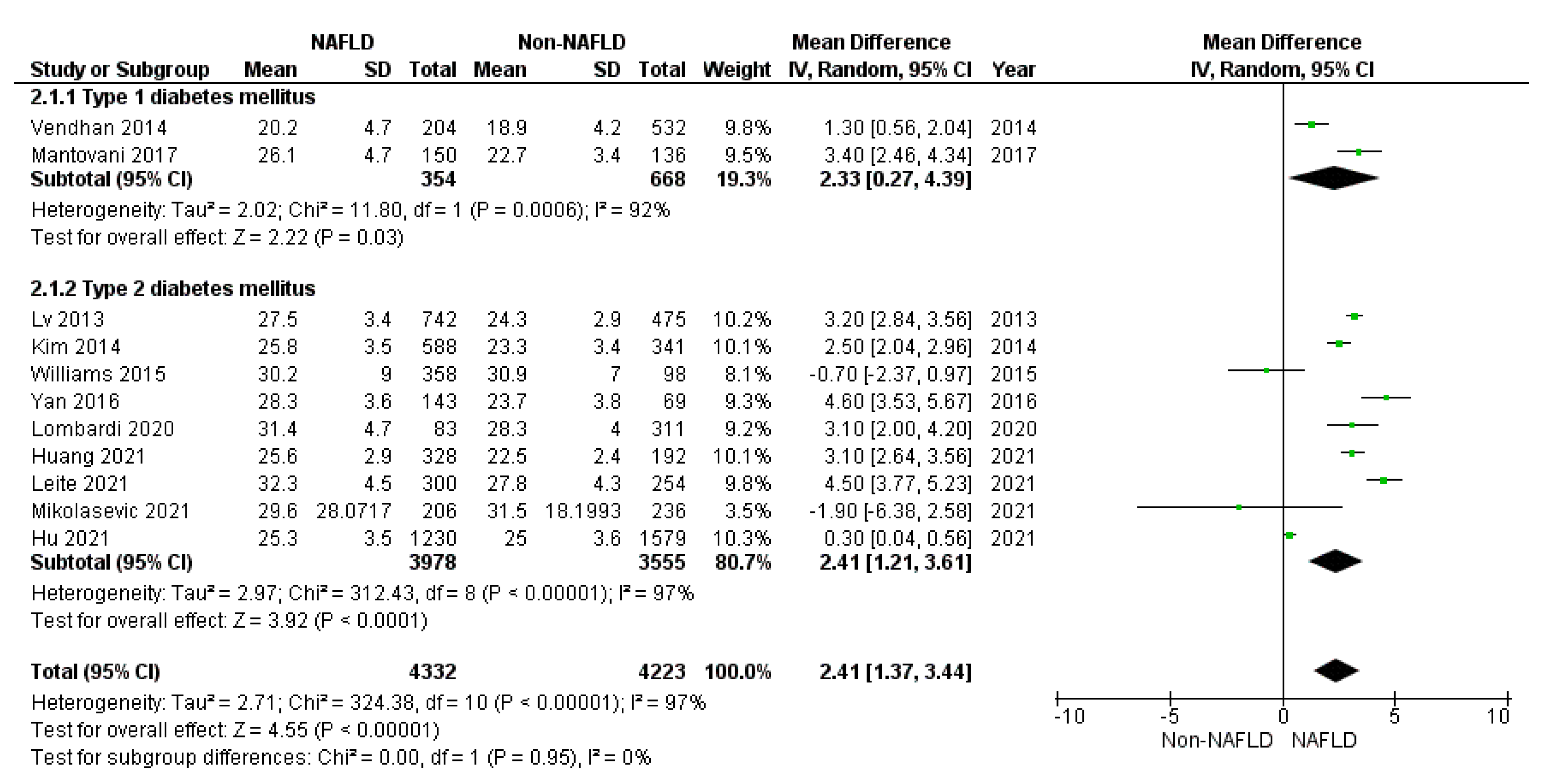
3.1. Secondary Endpoints
3.2. Sensitivity Analyses
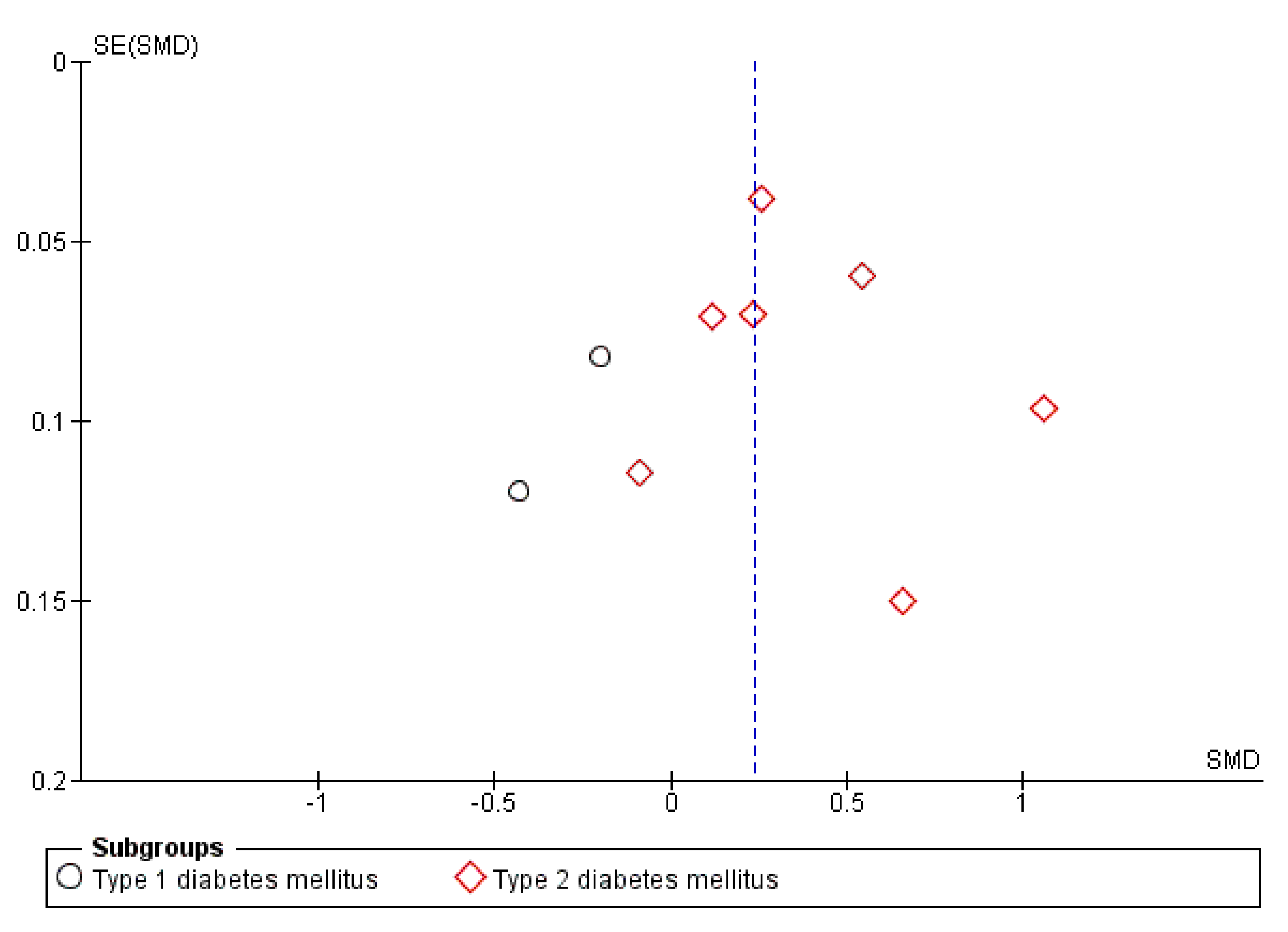
3.3. Publication Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tesfaye, S.; Boulton, A.J.; Dyck, P.J.; Freeman, R.; Horowitz, M.; Kempler, P.; Lauria, G.; Malik, R.A.; Spallone, V.; Vinik, A.; et al. Diabetic neuropathies: Update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care 2010, 33, 2285–2293. [Google Scholar] [CrossRef]
- Maser, R.E.; Steenkiste, A.R.; Dorman, J.S.; Nielsen, V.K.; Bass, E.B.; Manjoo, Q.; Drash, A.L.; Becker, D.J.; Kuller, L.H.; Greene, D.A. Epidemiological correlates of diabetic neuropathy. Report from Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes 1989, 38, 1456–1461. [Google Scholar] [CrossRef]
- Tesfaye, S.; Stevens, L.K.; Stephenson, J.M.; Fuller, J.H.; Plater, M.; Ionescu-Tirgoviste, C.; Nuber, A.; Pozza, G.; Ward, J.D. Prevalence of diabetic peripheral neuropathy and its relation to glycaemic control and potential risk factors: The EURODIAB IDDM Complications Study. Diabetologia 1996, 39, 1377–1384. [Google Scholar] [CrossRef]
- Martin, C.L.; Albers, J.W.; Pop-Busui, R.; DCCT/EDIC Research Group. Neuropathy and related findings in the diabetes control and complications trial/epidemiology of diabetes interventions and complications study. Diabetes Care 2014, 37, 31–38. [Google Scholar] [CrossRef]
- Albers, J.W.; Herman, W.H.; Pop-Busui, R.; Feldman, E.L.; Martin, C.L.; Cleary, P.A.; Waberski, B.H.; Lachin, J.M.; Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care 2010, 33, 1090–1096. [Google Scholar] [CrossRef]
- Young, M.J.; Boulton, A.J.; MacLeod, A.F.; Williams, D.R.; Sonksen, P.H. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia 1993, 36, 150–154. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 1998, 352, 837–853. [Google Scholar] [CrossRef]
- Ang, L.; Jaiswal, M.; Martin, C.; Pop-Busui, R. Glucose control and diabetic neuropathy: Lessons from recent large clinical trials. Curr. Diabetes Rep. 2014, 14, 528. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Lu, J.; Brooks, M.M.; Albert, S.; Althouse, A.D.; Escobedo, J.; Green, J.; Palumbo, P.; Perkins, B.A.; Whitehouse, F.; et al. Impact of glycemic control strategies on the progression of diabetic peripheral neuropathy in the Bypass Angioplasty Revascularization Investigation 2 Diabetes (BARI 2D) Cohort. Diabetes Care 2013, 36, 3208–3215. [Google Scholar] [CrossRef]
- Papanas, N.; Vinik, A.I.; Ziegler, D. Neuropathy in prediabetes: Does the clock start ticking early? Nat. Rev. Endocrinol. 2011, 7, 682–690. [Google Scholar] [CrossRef]
- Forsblom, C.M.; Sane, T.; Groop, P.H.; Tötterman, K.J.; Kallio, M.; Saloranta, C.; Laasonen, L.; Summanen, P.; Lepäntalo, M.; Laatikainen, L.; et al. Risk factors for mortality in Type II (non-insulin-dependent) diabetes: Evidence of a role for neuropathy and a protective effect of HLA-DR4. Diabetologia 1998, 41, 1253–1262. [Google Scholar] [CrossRef][Green Version]
- Soedamah-Muthu, S.S.; Chaturvedi, N.; Witte, D.R.; Stevens, L.K.; Porta, M.; Fuller, J.H.; EURODIAB Prospective Complications Study Group. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: The EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008, 31, 1360–1366. [Google Scholar] [CrossRef]
- Coppini, D.V.; Bowtell, P.A.; Weng, C.; Young, P.J.; Sönksen, P.H. Showing neuropathy is related to increased mortality in diabetic patients—A survival analysis using an accelerated failure time model. J. Clin. Epidemiol. 2000, 53, 519–523. [Google Scholar] [CrossRef]
- Tesfaye, S.; Chaturvedi, N.; Eaton, S.E.; Ward, J.D.; Manes, C.; Ionescu-Tirgoviste, C.; Witte, D.R.; Fuller, J.H.; EURODIAB Prospective Complications Study Group. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 2005, 352, 341–350. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 41. [Google Scholar] [CrossRef]
- Bönhof, G.J.; Herder, C.; Strom, A.; Papanas, N.; Roden, M.; Ziegler, D. Emerging Biomarkers, Tools, and Treatments for Diabetic Polyneuropathy. Endocr. Rev. 2019, 40, 153–192. [Google Scholar] [CrossRef]
- Ziegler, D.; Papanas, N.; Vinik, A.I.; Shaw, J.E. Epidemiology of polyneuropathy in diabetes and prediabetes. Handb. Clin. Neurol. 2014, 126, 3–22. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J. Hepatol. 2016, 64, 1388–1402. [Google Scholar] [CrossRef]
- Chalasani, N.; Younossi, Z.; Lavine, J.E.; Charlton, M.; Cusi, K.; Rinella, M.; Harrison, S.A.; Brunt, E.M.; Sanyal, A.J. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018, 67, 328–357. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Corey, K.E.; Lim, J.K. AGA Clinical Practice Update on Lifestyle Modification Using Diet and Exercise to Achieve Weight Loss in the Management of Nonalcoholic Fatty Liver Disease: Expert Review. Gastroenterology 2021, 160, 912–918. [Google Scholar] [CrossRef]
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Alkagiet, S.; Papagiannis, A.; Tziomalos, K. Associations between nonalcoholic fatty liver disease and ischemic stroke. World J. Hepatol. 2018, 10, 474–478. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Padovani, R.; Poli, F.; Scala, L.; Tessari, R.; Zenari, L.; Falezza, G. Increased prevalence of cardiovascular disease in Type 2 diabetic patients with non-alcoholic fatty liver disease. Diabet. Med. 2006, 23, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Tessari, R.; Zenari, L.; Day, C.; Arcaro, G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care 2007, 30, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Wijarnpreecha, K.; Thongprayoon, C.; Boonpheng, B.; Panjawatanan, P.; Sharma, K.; Ungprasert, P.; Pungpapong, S.; Cheungpasitporn, W. Nonalcoholic fatty liver disease and albuminuria: A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Li, C.; Wang, Z.; Zhao, Y.; Shen, B.; Zhao, W. Association of non-alcoholic fatty liver disease with diabetic retinopathy in type 2 diabetic patients: A meta-analysis of observational studies. J. Diabetes Investig. 2020, 12, 1471–1479. [Google Scholar] [CrossRef]
- Ludwig, J.; Viggiano, T.R.; McGill, D.B.; Oh, B.J. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin. Proc. 1980, 55, 434–438. [Google Scholar]
- Ziegler, D. Treatment of diabetic neuropathy and neuropathic pain: How far have we come? Diabetes Care 2008, 31 (Suppl. S2), S255–S261. [Google Scholar] [CrossRef]
- Panero, F.; Novelli, G.; Zucco, C.; Fornengo, P.; Perotto, M.; Segre, O.; Grassi, G.; Cavallo-Perin, P.; Bruno, G. Fasting plasma C-peptide and micro- and macrovascular complications in a large clinic-based cohort of type 1 diabetic patients. Diabetes Care 2009, 32, 301–305. [Google Scholar] [CrossRef]
- Sterne, J.A.; Egger, M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001, 54, 1046–1055. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Afarideh, M.; Aryan, Z.; Ghajar, A.; Ganji, M.; Ghaemi, F.; Saadat, M.; Heidari, B.; Mechanick, J.I.; Esteghamati, A. Association of non-alcoholic fatty liver disease with microvascular complications of type 2 diabetes. Prim. Care Diabetes 2019, 13, 505–514. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Q.; Min, R.; Deng, Y.; Xu, Y.; Gao, L. The association between serum uric acid and diabetic complications in patients with type 2 diabetes mellitus by gender: A cross-sectional study. PeerJ 2021, 9, e10691. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Jung, C.H.; Mok, J.O.; Kang, S.K.; Kim, C.H. Prevalences of diabetic retinopathy and nephropathy are lower in Korean type 2 diabetic patients with non-alcoholic fatty liver disease. J. Diabetes Investig. 2014, 5, 170–175. [Google Scholar] [CrossRef]
- Leite, N.C.; Cardoso, C.R.L.; Salles, G.F. Importance of non-invasive liver fibrosis scores for mortality and complications development in individuals with type 2 diabetes. J. Diabetes Complicat. 2021, 35, 107879. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. Liver fibrosis by FibroScan(®) independently of established cardiovascular risk parameters associates with macrovascular and microvascular complications in patients with type 2 diabetes. Liver Int. 2020, 40, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Lv, W.S.; Sun, R.X.; Gao, Y.Y.; Wen, J.P.; Pan, R.F.; Li, L.; Wang, J.; Xian, Y.X.; Cao, C.X.; Zheng, M. Nonalcoholic fatty liver disease and microvascular complications in type 2 diabetes. World J. Gastroenterol. 2013, 19, 3134–3142. [Google Scholar] [CrossRef]
- Mantovani, A.; Rigolon, R.; Mingolla, L.; Pichiri, I.; Cavalieri, V.; Salvotelli, L.; Stoico, V.; Zoppini, G.; Bonora, E.; Targher, G. Nonalcoholic fatty liver disease is associated with an increased prevalence of distal symmetric polyneuropathy in adult patients with type 1 diabetes. J. Diabetes Complicat. 2017, 31, 1021–1026. [Google Scholar] [CrossRef]
- Tripolino, C.; Irace, C.; Cutruzzolà, A.; Parise, M.; Barone, M.; Scicchitano, C.; Cortese, C.; Gnasso, A. Hepatic Steatosis Index Is Associated with Type 1 Diabetes Complications. Diabetes Metab. Syndr. Obes. 2019, 12, 2405–2410. [Google Scholar] [CrossRef]
- Vendhan, R.; Amutha, A.; Anjana, R.M.; Unnikrishnan, R.; Mohan, V. Clinical profile of nonalcoholic Fatty liver disease among young patients with type 1 diabetes mellitus seen at a diabetes speciality center in India. Endocr. Pract. Off. J. Am. Coll. Endocrinol. Am. Assoc. Clin. Endocrinol. 2014, 20, 1249–1257. [Google Scholar] [CrossRef]
- Williams, K.H.; Burns, K.; Constantino, M.; Shackel, N.A.; Prakoso, E.; Wong, J.; Wu, T.; George, J.; McCaughan, G.W.; Twigg, S.M. An association of large-fibre peripheral nerve dysfunction with non-invasive measures of liver fibrosis secondary to non-alcoholic fatty liver disease in diabetes. J. Diabetes Complicat. 2015, 29, 1240–1247. [Google Scholar] [CrossRef]
- Yan, L.H.; Mu, B.; Guan, Y.; Liu, X.; Zhao, N.; Pan, D.; Wang, S.Z. Assessment of the relationship between non-alcoholic fatty liver disease and diabetic complications. J. Diabetes Investig. 2016, 7, 889–894. [Google Scholar] [CrossRef]
- Zaharia, O.P.; Strassburger, K.; Strom, A.; Bönhof, G.J.; Karusheva, Y.; Antoniou, S.; Bódis, K.; Markgraf, D.F.; Burkart, V.; Müssig, K.; et al. Risk of diabetes-associated diseases in subgroups of patients with recent-onset diabetes: A 5-year follow-up study. Lancet Diabetes Endocrinol. 2019, 7, 684–694. [Google Scholar] [CrossRef]
- Zhao, L.; Ma, J.; Wang, S.; Xie, Y. Relationship between β-cell function, metabolic control, and microvascular complications in type 2 diabetes mellitus. Diabetes Technol. 2015, 17, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, R.; Airaghi, L.; Targher, G.; Serviddio, G.; Maffi, G.; Mantovani, A.; Maffeis, C.; Colecchia, A.; Villani, R.; Rinaldi, L.; et al. NAFLD fibrosis score (NFS) can be used in outpatient services to identify chronic vascular complications besides advanced liver fibrosis in type 2 diabetes. J. Diabetes Complicat. 2020, 34, 107684. [Google Scholar] [CrossRef]
- Huang, J.; Li, R.; Liu, N.; Yi, N.; Zheng, H.; Zhang, Q.; Zhou, L.; Zhou, L.; Hu, R.; Lu, B. Liver Fibrosis is Independently Associated with Diabetic Peripheral Neuropathy in Type 2 Diabetes Mellitus. J. Diabetes Investig. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mikolasevic, I.; Rahelic, D.; Turk-Wensween, T.; Ruzic, A.; Domislovic, V.; Hauser, G.; Matic, T.; Radic-Kristo, D.; Krznaric, Z.; Radic, M.; et al. Significant liver fibrosis, as assessed by fibroscan, is independently associated with chronic vascular complications of type 2 diabetes: A multicenter study. Diabetes Res. Clin. Pract. 2021, 177, 108884. [Google Scholar] [CrossRef]
- Casoinic, F.; Sâmpelean, D.; Bădău, C.; Prună, L. Nonalcoholic fatty liver disease--a risk factor for microalbuminuria in type 2 diabetic patients. Rom. J. Intern. Med. 2009, 47, 55–59. [Google Scholar] [PubMed]
- Targher, G.; Bertolini, L.; Rodella, S.; Zoppini, G.; Lippi, G.; Day, C.; Muggeo, M. Non-alcoholic fatty liver disease is independently associated with an increased prevalence of chronic kidney disease and proliferative/laser-treated retinopathy in type 2 diabetic patients. Diabetologia 2008, 51, 444–450. [Google Scholar] [CrossRef]
- Bugianesi, E.; McCullough, A.J.; Marchesini, G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology 2005, 42, 987–1000. [Google Scholar] [CrossRef]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metab. Clin. Exp. 2016, 65, 1038–1048. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Moschen, A.R. IL-1 cytokine family members and NAFLD: Neglected in metabolic liver inflammation. J. Hepatol. 2011, 55, 960–962. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.; Spaggiari, G.; Greco, C.; Lazzaretti, C.; Paradiso, E.; Casarini, L.; Potì, F.; Brigante, G.; Simoni, M. The “Hitchhiker’s Guide to the Galaxy” of Endothelial Dysfunction Markers in Human Fertility. Int. J. Mol. Sci. 2021, 22, 2584. [Google Scholar] [CrossRef]
- Dyson, J.K.; Anstee, Q.M.; McPherson, S. Non-alcoholic fatty liver disease: A practical approach to diagnosis and staging. Front. Gastroenterol. 2014, 5, 211–218. [Google Scholar] [CrossRef] [PubMed]
- McPherson, S.; Stewart, S.F.; Henderson, E.; Burt, A.D.; Day, C.P. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut 2010, 59, 1265–1269. [Google Scholar] [CrossRef]
- Mofrad, P.; Contos, M.J.; Haque, M.; Sargeant, C.; Fisher, R.A.; Luketic, V.A.; Sterling, R.K.; Shiffman, M.L.; Stravitz, R.T.; Sanyal, A.J. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology 2003, 37, 1286–1292. [Google Scholar] [CrossRef]
- Ballestri, S.; Nascimbeni, F.; Lugari, S.; Lonardo, A.; Francica, G. A critical appraisal of the use of ultrasound in hepatic steatosis. Expert Rev. Gastroenterol. Hepatol. 2019, 13, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Deprince, A.; Haas, J.T.; Staels, B. Dysregulated lipid metabolism links NAFLD to cardiovascular disease. Mol. Metab. 2020, 42, 101092. [Google Scholar] [CrossRef] [PubMed]
- Elosua-Bayés, I.; Beloqui Ruiz, Ó. Association between non alcoholic fatty liver disease, metabolic and vascular risk. Clin. Investig. Arter. 2020, 32, 200–205. [Google Scholar] [CrossRef]
- Chen, T.P.; Lai, M.; Lin, W.Y.; Huang, K.C.; Yang, K.C. Metabolic profiles and fibrosis of nonalcoholic fatty liver disease in the elderly: A community-based study. J. Gastroenterol. Hepatol. 2020, 35, 1636–1643. [Google Scholar] [CrossRef]



| Author, Year | Country | Study Design | Aim of the Study | Enrolled Patients (Number) and Type of DM | Sex | NAFLD Diagnosis | DPN Diagnosis |
|---|---|---|---|---|---|---|---|
| Afarideh, 2019 | Iran | Cross-sectional | To evaluate associations of serum liver enzymes and NAFLD with chronic microvascular complications in patients with T2D | 935 New-onset T2DM | Males 450 (48.1%) Females 485 (51.9%) | US | DNS score |
| Hu, 2021 | China | Cross-sectional | To examine whether serum uric acid in T2DM is influenced by age, gender, BMI, lipid, renal function and other characteristics | 2809 T2DM | Males 1784 (63.5%) Females 1025 (36.5%) | US (+reduced alcohol intake) | Physical examination and NCS |
| Kim, 2014 | Korea | Cross-sectional | To assess association between NAFLD and macro- and micro-vascular complications | 929 T2DM | Males 489 (52.6%) Females 440 (47.4%) | US | Physical examination and NCS |
| Leite, 2021 | Brazil | Cross-sectional | To evaluate the NAFLD fibrosis score as predictors of complications development and mortality | 554 T2DM | Males 218 (39.4%) Females 336 (60.6%) | US + NFS | Physical examination |
| Lombardi, 2020 | Italy | Cross-sectional | To evaluate whether FibroScan® is able to detect an association between hepatic steatosis and micro- and macro-vascular complications | 394 T2DM > 5 years | Males 210 (53.4%) Females 184 (46.6%) | US +NFS +FibroScan | Physical examination and NCS |
| Lv, 2013 | Cina | Cross-sectional | To determine the prevalence and risk factors for NAFLD and evaluated its correlations with microvascular complications | 1217 T2DM | Males 460 (37.8%) Females 757 (62.2%) | US (+absence of a secondary cause of steatosis) | Physical examination |
| Mantovani, 2017 | Italy | Cross-sectional | To assess association between NAFLD and DPN | 286 T1DM | Males 121 (42.3%) Females 165 (57.7%) | US | MNSI score and VPT |
| Mikolasevic 2021 | Croatia | Cross-sectional | To examine whether NAFLD is associated with chronic vascular complications of T2DM | 442 T2DM | Males 209 (47.3%) Females 233 (52.7%) | FibroScan | Physical examination and NCS |
| Tripolino, 2019 | Italy | Cross-sectional | To evaluate association between NAFLD and complications | 124 T1DM | Males 68 (60.7%) Females 44 (39.3%) | HSI | Physical examination and NCS |
| Vendhan, 2014 | India | Cross-sectional | To estimate the prevalence and clinical profile of NAFLD | 736 T1DM | Males 384 (52%) Females 354 (48%) | US | VPT |
| Williams, 2015 | Australia | Cross-sectional | To examine the association between distal VPT and NAFLD | 456 T2DM | Males 270 (59.2%) Females 186 (40.8%) | US | VPT |
| Yan, 2016 | China | Cross-sectional | To explore differences in complications when NAFLD developed with pre-existing T2DM | 212 T2DM | Males 120 (56.6%) Females 92 (43.4%) | US | Physical examination |
| Huang, 2021 | China | Cross-sectional | To evaluate the relationship between NAFLD and DPN | 520 T2DM | Males 227 (43.7%) Females 293 (56.3%) | FibroScan | Physical examination and NCS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, C.; Nascimbeni, F.; Carubbi, F.; Andreone, P.; Simoni, M.; Santi, D. Association of Nonalcoholic Fatty Liver Disease (NAFLD) with Peripheral Diabetic Polyneuropathy: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 4466. https://doi.org/10.3390/jcm10194466
Greco C, Nascimbeni F, Carubbi F, Andreone P, Simoni M, Santi D. Association of Nonalcoholic Fatty Liver Disease (NAFLD) with Peripheral Diabetic Polyneuropathy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(19):4466. https://doi.org/10.3390/jcm10194466
Chicago/Turabian StyleGreco, Carla, Fabio Nascimbeni, Francesca Carubbi, Pietro Andreone, Manuela Simoni, and Daniele Santi. 2021. "Association of Nonalcoholic Fatty Liver Disease (NAFLD) with Peripheral Diabetic Polyneuropathy: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 19: 4466. https://doi.org/10.3390/jcm10194466
APA StyleGreco, C., Nascimbeni, F., Carubbi, F., Andreone, P., Simoni, M., & Santi, D. (2021). Association of Nonalcoholic Fatty Liver Disease (NAFLD) with Peripheral Diabetic Polyneuropathy: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(19), 4466. https://doi.org/10.3390/jcm10194466








