Ulipristal Acetate Modifies miRNA Expression in Both Superficial and Basal Layers of the Human Endometrium
Abstract
:1. Introduction
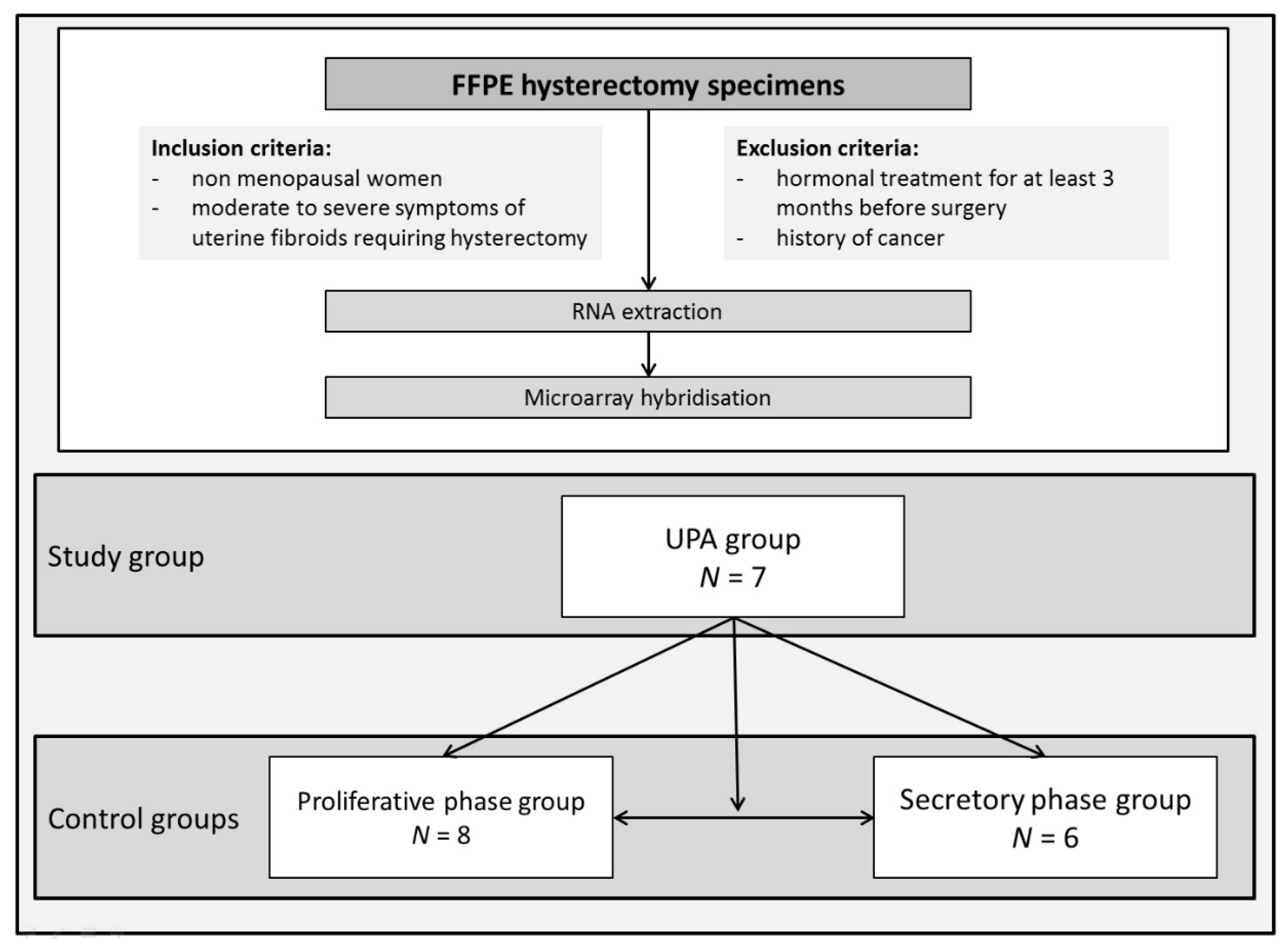
2. Materials and Methods
2.1. Sample Collection
2.2. Endometrial Dating
2.3. RNA Extraction from FFPE Tissues, Microarray Hybridization and DATA Analysis
2.4. Statistical Analysis
3. Results
3.1. Population and Endometrium Characteristics
3.2. Effect of UPA on Global miRNA Expression in Endometrial Samples (GEO: GSE150231)
3.3. Functional Analyses
3.3.1. Gene Ontology Analysis
3.3.2. Modified State of Canonical Pathways
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chabbert-Buffet, N.; Pintiaux, A.; Bouchard, P. The immninent dawn of SPRMs in obstetrics and gynecology. Mol. Cell. Endocrinol. 2012, 358, 232–243. [Google Scholar] [CrossRef]
- Glasier, A.F.; Cameron, S.T.; Fine, P.M.; Logan, S.J.S.; Casale, W.; Van Horn, J.; Sogor, L.; Blithe, D.L.; Scherrer, B.; Mathe, H.; et al. Ulipristal acetate versus levonorgestrel for emergency contraception: A randomised non-inferiority trial and meta-analysis. Lancet 2010, 375, 555–562. [Google Scholar] [CrossRef]
- Luyckx, M.; Squifflet, J.-L.; Jadoul, P.; Votino, R.; Dolmans, M.-M.; Donnez, J. First series of 18 pregnancies after ulipristal acetate treatment for uterine fibroids. Fertil. Steril. 2014, 102, 1404–1409. [Google Scholar] [CrossRef]
- De Gasperis-Brigante, C.; Singh, S.S.; Vilos, G.; Kives, S.; Murji, A. Pregnancy Outcomes Following Ulipristal Acetate for Uterine Fibroids: A Systematic Review. J. Obstet. Gynaecol. Can. 2018, 40, 1066–1076.e2. [Google Scholar] [CrossRef]
- Nogales, F.F.; Crespo-Lora, V.; Cruz-Viruel, N.; Chamorro-Santos, C.; Bergeron, C. Endometrial Changes in Surgical Specimens of Perimenopausal Patients Treated With Ulipristal Acetate for Uterine Leiomyomas. Int. J. Gynecol. Pathol. 2018, 37, 575–580. [Google Scholar] [CrossRef]
- Williams, A.R.W.; Bergeron, C.; Barlow, D.H.; Ferenczy, A. Endometrial morphology after treatment of uterine fibroids with the selective progesterone receptor modulator, ulipristal acetate. Int. J. Gynecol. Pathol. 2012, 31, 556–569. [Google Scholar] [CrossRef]
- Mutter, G.L.; Bergeron, C.; Deligdisch, L.; Ferenczy, A.; Glant, M.; Merino, M.; Williams, A.R.W.; Blithe, D.L. The spectrum of endometrial pathology induced by progesterone receptor modulators. Mod. Pathol. 2008, 21, 591–598. [Google Scholar] [CrossRef]
- Donnez, J.; Tatarchuk, T.F.; Bouchard, P.; Puscasiu, L.; Zakharenko, N.F.; Ivanova, T.; Ugocsai, G.; Mara, M.; Jilla, M.P.; Bestel, E.; et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N. Engl. J. Med. 2012, 366, 409–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szydłowska, I.; Grabowska, M.; Nawrocka-Rutkowska, J.; Piasecka, M.; Starczewski, A. Markers of Cellular Proliferation, Apoptosis, Estrogen/Progesterone Receptor Expression and Fibrosis in Selective Progesterone Receptor Modulator (Ulipristal Acetate)-Treated Uterine Fibroids. J. Clin. Med. 2021, 10, 562. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska, I.; Grabowska, M.; Nawrocka-Rutkowska, J.; Kram, A.; Piasecka, M.; Starczewski, A. Markers of Inflammation and Vascular Parameters in Selective Progesterone Receptor Modulator (Ulipristal Acetate)-Treated Uterine Fibroids. J. Clin. Med. 2021, 10, 3721. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, L.H.R.; Murray, A.A.; Matthews, R.; Shaw, G.; Williams, A.R.W.; Saunders, P.T.K.; Critchley, H.O.D. Selective progesterone receptor modulator (SPRM) ulipristal acetate (UPA) and its effects on the human endometrium. Hum. Reprod. 2017, 32, 531–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lira-Albarrán, S.; Durand, M.; Larrea-Schiavon, M.F.; González, L.; Barrera, D.; Vega, C.; Gamboa-Domínguez, A.; Rangel, C.; Larrea, F. Ulipristal acetate administration at mid-cycle changes gene expression profiling of endometrial biopsies taken during the receptive period of the human menstrual cycle. Mol. Cell. Endocrinol. 2017, 447, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in Control of Gene Expression: An Overview of Nuclear Functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Niu, Z.; Li, Q.; Pang, R.T.K.; Chiu, P.C.N.; Yeung, W.S.-B. MicroRNA and Embryo Implantation. Am. J. Reprod. Immunol. 2016, 75, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Qiao, J.; Wang, L.; Li, L.; Zhen, X.; Liu, P.; Zheng, X. MicroRNA array and microarray evaluation of endometrial receptivity in patients with high serum progesterone levels on the day of hCG administration. Reprod. Biol. Endocrinol. 2011, 9, 29. [Google Scholar] [CrossRef] [Green Version]
- Munro, M.G.; Critchley, H.O.D.; Broder, M.S.; Fraser, I.S.; FIGO Working Group on Menstrual Disorders. FIGO classification system (PALM-COEIN) for causes of abnormal uterine bleeding in nongravid women of reproductive age. Int. J. Gynaecol. Obstet. 2011, 113, 3–13. [Google Scholar] [CrossRef]
- Noyes, R.W.; Hertig, A.T.; Rock, J. Dating the endometrial biopsy. Am. J. Obstet. Gynecol. 1975, 122, 262–263. [Google Scholar] [CrossRef]
- Bouchard, P.; Marraoui, J.; Massai, M.R.; Medalie, D.A.; De Ziegler, D.; Perrot-Applanat, M.; Frydman, R.; Bergeron, C. Immunocytochemical localization of oestradiol and progesterone receptors in human endometrium: A tool to assess endometrial maturation. Bailliere’s Clin. Obstet. Gynaecol. 1991, 5, 107–115. [Google Scholar] [CrossRef]
- Kolanska, K.; Varinot, J.; Canlorbe, G.; Bergeron, C.; Mekinian, A.; Capmas, P.; Koskas, M.; Daraï, E.; Aractingi, S.; Bendifallah, S.; et al. Absence of predictable long-term molecular effect of ulipristal acetate (UPA) on the endometrium. Reprod. Biomed. Online 2019, 38, 825–834. [Google Scholar] [CrossRef]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Irizarry, R.A.; Hobbs, B.; Collin, F.; Beazer-Barclay, Y.D.; Antonellis, K.J.; Scherf, U.; Speed, T.P. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003, 4, 249–264. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Shen, H.; Fan, L.-J.; Guan, J.; Zheng, X.-B.; Chen, X.; Liang, R.; Zhang, X.-W.; Cui, Q.-H.; Sun, K.-K.; et al. Endometrial MicroRNA Signature during the Window of Implantation Changed in Patients with Repeated Implantation Failure. Chin. Med. J. 2017, 130, 566–573. [Google Scholar] [CrossRef]
- Choi, Y.; Kim, H.-R.; Lim, E.J.; Park, M.; Yoon, J.A.; Kim, Y.S.; Kim, E.-K.; Shin, J.-E.; Kim, J.H.; Kwon, H.; et al. Integrative Analyses of Uterine Transcriptome and MicroRNAome Reveal Compromised LIF-STAT3 Signaling and Progesterone Response in the Endometrium of Patients with Recurrent/Repeated Implantation Failure (RIF). PLoS ONE 2016, 11, e0157696. [Google Scholar] [CrossRef] [Green Version]
- Hiroki, E.; Akahira, J.-I.; Suzuki, F.; Nagase, S.; Ito, K.; Suzuki, T.; Sasano, H.; Yaegashi, N. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas. Cancer Sci. 2010, 101, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Jurcevic, S.; Olsson, B.; Klinga-Levan, K. MicroRNA expression in human endometrial adenocarcinoma. Cancer Cell Int. 2014, 14, 88. [Google Scholar] [CrossRef] [PubMed]
- Kolanska, K.; Bendifallah, S.; Canlorbe, G.; Mekinian, A.; Touboul, C.; Aractingi, S.; Chabbert-Buffet, N.; Daraï, E. Role of miRNAs in Normal Endometrium and in Endometrial Disorders: Comprehensive Review. J. Clin. Med. 2021, 10, 3457. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, P.; Chabbert-Buffet, N.; Fauser, B.C.J.M. Selective progesterone receptor modulators in reproductive medicine: Pharmacology, clinical efficacy and safety. Fertil. Steril. 2011, 96, 1175–1189. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Péault, B.; Yan, G.; Sun, H.; Hu, Y.; Ding, L. Stem Cells and Endometrial Regeneration: From Basic Research to Clinical Trial. Curr. Stem Cell Res. Ther. 2019, 14, 293–304. [Google Scholar] [CrossRef]
- Gaide Chevronnay, H.P.; Galant, C.; Lemoine, P.; Courtoy, P.J.; Marbaix, E.; Henriet, P. Spatiotemporal coupling of focal extracellular matrix degradation and reconstruction in the menstrual human endometrium. Endocrinology 2009, 150, 5094–5105. [Google Scholar] [CrossRef] [Green Version]
- Xi, Y.; Nakajima, G.; Gavin, E.; Morris, C.G.; Kudo, K.; Hayashi, K.; Ju, J. Systematic analysis of microRNA expression of RNA extracted from fresh frozen and formalin-fixed paraffin-embedded samples. RNA 2007, 13, 1668–1674. [Google Scholar] [CrossRef] [Green Version]
- Howe, K. Extraction of miRNAs from Formalin-Fixed Paraffin-Embedded (FFPE) Tissues. Methods Mol. Biol. 2017, 1509, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Becker, M.; Schumann, T.; Speer, T.; Fehlmann, T.; Keller, A.; Meese, E. Bias in recent miRBase annotations potentially associated with RNA quality issues. Sci. Rep. 2017, 7, 5162. [Google Scholar] [CrossRef] [PubMed]

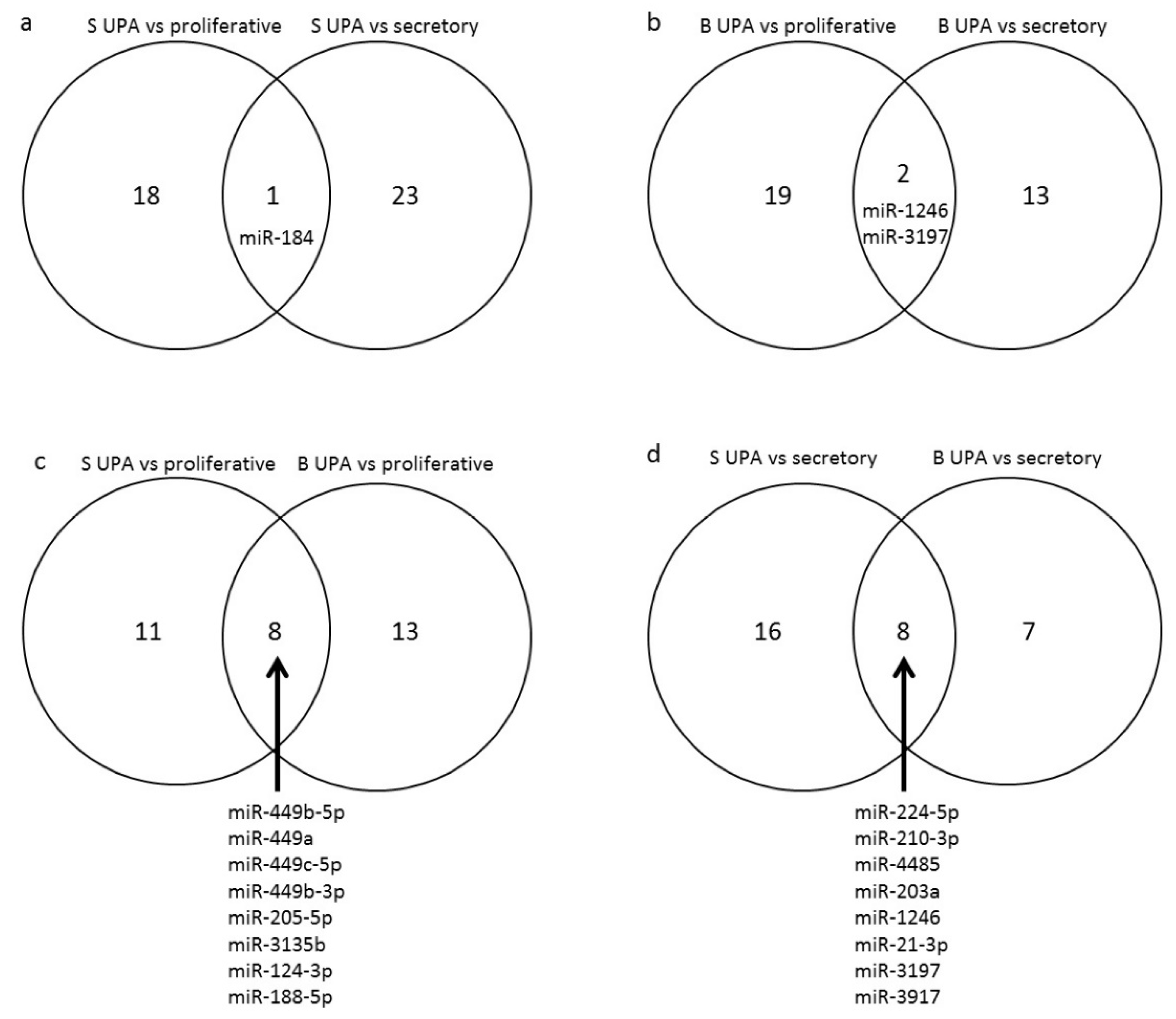
| miRNA | p | FC | Number of Experimentally Proven * Regulated mRNA | |||
|---|---|---|---|---|---|---|
| Superficial layer | Different from proliferative | Up-regulated | hsa-miR-184 | 0.016 | 3.79 | 3 |
| hsa-miR-3613-5p | 0.044 | 3.42 | ||||
| Down-regulated | hsa-miR-449b-5p 2 | 0.003 | −14.97 | 269 | ||
| hsa-miR-449a 1 | 0.011 | −11.72 | ||||
| hsa-miR-449c-5p 3 | 0.013 | −10.83 | ||||
| hsa-miR-449b-3p 4 | 0.010 | −4.42 | ||||
| hsa-miR-205-5p 5 | 0.002 | −4.40 | ||||
| hsa-miR-3135b 6 | 0.011 | −2.85 | ||||
| hsa-miR-362-5p | 0.032 | −2.59 | ||||
| hsa-miR-370-3p | 0.029 | −2.57 | ||||
| hsa-miR-124-3p 7 | 0.038 | −2.51 | ||||
| hsa-miR-542-5p | 0.033 | −2.50 | ||||
| hsa-miR-663b | 0.003 | −2.47 | ||||
| hsa-miR-188-5p 8 | 0.039 | −2.41 | ||||
| hsa-miR-339-5p | 0.034 | −2.29 | ||||
| hsa-miR-1244 | 0.045 | −2.14 | ||||
| hsa-miR-6728-5p | 0.005 | −2.04 | ||||
| hsa-miR-193b-3p | 0.023 | −2.01 | ||||
| hsa-miR-501-3p | 0.036 | −2.00 | ||||
| Different from secretory | Up-regulated | hsa-miR-184 | 0.020 | 3.99 | 23 | |
| hsa-miR-429 | 0.008 | 2.02 | ||||
| Down-regulated | hsa-miR-224-5p 9 | 0.0003 | −4.93 | 125 | ||
| hsa-miR-210-3p 10 | 0.002 | −3.34 | ||||
| hsa-miR-1298-3p | 0.013 | −3.32 | ||||
| hsa-miR-4485 11 | 0.001 | −3.12 | ||||
| hsa-miR-203a 12 | 0.017 | −3.08 | ||||
| hsa-miR-30b-5p | 0.022 | −2.91 | ||||
| hsa-miR-1247-5p | 0.032 | −2.89 | ||||
| hsa-miR-28-5p | 0.038 | −2.41 | ||||
| hsa-miR-4655-5p | 0.004 | −2.41 | ||||
| hsa-miR-1246 13 | 0.018 | −2.40 | ||||
| hsa-miR-4428 | 0.010 | −2.40 | ||||
| hsa-miR-3163 | 0.000 | −2.39 | ||||
| hsa-miR-21-3p 14 | 0.041 | −2.36 | ||||
| hsa-miR-1973 | 0.044 | −2.27 | ||||
| hsa-miR-6718-5p | 0.013 | −2.25 | ||||
| hsa-miR-4667-5p | 0.017 | −2.25 | ||||
| hsa-miR-30d-5p | 0.006 | −2.22 | ||||
| hsa-miR-4513 | 0.005 | −2.20 | ||||
| hsa-miR-4685-5p | 0.012 | −2.12 | ||||
| hsa-miR-3197 15 | 0.042 | −2.11 | ||||
| hsa-miR-3917 16 | 0.044 | −2.04 | ||||
| hsa-miR-7846-3p | 0.015 | −2.02 | ||||
| Basal layer | Different from proliferative | Down-regulated | hsa-miR-449c-5p 3 | 0.006 | −14.00 | 444 |
| hsa-miR-449b-5p 2 | 0.009 | −10.13 | ||||
| hsa-miR-449a 1 | 0.018 | −9.72 | ||||
| hsa-miR-205-5p 5 | 0.0004 | −5.59 | ||||
| hsa-miR-124-3p 7 | 0.002 | −4.01 | ||||
| hsa-miR-3135b 6 | 0.002 | −3.69 | ||||
| hsa-miR-449b-3p 4 | 0.047 | −3.09 | ||||
| hsa-miR-887-3p | 0.016 | −2.67 | ||||
| hsa-miR-15a-5p | 0.047 | −2.59 | ||||
| hsa-miR-378f | 0.042 | −2.55 | ||||
| hsa-miR-378d | 0.046 | −2.46 | ||||
| hsa-miR-188-5p 8 | 0.037 | −2.44 | ||||
| hsa-miR-7162-3p | 0.022 | −2.36 | ||||
| hsa-miR-500b-3p | 0.030 | −2.30 | ||||
| hsa-miR-125b-2-3p | 0.049 | −2.26 | ||||
| hsa-miR-4788 | 0.005 | −2.25 | ||||
| hsa-miR-1246 13 | 0.019 | −2.23 | ||||
| hsa-miR-3907 | 0.003 | −2.16 | ||||
| hsa-miR-6824-5p | 0.039 | −2.10 | ||||
| hsa-miR-6875-5p | 0.014 | −2.07 | ||||
| hsa-miR-3197 15 | 0.038 | −2.04 | ||||
| Different from secretory | Up-regulated | hsa-miR-196a-5p | 0.0074 | 9.37 | 11 | |
| hsa-miR-3613-5p | 0.0429 | 3.78 | ||||
| hsa-miR-615-3p | 0.0327 | 2.60 | ||||
| Down-regulated | hsa-miR-224-5p 9 | 0.0012 | −4.10 | 103 | ||
| hsa-miR-203a 12 | 0.0325 | −2.73 | ||||
| hsa-miR-210-3p 10 | 0.0108 | −2.66 | ||||
| hsa-miR-4485 11 | 0.0039 | −2.65 | ||||
| hsa-miR-21-3p 14 | 0.0282 | −2.53 | ||||
| hsa-miR-146b-5p | 0.0156 | −2.52 | ||||
| hsa-miR-3917 16 | 0.0102 | −2.51 | ||||
| hsa-miR-1246 13 | 0.0247 | −2.29 | ||||
| hsa-miR-3911 | 0.0034 | −2.28 | ||||
| hsa-miR-3197 15 | 0.0291 | −2.24 | ||||
| hsa-miR-7111-5p | 0.0357 | −2.06 | ||||
| hsa-miR-4669 | 0.0169 | −2.01 | ||||
| UPA Action on miRNA | Embryo Implantation | Endometrial Cancer | |
|---|---|---|---|
| miR-21-3p | down-regulated compared to secretory phase | down-regulated in RIF during implantation window [23] | |
| miR-28-5p | down-regulated compared to secretory phase | up-regulated in endometrial cancer [25] | |
| miR-30b-5p | down-regulated compared to secretory phase | up-regulated in RIF during implantation window [22,23] | |
| miR-188-5p | down-regulated compared to proliferative phase | down-regulated in RIF during implantation window [23] | up-regulated in endometrial cancer compared to normal endometrium [24] |
| miR-196a-5p | up-regulated compared to secretory phase | up-regulated in RIF during implantation window [22] | |
| miR-205-5p | down-regulated compared to proliferative phase | down-regulated in RIF during implantation window [23] | |
| miR-339-5p | down-regulated compared to proliferative phase | up-regulated in endometrial cancer compared to normal endometrium [25] | |
| miR-429 | up-regulated compared to secretory phase | up-regulated in endometrial cancer compared to normal endometrium samples [25] | |
| miR-449a | down-regulated compared to proliferative phase | up-regulated in RIF during implantation window [22] | |
| miR-449b-3p | down-regulated compared to proliferative phase | up-regulated in RIF during implantation window [22] | |
| miR-449b-5p | down-regulated compared to proliferative phase | up-regulated in RIF during implantation window [22] | |
| miR-542-5p | down-regulated compared to proliferative phase | down-regulated in endometrial cancer compared to normal endometrium [25] | |
| miR-663b | down-regulated compared to proliferative phase | up-regulated in endometrial cancer compared to normal endometrium [25] | |
| miR-449c-5p | down-regulated compared to proliferative phase | up-regulated in RIF during implantation window [22] | |
| miR-1246 | down-regulated compared to secretory phase | up-regulated in RIF during implantation window [22,23] | |
| miR-1973 | down-regulated compared to secretory phase | up-regulated in RIF during implantation window [23,24] | |
| miR-4485 | down-regulated compared to secretory phase | up-regulated in RIF during implantation window [22,23] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolanska, K.; Sbeih, M.; Canlorbe, G.; Mekinian, A.; Varinot, J.; Capmas, P.; Koskas, M.; Aractingi, S.; Daraï, E.; Chabbert-Buffet, N. Ulipristal Acetate Modifies miRNA Expression in Both Superficial and Basal Layers of the Human Endometrium. J. Clin. Med. 2021, 10, 4442. https://doi.org/10.3390/jcm10194442
Kolanska K, Sbeih M, Canlorbe G, Mekinian A, Varinot J, Capmas P, Koskas M, Aractingi S, Daraï E, Chabbert-Buffet N. Ulipristal Acetate Modifies miRNA Expression in Both Superficial and Basal Layers of the Human Endometrium. Journal of Clinical Medicine. 2021; 10(19):4442. https://doi.org/10.3390/jcm10194442
Chicago/Turabian StyleKolanska, Kamila, Maria Sbeih, Geoffroy Canlorbe, Arsène Mekinian, Justine Varinot, Perrine Capmas, Martin Koskas, Selim Aractingi, Emile Daraï, and Nathalie Chabbert-Buffet. 2021. "Ulipristal Acetate Modifies miRNA Expression in Both Superficial and Basal Layers of the Human Endometrium" Journal of Clinical Medicine 10, no. 19: 4442. https://doi.org/10.3390/jcm10194442
APA StyleKolanska, K., Sbeih, M., Canlorbe, G., Mekinian, A., Varinot, J., Capmas, P., Koskas, M., Aractingi, S., Daraï, E., & Chabbert-Buffet, N. (2021). Ulipristal Acetate Modifies miRNA Expression in Both Superficial and Basal Layers of the Human Endometrium. Journal of Clinical Medicine, 10(19), 4442. https://doi.org/10.3390/jcm10194442






