A Systematic Review of the Cyclooxygenase-2 (COX-2) Expression in Rectal Cancer Patients Treated with Preoperative Radiotherapy or Radiochemotherapy
Abstract
1. Introduction
2. Guidelines for the Colorectal Cancer Treatment
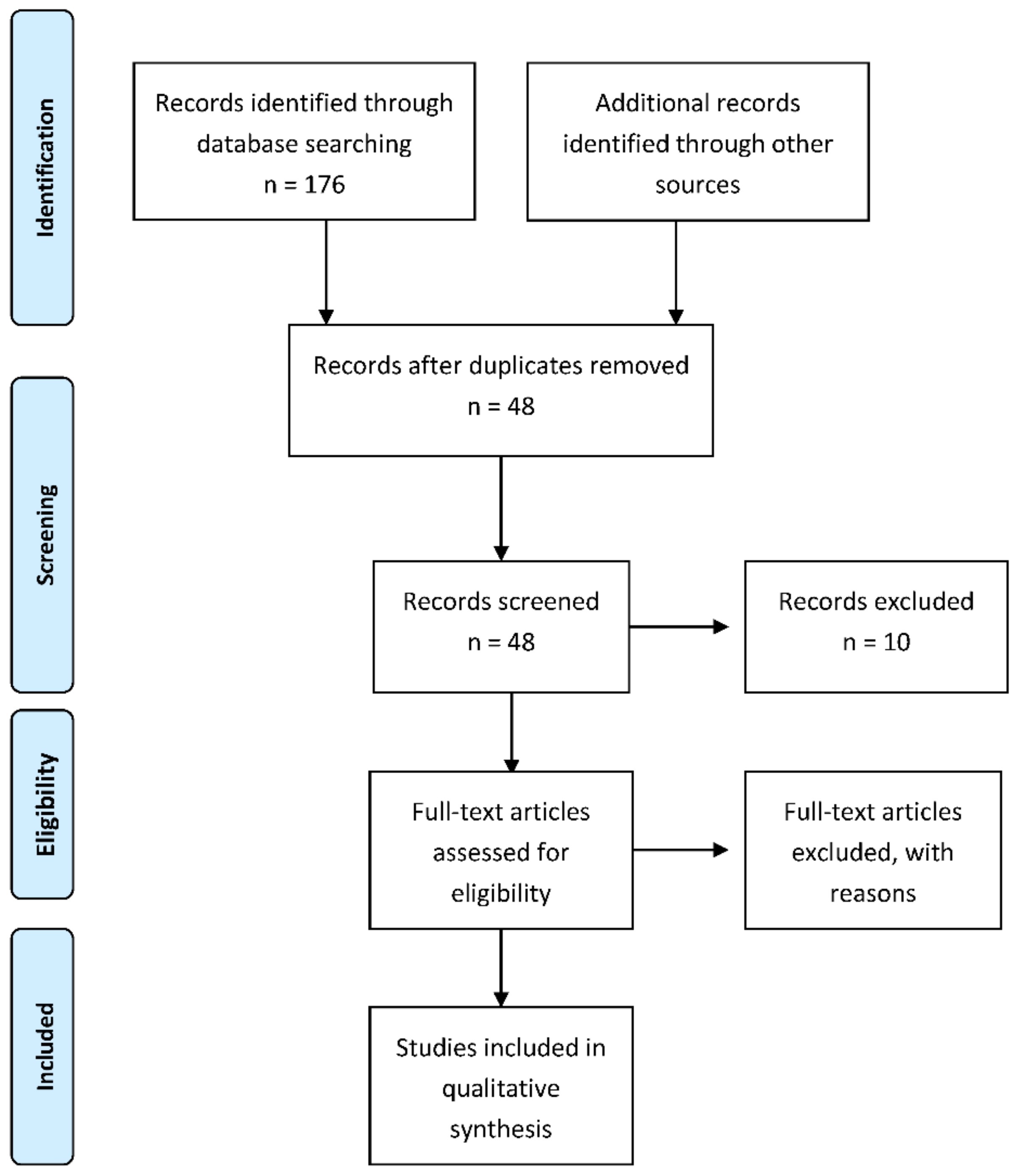
3. Patients and Methods
3.1. Assessment of Methodological Quality
3.2. Outcome Measures
4. Results
| Ref. | Study | Year | Country | Study Design | Analyzed Samples | Total Number of Patients | Treatment Strategy | COX-2 Expression Measurement |
|---|---|---|---|---|---|---|---|---|
| [76] | Pachkoria et al. | 2005 | Sweden | Prospective randomized trial of preoperative radiotherapy | Samples: - distal normal mucosa (n = 28) - adjacent normal mucosa (n = 108) - primary cancer (n = 138) - lymph node metastasis (n = 30) - biopsy (n = 85) | 75 (138—total 75—RTH + surgery 63—surgery alone) | Short-term radiation (radiotherapy 5 × 5 Gy to total dose of 25 Gy) followed by surgery | IHC Western Blott |
| [75] | de Heer et al. | 2007 | Netherlands | Retrospective multicenter randomized clinical trial | Archival tumor material | 1038 (924—RTH + surgery 927—surgery alone) | Short-term radiation (radiotherapy 5 × 5 Gy to total dose of 25 Gy) followed by TME surgery | IHC |
| [77] | Giralt et al. | 2007 | Spain | Retrospective study | Preirridation diagnostic biopsies | 34 (81—total 34—radiotherapy 47—radiochemotherapy) | Long-term radiation (radiotherapy: conventional fractionation 1.8 Gy/day to a total dose of 45 Gy; additionally, boost to 50.4 Gy in 8 cases) followed by TME surgery | IHC |
| [78] | Bouzourene et al. | 2008 | Switzerland | Retrospective multicenter cohort study | Pretherapeutic tumor biopsies (n = 26) and surgical specimens (n = 88) | 104 (88 specimens 26 pretherapeutic biopsies) | Hyperfractionated radiotherapy (HART) followed by surgery (APR or low anterior resection) | IHC |
| [79] | Wen et al. | 2020 | Sweden | Randomized clinical trial | Surgical samples | 219 (127—RTH + surgery 92—surgery alone) | Radiotherapy (25 Gy in 5 fractions during a median of 6 days (range, 5–12)) followed by surgery | IHC |
| Ref. | Study | Year | Country | Study Design | Analyzed Samples | Total Number of Patients | Treatment Strategy | COX-2 Expression Measurement |
|---|---|---|---|---|---|---|---|---|
| [80] | Yeoh et al. | 2005 | Australia | Retrospective study | Samples obtained from patiets treated with preoperative radiotherapy | 28 |
| IHC |
| [81] | Smith et al. | 2006 | Ireland | Retrospective and prospective study | Pretreatment specimens | 49 |
| IHC |
| [77] | Giralt et al. | 2007 | Spain | Retrospective study | Preirridation diagnostic biopsies | 47 (81—total 34—radiotherapy 47—radiochemotherapy) |
| IHC |
| [82] | Min et al. | 2008 | Korea | Prospective study | Pretreatment biopsy specimens | 30 |
| IHC |
| [83] | Edden et al. | 2010 | USA | Retrospective study | Preatrement and surgical specimens | 152 |
| IHC |
| [84] | Peng et al. | 2016 | China | Retrospective study | Pretreatment biopsies | 82 |
| IHC |
| [85] | Jafarian et al. | 2016 | Iran | Retrospective cohort study | Pretreatment specimens | 55 |
| |
| [86] | Sole et al. | 2016 | Spain | Prospective study | Pretreatment endoscopic biopsy and surgical specimens | 38 |
| IHC |
| [87] | Shinto et al. | 2020 | Japan | Retrospective and prospective study | Pretreatment biopsies | 144 (95 in the retrospective study 49 in the prospective study) | In the retrospective study:
In the prospective study:
| IHC |
| Ref. | Study | Total Number of Patients | Male | Female | Median Age | Age Range |
|---|---|---|---|---|---|---|
| [75] | de Heer et al. | 1038 (924—RTH + surgery, 927—surgery alone) | 573 | 324 | 65 | 26–88 |
| [78] | Bouzourene et al. | 104 | ND | ND | 63 | 28–85 |
| [76] | Pachkoria et al. | 75 | 40 | 23 | 67 | 36–85 |
| [81] | Smith et al. | 49 | 31 | 18 | ND | ND |
| [77] | Giralt et al. | 81 | 54 | 27 | 64.8 | 34–92 |
| [83] | Edden et al. | 152 | 77 | 75 | 58.1 | 31–82 |
| [80] | Yeoh et al. | 28 | 21 | 7 | ND | ND |
| [82] | Min et al. | 30 | 26 | 4 | 48.0 | 31–69 |
| [87] | Shinto et al. | 144 | 100 | 44 | 61,8 | ND |
| [79] | Wen et al. | 219 (127—RTH+ surgery, 92—surgery alone) | ND | ND | ND | ND |
| [84] | Peng et al. | 82 | 57 | 25 | 57 | 15–75 |
| [86] | Sole et al. | 38 | 27 | 11 | 62 | 43–77 |
| [85] | Jafarian et al. | 55 | 27 | 18 | 52 | 18–87 |
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Goradel, N.H.; Najafi, M.; Salehi, E.; Farhood, B.; Mortezaee, K. Cyclooxygenase-2 in cancer: A review. J. Cell. Physiol. 2019, 234, 5683–5699. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, T.; Ohta, T.; Oyama, K.; Miyashita, T.; Miwa, K. Role of cyclooxygenase-2 in the carcinogenesis of gastrointestinal tract cancers: A review and report of personal experience. World J. Gastroenterol. 2006, 12, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Simmons, D.L.; Botting, R.M.; Hla, T. Cyclooxygenase isozymes: The biology of prostaglandin synthesis and inhibition. Pharmacol. Rev. 2004, 56, 387–437. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, F.A. Cyclooxygenase enzymes: Regulation and function. Curr. Pharm. Des. 2004, 10, 577–588. [Google Scholar] [CrossRef]
- Jackson, L.M.; Wu, K.C.; Mahida, Y.R.; Jenkins, D.; Hawkey, C.J. Cyclooxygenase (COX) 1 and 2 in normal, inflamed, and ulcerated human gastric mucosa. Gut 2000, 47, 762–770. [Google Scholar] [CrossRef]
- Davies, N.M.; Good, R.L.; Roupe, K.A.; Yáñez, J.A. Cyclooxygenase-3: Axiom, dogma, anomaly, enigma or splice error?—Not as easy as 1, 2, 3. J. Pharm. Pharm. Sci. 2004, 7, 217–226. [Google Scholar]
- Liu, B.; Qu, L.; Yan, S. Cyclooxygenase-2 promotes tumor growth and suppresses tumor immunity. Cancer Cell Int. 2015, 15, 106. [Google Scholar] [CrossRef]
- Stasinopoulos, I.; Shah, T.; Penet, M.-F.; Krishnamachary, B.; Bhujwalla, Z.M. COX-2 in cancer: Gordian knot or achilles heel? Front. Pharmacol. 2013, 4, 34. [Google Scholar] [CrossRef]
- Saba, N.F.; Choi, M.; Muller, S.; Shin, H.J.C.; Tighiouart, M.; Papadimitrakopoulou, V.A.; El-Naggar, A.K.; Khuri, F.R.; Chen, Z.G.; Shin, D.M. Role of cyclooxygenase-2 in tumor progression and survival of head and neck squamous cell carcinoma. Cancer Prev. Res. 2009, 2, 823–829. [Google Scholar] [CrossRef]
- Marrogi, A.; Pass, H.I.; Khan, M.; Metheny-Barlow, L.J.; Harris, C.C.; Gerwin, B.I. Human mesothelioma samples overexpress both cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (NOS2): In Vitro antiproliferative effects of a COX-2 inhib-itor. Cancer Res. 2000, 60, 3696–3700. [Google Scholar]
- Cervello, M.; Montalto, G. Cyclooxygenases in hepatocellular carcinoma. World J. Gastroenterol. 2006, 12, 5113–5121. [Google Scholar] [CrossRef] [PubMed]
- Hahm, K.-B.; Lim, H.-Y.; Sohn, S.; Kwon, H.-J.; Lee, K.-M.; Lee, J.-S.; Surh, Y.-J.; Kim, Y.-B.; Joo, H.-J.; Kim, W.-S.; et al. In Vitro evidence of the role of COX-2 in attenuating gastric inflammation and promoting gastric carcinogenesis. J. Environ. Pathol. Toxicol. Oncol. 2002, 21, 165–176. [Google Scholar] [CrossRef]
- Harris, R.E.; Casto, B.C.; Harris, Z.M. Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J. Clin. Oncol. 2014, 5, 677–692. [Google Scholar] [CrossRef]
- Petkova, D.; Clelland, C.; Ronan, J.; Pang, L.; Coulson, J.; Lewis, S.; Knox, A. Overexpression of cyclooxygenase-2 in non-small cell lung cancer. Respir. Med. 2004, 98, 164–172. [Google Scholar] [CrossRef]
- Crosby, C.G.; Dubois, R.N. The cyclooxygenase-2 pathway as a target for treatment or prevention of cancer. Expert Opin. Emerg. Drugs 2003, 8, 1–7. [Google Scholar] [CrossRef]
- Yu, T.; Lao, X.; Zheng, H. Influencing COX-2 Activity by COX Related Pathways in Inflammation and Cancer. Mini Rev. Med. Chem. 2016, 16, 1230–1243. [Google Scholar] [CrossRef]
- Bakhle, Y.S. COX-2 and cancer: A new approach to an old problem. Br. J. Pharmacol. 2001, 134, 1137–1150. [Google Scholar] [CrossRef]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef]
- Liu, C.H.; Chang, S.-H.; Narko, K.; Trifan, O.C.; Wu, M.-T.; Smith, E.; Haudenschild, C.; Lane, T.F.; Hla, T. Overexpression of Cyclooxygenase-2 is sufficient to induce tumorigenesis in transgenic mice. J. Biol. Chem. 2001, 276, 18563–18569. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Sun, H.; Yu, F.-B.; Li, B.; Zhang, Y.; Zhu, Y.-T. The role of cyclooxygenase-2 in colorectal cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; DuBois, R.N. The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 2009, 29, 781–788. [Google Scholar] [CrossRef]
- Roelofs, H.M.; Morsche, R.H.T.; Van Heumen, B.W.; Nagengast, F.M.; Peters, W.H. Over-expression of COX-2 mRNA in colorectal cancer. BMC Gastroenterol. 2014, 14, 1. [Google Scholar] [CrossRef]
- Soslow, R.A.; Dannenberg, A.J.; Rush, D.; Woerner, B.M.; Khan, K.N.; Masferrer, J.; Koki, A.T. COX-2 is expressed in human pulmonary, colonic, and mammary tumors. Cancer 2000, 89, 2637–2645. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, X.F. Overexpression of cyclooxygenase-2 correlates with advanced stages of colorectal cancer. Am. J. Gastroen-terol. 2002, 97, 1037–1041. [Google Scholar] [CrossRef] [PubMed]
- Eberhart, C.E.; Coffrey, R.J.; Radhika, A.; Giardiello, F.M.; Ferrenbach, S.; DuBois, R.N. Up-regulation of cyclooxygenase 2 gene ex-pression in human colorectal adenomas and adenocarcinomas. Gastroenterology 1994, 107, 1183–1188. [Google Scholar] [CrossRef]
- Masferrer, J.L.; Leahy, K.M.; Koki, A.T.; Zweifel, B.S.; Settle, S.L.; Woerner, B.M.; Edwards, D.A.; Flickinger, A.G.; Moore, R.J.; Seibert, K. Antiangiogenic and antitumor activities of cy-clooxygenase-2 inhibitors. Cancer Res. 2000, 60, 1306–1311. [Google Scholar] [PubMed]
- Sheehan, K.M.; Sheahan, K.; O’Donoghue, D.P.; MacSweeney, F.; Conroy, R.M.; Fitzgerald, D.J.; Murray, F.E. The relationship between cy-clooxygenase-2 expression and colorectal cancer. JAMA 1999, 282, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Cianchi, F.; Cortesini, C.; Bechi, P.; Fantappie, O.; Messerini, L.; Vannacci, A.; Sardi, I.; Baroni, G.; Boddi, V.; Mazzanti, R.; et al. Upregulation of cyclooxygenase 2 gene expres-sion correlates with tumor angiogenesis in human colorectal cancer. Gastroenterology 2001, 121, 1339–1347. [Google Scholar] [CrossRef]
- Markman, B.; Ramos, F.J.; Capdevila, J.; Tabernero, J. EGFR and KRAS in colorectal cancer. Adv. Virus Res. 2010, 51, 71–119. [Google Scholar] [CrossRef]
- Nakayama, M.; Oshima, M. Mutant p53 in colon cancer. J. Mol. Cell Biol. 2019, 11, 267–276. [Google Scholar] [CrossRef]
- Nojadeh, J.N.; Sharif, S.B.; Sakhinia, E. Microsatellite instability in colorectal cancer. EXCLI J. 2018, 17, 159–168. [Google Scholar] [CrossRef]
- Dinu, D.; Dobre, M.; Panaitescu, E.; Bîrlă, R.; Iosif, C.; Hoara, P.; Caragui, A.; Boeriu, M.; Constantinoiu, S.; Ardeleanu, C. Prognostic significance of KRAS gene mutations in colorectal cancer—preliminary study. J. Med. Life 2015, 7, 581–587. [Google Scholar]
- Wang, P.; Liang, J.; Wang, Z.; Hou, H.; Shi, L.; Zhou, Z. The prognostic value of p53 positive in colorectal cancer: A retrospective cohort study. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.-W.; Chen, Y.-T.; Tsai, H.-L.; Yeh, Y.-S.; Su, W.-C.; Ma, C.-J.; Tsai, T.-N.; Wang, J.-Y. EGFR expression in patients with stage III colorectal cancer after adjuvant chemotherapy and on cancer cell function. Oncotarget 2017, 8, 114663–114676. [Google Scholar] [CrossRef]
- Kang, S.; Na, Y.; Joung, S.Y.; Lee, S.I.; Oh, S.C.; Min, B.W. The significance of microsatellite instability in colorectal cancer after controlling for clinicopathological factors. Medicine 2018, 97, e0019. [Google Scholar] [CrossRef]
- Kunzmann, A.T.; Murray, L.J.; Cardwell, C.; McShane, C.M.; McMenamin, Ú.; Cantwell, M.M. PTGS2 (cyclooxygenase-2) expression and survival among colorectal cancer patients: A systematic review. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Su, C.-W.; Zhang, Y.; Zhu, Y.-T. Stromal COX-2 signaling are correlated with colorectal cancer: A review. Crit. Rev. Oncol. 2016, 107, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Negi, R.R.; Rana, S.V.; Gupta, V.; Gupta, R.; Chadha, V.D.; Prasad, K.K.; Dhawan, D.K. Over-expression of cyclooxygenase-2 in colorectal cancer patients. Asian Pac. J. Cancer Prev. 2019, 20, 1675–1681. [Google Scholar] [CrossRef] [PubMed]
- Anania, G.; Resta, G.; Marino, S.; Fabbri, N.; Scagliarini, L.; Marchitelli, I.; Fiorica, F.; Cavallesco, G. Treatment of colorectal cancer: A multidisciplinary approach. J. Gastrointest. Cancer 2019, 50, 458–468. [Google Scholar] [CrossRef]
- Xue, L.; Williamson, A.; Gaines, S.; Andolfi, C.; Paul-Olson, T.; Neerukonda, A.; Steinhagen, E.; Smith, R.; Cannon, L.; Polite, B.; et al. An update on colorectal cancer. Curr. Probl. Surg. 2018, 55, 76–116. [Google Scholar] [CrossRef]
- Mirnezami, R.; Chang, G.J.; Das, P.; Chandrakumaran, K.; Tekkis, P.; Darzi, A.; Mirnezami, A.H. Intraoperative radiotherapy in colorectal cancer: Systematic review and meta-analysis of techniques, long-term outcomes, and complications. Surg. Oncol. 2013, 22, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A. Targeting cyclooxygenase-2 for prevention and therapy of colorectal cancer. Mol. Carcinog. 2006, 45, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Sinicrope, F.A.; Gill, S. Role of cyclooxygenase-2 in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Moshkowitz, M.; Arber, N. Cyclo-oxygenase-2 inhibitors in colorectal cancer prevention. Am. J. Cancer 2006, 5, 357–362. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Fuloria, J.; Prakash, O. Cyclooxygenase 2 inhibitors and colon cancer. Ochsner J. 2002, 4, 176–179. [Google Scholar] [PubMed]
- Rahman, M.; Selvarajan, K.; Hasan, M.R.; Chan, A.P.; Jin, C.; Kim, J.; Chan, S.K.; Le, N.D.; Kim, Y.-B.; Tai, I.T. Inhibition of COX-2 in colon cancer modulates tumor growth and MDR-1 expression to enhance tumor regression in therapy-refractory cancers In Vivo. Neoplasia 2012, 14, 624-IN18. [Google Scholar] [CrossRef]
- Hidalgo-Estévez, A.M.; Stamatakis, K.; Jiménez-Martínez, M.; López-Pérez, R.; Fresno, M. Cyclooxygenase 2-regulated genes an alternative avenue to the development of new therapeutic drugs for colorectal cancer. Front. Pharmacol. 2020, 11, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Kaiser, H.E.; Boulware, D.; Hakam, A.; Zhao, H.; Yeatman, T.; Barthel, J.; Coppola, D. Cyclooxygenase-2 expression in right- and left-sided colon cancer: A rationale for optimization of cyclooxygenase-2 inhibitor therapy. Clin. Color. Cancer 2004, 3, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Abate, D.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdel-Rahman, O.; Abdelalim, A.; Abdoli, A.; Abdollahpour, I.; Abdulle, A.S.; et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol. 2019, 5, 1749–1768. [Google Scholar]
- Smalley, W.; Ray, W.A.; Daugherty, J.; Griffin, M.R. Use of nonsteroidal anti-inflammatory drugs and incidence of colorectal cancer. Arch. Intern. Med. 1999, 159, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Pardini, B.; Kumar, R.; Naccarati, A.; Novotny, J.; Prasad, R.; Forsti, A.; Hemminki, K.; Vodicka, P.; Bermejo, J.L. 5-Fluorouracil-based chemotherapy for colorectal cancer and MTHFR/MTRR genotypes. Br. J. Clin. Pharmacol. 2010, 72, 162–163. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Kubota, Y.; Ishida, H.; Sasaki, Y. Irinotecan, a key chemotherapeutic drug for metastatic colorectal cancer. World J. Gastroenterol. 2015, 21, 12234–12248. [Google Scholar] [CrossRef]
- Zafar, S.Y.; Hirsch, B.R. Capecitabine in the management of colorectal cancer. Cancer Manag. Res. 2011, 3, 79–89. [Google Scholar] [CrossRef][Green Version]
- Comella, P. Role of oxaliplatin in the treatment of colorectal cancer. Ther. Clin. Risk Manag. 2009, 5, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Burness, C.B.; Duggan, S.T. Trifluridine/tipiracil: A review in metastatic colorectal cancer. Drugs 2016, 76, 1393–1402. [Google Scholar] [CrossRef]
- Coupez, D.; Hulo, P.; Touchefeu, Y.; Bossard, C.; Bennouna, J. Pembrolizumab for the treatment of colorectal cancer. Expert Opin. Biol. Ther. 2020, 20, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Desai, J. Nivolumab for the treatment of colorectal cancer. Expert Rev. Anticancer. Ther. 2018, 18, 611–618. [Google Scholar] [CrossRef]
- Morse, M.A.; Overman, M.J.; Hartman, L.; Khoukaz, T.; Brutcher, E.; Lenz, H.; Atasoy, A.; Shangguan, T.; Zhao, H.; El-Rayes, B. Safety of nivolumab plus low-dose ipilimumab in previously treated microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer. Oncologist 2019, 24, 1453–1461. [Google Scholar] [CrossRef]
- Wolmark, N.; Wieand, H.S.; Hyams, D.M.; Colangelo, L.; Dimitrov, N.V.; Romond, E.H.; Wexler, M.; Prager, D.; Cruz, A.B.; Gordon, P.H.; et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National surgical adjuvant breast and bowel project protocol R-02. J. Natl. Cancer Inst. 2000, 92, 388–396. [Google Scholar] [CrossRef]
- Thomas, P.R.; Lindblad, A.S. Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: A review of the gastrointestinal tumor study group experience. Radiother. Oncol. 1988, 13, 245–252. [Google Scholar] [CrossRef]
- Glimelius, B.; Tiret, E.; Cervantes, A.; Arnold, D. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013, 24, vi81–vi88. [Google Scholar] [CrossRef]
- Glimelius, B. Optimal time intervals between pre-operative radiotherapy or chemoradiotherapy and surgery in rectal cancer? Front. Oncol. 2014, 4, 50. [Google Scholar] [CrossRef][Green Version]
- Glynn, L. A critical appraisal tool for library and information research. Libr. Hi Tech 2006, 24, 387–399. [Google Scholar] [CrossRef]
- Lim, S.-C.; Lee, T.-B.; Choi, C.-H.; Ryu, S.-Y.; Kim, K.-J.; Min, Y.-D. Expression of cyclooxygenase-2 and its relationship to p53 accumulation in colorectal cancers. Yonsei Med. J. 2007, 48, 495–501. [Google Scholar] [CrossRef]
- Grösch, S.; Tegeder, I.; Niederberger, E.; Bräutigam, L.; Geisslinger, G. COX-2 independent induction of cell cycle arrest and apoptosis in colon cancer cells by the selective COX-2 inhibitor celecoxib. FASEB J. 2001, 15, 2742–2744. [Google Scholar] [CrossRef]
- Sheng, H.; Shao, J.; Morrow, J.D.; Beauchamp, R.D.; Dubois, R.N. Modulation of apoptosis and Bcl-2 expression by prostaglandin E2 in human colon cancer cells. Cancer Res. 1998, 58, 9443418. [Google Scholar]
- Xiong, B.; Sun, T.-J.; Yuan, H.-Y.; Hu, M.-B.; Hu, W.-D.; Cheng, F.-L. Cyclooxygenase-2 expression and angiogenesis in colorectal cancer. World J. Gastroenterol. 2003, 9, 1237–1240. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; You, K.; Zhang, R.; Xi, S.; Zhang, T.; Dong, J.; Cai, M.; Wang, C.; Zhang, H.; Zhou, T.; et al. Predictive value of APAF-1 and COX-2 expression in pathologic complete response to neoadjuvant chemoradiotherapy for patients with locally advanced rectal adenocarcinoma. Oncotarget 2016, 7, 35233–35240. [Google Scholar] [CrossRef] [PubMed]
- Masunaga, R.; Kohno, H.; Dhar, D.K.; Ohno, S.; Shibakita, M.; Kinugasa, S.; Yoshimura, H.; Tachibana, M.; Kubota, H.; Nagasue, N. Cyclooxygenase-2 expression correlates with tumor neovascularization and prognosis in human colorectal carcinoma patients. Clin. Cancer Res. 2000, 6, 4064–4068. [Google Scholar] [PubMed]
- Sakuma, K.; Fujimori, T.; Hirabayashi, K.; Terano, A. Cyclooxygenase (COX)-2 immunoreactivity and relationship to p53 and Ki-67 expression in colorectal cancer. J. Gastroenterol. 1999, 34, 189–194. [Google Scholar] [CrossRef]
- Chu, A.J.; Chou, T.H.; Chen, B.D. Prevention of colorectal cancer using COX-2 inhibitors: Basic science and clinical applications. Front. Biosci. 2004, 9, 2697–2713. [Google Scholar] [CrossRef]
- Ceccarelli, C.; Piazzi, G.; Paterini, P.; Pantaleo, M.A.; Taffurelli, M.; Santini, D.; Martinelli, G.N.; Biasco, G. Concurrent EGFr and Cox-2 expression in colorectal cancer: Proliferation impact and tumour spreading. Ann. Oncol. 2005, 16, iv74–iv79. [Google Scholar] [CrossRef]
- Nakamoto, R.H.; Uetake, H.; Iida, S.; Kolev, Y.V.; Soumaoro, L.T.; Takagi, Y.; Yasuno, M.; Sugihara, K. Correlations between Cyclooxygenase-2 expression and angiogenic factors in primary tumors and liver metastases in colorectal cancer. Jpn. J. Clin. Oncol. 2007, 37, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Heer, P.D.; Gosens, M.J.; Bruin, E.C.D.; Dekker-Ensink, N.G.; Putter, H.; Marijnen, C.A.; van den Brule, A.J.; van Krieken, J.H.J.; Rutten, H.J.; Kuppen, P.J.; et al. Cyclooxygenase 2 expression in rectal cancer is of prognostic significance in patients receiving preoperative radiotherapy. Clin. Cancer Res. 2007, 13, 2955–2960. [Google Scholar] [CrossRef]
- Pachkoria, K.; Zhang, H.; Adell, G.; Jarlsfelt, I.; Sun, X.-F. Significance of Cox-2 expression in rectal cancers with or without preoperative radiotherapy. Int. J. Radiat. Oncol. 2005, 63, 739–744. [Google Scholar] [CrossRef]
- Giralt, J.; Navalpotro, B.; Hermosilla, E.; De Torres, I.; Espin, E.; Reyes, V.; Cerezo, L.; De Las Heras, M.; y Cajal, S.R.; Armengol, M. Prognostic significance of vascular endothelial growth factor and cyclooxygenase-2 in patients with rectal cancer treated with preoperative radiotherapy. Oncology 2006, 71, 312–319. [Google Scholar] [CrossRef]
- Bouzourene, H.; Yan, P.; Sandmeier, D.; Zouhair, A.; Matter, M.; Vuilleumier, H.; Coucke, P. The role of COX-2 in rectal cancer treated with preoperative radiotherapy. Virchows Arch. 2008, 452, 499–505. [Google Scholar] [CrossRef][Green Version]
- Wen, Y.; Zhao, S.; Holmqvist, A.; Hahn-Stromberg, V.; Adell, G.; Holmlund, B.; Pathak, S.; Peng, Z.; Sun, X.F. Predictive role of biopsy based Bi-omarkers for radiotherapy treatment in rectal cancer. J. Pers. Med. 2020, 13, 168. [Google Scholar]
- Yeoh, A.S.; Bowen, J.M.; Gibson, R.J.; Keefe, D.M. Nuclear factor κB (NFκB) and cyclooxygenase-2 (Cox-2) expression in the irradiated colorectum is associated with subsequent histopathological changes. Int. J. Radiat. Oncol. 2005, 63, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.M.; Reynolds, J.V.; Kay, E.W.; Crotty, P.; Murphy, J.O.; Hollywood, D.; Gaffney, E.F.; Stephens, R.B.; Kennedy, M.J. COX-2 overexpression in pretreatment biopsies predicts response of rectal cancers to neoadjuvant radiochemotherapy. Int. J. Radiat. Oncol. 2006, 64, 466–472. [Google Scholar] [CrossRef]
- Min, B.S.; Choi, Y.J.; Pyo, H.R.; Kim, N.-K.; Seong, J.; Chung, H.C.; Rha, S.Y. Cyclooxygenase-2 expression in pretreatment biopsy as a predictor of tumor responses after preoperative chemoradiation in rectal cancer. Arch. Surg. 2008, 143, 1091. [Google Scholar] [CrossRef]
- Edden, Y.; Wexer, S.D.; Berhof, M. The use of molecular markers as a method to predict the response to neoadjuvant therapy for advanced stage rectal adenocarcinoma. Colorectal Dis. 2011, 14, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Zlobec, I.; Minoo, P.; Baker, K.; Haegert, D.; Khetani, K.; Tornillo, L.; Terracciano, L.; Jass, J.R.; Lugli, A. Loss of APAF-1 expression is associated with tumour progression and adverse prognosis in colorectal cancer. Eur. J. Cancer 2007, 43, 1101–1107. [Google Scholar] [CrossRef]
- Kermani, A.T.; Jafarian, A.; Esmaeili, J.; Roshan, N.; Seilanian-Toosi, M.; Omidi, A.; Shahri, M.K. The role of COX-2 and Ki-67 over-expression in the prediction of pathologic response of rectal cancer to neoadjuvant chemoradiation therapy. Indian J. Cancer 2016, 53, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Sole, C.V.; Calvo, F.A.; Alvarez, E.; Carreras, J.L. Metabolic and molecular relative percentage coreduction in patients with locally advanced rectal cancer treated with neoadjuvant therapy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Shinto, E.; Omata, J.; Sikina, A.; Sekizawa, A.; Kajiwara, Y.; Hayashi, K.; Hashiguchi, Y.; Hase, K.; Ueno, H. Predictive immunohistochemical features for tumour response to chemoradiotherapy in rectal cancer. BJS Open 2020, 4, 301–309. [Google Scholar] [CrossRef]
- Kalinski, P. Regulation of immune responses by prostaglandin E2. J. Immunol. 2011, 188, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Hla, T.; Maciag, T. Cyclooxygenase is an immediate-early gene induced by interleukin-1 in human endothelial cells. J. Biol. Chem. 1990, 265, 10805–10808. [Google Scholar] [CrossRef]
- Hueman, M.; Wang, H.; Henson, D.; Chen, D. Expanding the TNM for cancers of the colon and rectum using machine learning: A demonstration. ESMO Open 2019, 4, e000518. [Google Scholar] [CrossRef]
- Diaconescu, M.; Burada, F. T4 colon cancer—current management. Curr. Health Sci. J. 2018, 44, 5–13. [Google Scholar] [CrossRef]
- Hallak, A.; Alon-Baron, L.; Shamir, R.; Moshkowitz, M.; Bulvik, B.; Brazowski, E.; Halpern, Z.; Arber, N. Rofecoxib reduces polyp recurrence in familial polyposis. Dig. Dis. Sci. 2003, 48, 1998–2002. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Giardiello, F.M. Chemoprevention in familial adenomatous polyposis. Best Pr. Res. Clin. Gastroenterol. 2011, 25, 607–622. [Google Scholar] [CrossRef]
- Wang, L.-W.; Hsiao, C.-F.; Chen, W.T.-L.; Lee, H.-H.; Lin, T.-C.; Chen, H.-C.; Chen, H.-H.; Chien, C.-R.; Lin, T.-Y.; Liu, T.-W. Celecoxib plus chemoradiotherapy for locally advanced rectal cancer: A phase II TCOG study. J. Surg. Oncol. 2014, 109, 580–585. [Google Scholar] [CrossRef]
- Choy, H. Enhancing radiotherapy with cyclooxygenase-2 enzyme inhibitors: A rational advance? J. Natl. Cancer Inst. 2003, 95, 1440–1452. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, N.; Chaki, R.; Mandal, V.; Mandal, S.C. COX-2 as a target for cancer chemotherapy. Pharmacol. Rep. 2010, 62, 233–244. [Google Scholar] [CrossRef]
- Li, S.; Jiang, M.; Wang, L.; Yu, S. Combined chemotherapy with cyclooxygenase-2 (COX-2) inhibitors in treating human cancers: Recent advancement. Biomed. Pharmacother. 2020, 129, 110389. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.-B.; Sun, G.-P. Expression of COX-2 and HER-2 in colorectal cancer and their correlation. World J. Gastroenterol. 2015, 21, 6206–6214. [Google Scholar] [CrossRef] [PubMed]
- Koehne, C.H.; Dubois, R.N. COX-2 inhibition and colorectal cancer. Semin Oncol. 2004, 31, 12–21. [Google Scholar] [CrossRef] [PubMed]
| Region | Preoperative Therapy Description |
|---|---|
| Europe | A short-course radiotherapy—1 week of radiation without chemotherapy (5 Gy × 5) followed by surgery the next week (TME < 10 days from the first radiation fraction. |
| United States and Canada | A long-course chemoradiotherapy—45–50.4 Gy, 1.8–2 Gy/fraction without or with 5-Fluorouracil (5-FU; bolus injections with leucovorin at 6–10 times during the radiation or continuous infusion or oral capecitabine), followed by radical surgery 6–8 weeks later |
| Ref. | Study | TNM Stage | Preoperative Treatment | Type of Resection | Effect of Preoperative Treatment (%) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | I | II | III | IV | pT1 | pT2 | pT3 | pT4 | pN0 | pN1-2 | M | - | Hartmann | Rectal Amputation/Low Anterior | Abdominoperineal Resection | Unknown | Complete Tumor Regression (pCR) | Partial Regression | Absence of Tumor Regression | Overall Survival—OS | ||
| [75] | de Heer et al. | 11 | 265 | 252 | 300 | 61 | RTH | 50 | 579 | 251 | 1 | 82% (at 24 months) | ||||||||||
| [78] | Bouzourene et al. | 2 | 21 | 66 | 14 | RTH | 50 | 51 | 2 | 0% | 79% | 20% | Median—53 months | |||||||||
| [76] | Pachkoria et al. | RTH | 25 | 38 | No data | |||||||||||||||||
| [81] | Smith et al. | 4 | 7 | 26 | 6 | 32 | 16 | CRTH | 39 | 10 | 10% (pCR) + 33% (near pCR) | 22% | 35% | No data | ||||||||
| [77] | Giralt et al. | 6 | 62 | 13 | 48 | 27 | CRTH | 48 | 33 | Median—53 months | ||||||||||||
| [83] | Edden et al. | CRTH | 103 | 46 | 24.5% (pCR) + 15.1% (near pCR) | 39.40% | 21% | No data | ||||||||||||||
| [80] | Yeoh et al. | RTH | No data | |||||||||||||||||||
| [82] | Min et al. | CRTH | 1 | 16 | 16,70% | 50% | 26.70% | No data | ||||||||||||||
| [87] | Shinto et al. | 35 | 98 | 78 | 66 | CRTH | ||||||||||||||||
| [79] | Wen et al. | RTH | ||||||||||||||||||||
| [84] | Peng et al. | Neo-CRTH | 28% | |||||||||||||||||||
| [86] | Sole et al. | Neo-CRTH | ||||||||||||||||||||
| [85] | Jafarian et al. | Neo-CRTH | ||||||||||||||||||||
| Ref. | Study | COX-2 Expression Level | |
|---|---|---|---|
| [75] | De Heer et al. (2015) | Irradiated Specimens: | |
| Absent | 0.4% | ||
| Weak | 12.4% | ||
| Moderate | 59.2% | ||
| Strong | 28% | ||
| [78] | Bouzourene et al. (2008) | Non-irradiated specimens: | |
| Absent | 50% | ||
| Weak | 15.4% | ||
| Moderate | 15.4% | ||
| Strong | 19.2% | ||
| Irradiated specimens: | |||
| Absent | 11% | ||
| Weak | 44% | ||
| Moderate | 28% | ||
| Strong | 17% | ||
| [76] | Pachkoria et al. (2005) | Non-irradiated specimens: | |
| Weak | 22% | ||
| Strong | 51% | ||
| Irradiated specimens: | |||
| Weak | 18% | ||
| Strong | 53% | ||
| [82] | Min et al. (2008) | ND | |
| [81] | Smith et al. (2006) | COX-2 overexpression | Tumor regression grade |
| 0% | Complete | ||
| 10% | Moderate | ||
| 8% | Poor | ||
| 20% | Absent | ||
| [77] | Giralt et al. (2007) | Irradiated specimens: | |
| Absent | 48.6% | ||
| Present | 51.4% | ||
| [83] | Edden et al. (2010) | Pretreatment biopsies: | |
| Weak | 32.9% | ||
| Moderate | 34.9% | ||
| Strong | 32.2% | ||
| [80] | Yeoh et al. (2005) | ND | |
| [87] | Shinto et al. (2020) | Irradiated specimens: | |
| Retrospective cohort: | |||
| Low | 21.1% | ||
| High | 78.9% | ||
| Prospective cohort: | |||
| Low | 30.6% | ||
| High | 69.4% | ||
| [79] | Wen et al. (2020) | Non-irradiated specimens: | |
| Absent | 67.7% | ||
| Present | 32.3% | ||
| Irradiated specimens: | |||
| Absent | 52.2% | ||
| Present | 47.8% | ||
| [84] | Peng et al. (2016) | Irradiated specimens: | |
| Low | 58.5% | ||
| High | 41.5% | ||
| [86] | Sole et al. (2016) | ND | |
| [85] | Jafarian et al. (2016) | COX-2 expression was observed in 95.6% of cases with various extent and intensities. | |
| Ref. | Study | COX-2 Expression versus Treatment Effects |
COX-2 Expression versus Tumor Prognosis:
|
|---|---|---|---|
| [75] | De Heer et al. (2015) | High COX-2 expression is an independent poor prognostic factor for disease-free and overall survival in irradiated rectal cancer patients |
|
| [78] | Bouzourene et al. (2008) | (1) Inconclusive data (2) COX-2 is overexpressed in the majority of rectal cancer treated with radiotherapy and it plays a role in local relapse |
|
| [76] | Pachkoria et al. (2005) | COX-2 expression is an early event involved in rectal cancer development |
|
| [82] | Min et al. (2008) | COX-2 overexpression is a predictor of poor tumor regression |
|
| [81] | Smith et al. (2006) | COX-2 overexpression significantly associated with poor response to RCT |
|
| [77] | Giralt et al. (2007) | Value of COX-2 as a biomarker is controversial |
|
| [83] | Edden et al. (2010) | Evaluation of pretreatment COX-2 expression may predict tumor response to neoadjuvant rectal cancer therapy |
|
| [80] | Yeoh et al. (2005) |
| |
| [87] | Shinto et al. (2020) | The expression of COX-2 was significant predictor of tumour response to preoperative RCT. However, expression levels of COX-2 showed no statistical significance. |
|
| [79] | Wen et al. (2020) | The expression of COX-2 had diagnostic value for rectal cancer patients preoperatively (the expression in biopsy sample was higher than that in surgical samples including distance normal mucosa (histologically free from tumor cells), p < 0.05) |
|
| [84] | Peng et al. (2016) | Low expression of COX-2 was associated with achieving the highest pCR rate, which was significantly higher than those with high expression of COX-2. |
|
| [86] | Sole et al. (2016) | No significant differences in COX-2 expression level. |
|
| [85] | Jafarian et al. (2016) | The mean COX-2 immunoreactivity extent in pre-RCT samples was significantly higher in cases with post-RCT biopsies showing >50% necrosis than those with <50% necrosis (p < 0.01) |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berbecka, M.; Forma, A.; Baj, J.; Furtak-Niczyporuk, M.; Maciejewski, R.; Sitarz, R. A Systematic Review of the Cyclooxygenase-2 (COX-2) Expression in Rectal Cancer Patients Treated with Preoperative Radiotherapy or Radiochemotherapy. J. Clin. Med. 2021, 10, 4443. https://doi.org/10.3390/jcm10194443
Berbecka M, Forma A, Baj J, Furtak-Niczyporuk M, Maciejewski R, Sitarz R. A Systematic Review of the Cyclooxygenase-2 (COX-2) Expression in Rectal Cancer Patients Treated with Preoperative Radiotherapy or Radiochemotherapy. Journal of Clinical Medicine. 2021; 10(19):4443. https://doi.org/10.3390/jcm10194443
Chicago/Turabian StyleBerbecka, Monika, Alicja Forma, Jacek Baj, Marzena Furtak-Niczyporuk, Ryszard Maciejewski, and Robert Sitarz. 2021. "A Systematic Review of the Cyclooxygenase-2 (COX-2) Expression in Rectal Cancer Patients Treated with Preoperative Radiotherapy or Radiochemotherapy" Journal of Clinical Medicine 10, no. 19: 4443. https://doi.org/10.3390/jcm10194443
APA StyleBerbecka, M., Forma, A., Baj, J., Furtak-Niczyporuk, M., Maciejewski, R., & Sitarz, R. (2021). A Systematic Review of the Cyclooxygenase-2 (COX-2) Expression in Rectal Cancer Patients Treated with Preoperative Radiotherapy or Radiochemotherapy. Journal of Clinical Medicine, 10(19), 4443. https://doi.org/10.3390/jcm10194443







