Acute-on-Chronic Liver Failure in Cirrhosis
Abstract
:1. Definition of Acute-on-Chronic Liver Failure
2. Clinical Features
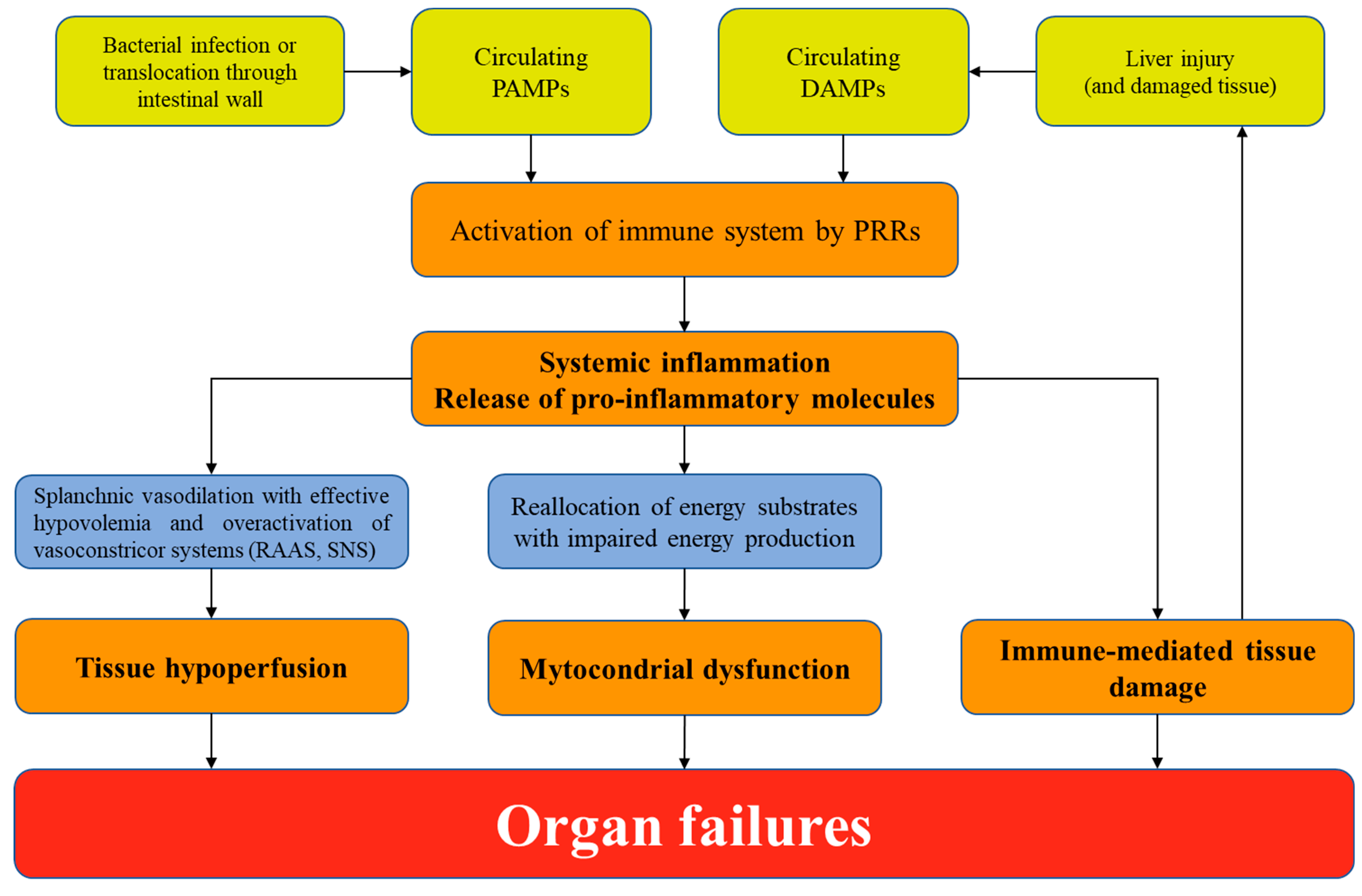
3. Pathophysiology
4. Prognostic Stratification
5. Management of ACLF
5.1. Admission to Intensive Care Unit
| Kidney | Circulation | Coagulation | Lung | Brain | Infections |
|---|---|---|---|---|---|
| Assess AKI severity using ICA Criteria * Taper/withdraw from diuretics and beta-blockers, withdraw from nephrotoxic drugs | Assess hemodynamic state early; consider a MAP ≥ 65 mmHg as target | Assess complete blood count and coagulation tests | Assess respiratory state by using also imaging techniques Calculate PaO2/FiO2 or SpO2/FiO2 | Assess hepatic encephalopathy using West Haven criteria. Identify and treat the underlying cause | Perform a complete work up for infection at ACLF diagnosis |
| Administer albumin (1 g/kg for 48 h) if AKI stage > 1a * to volume expansion; if HRS-AKI, administer terlipressin by continuos infusion (2 mg/24 h) and albumin (20/40 g/day) | Administer crystalloids and 5% albumin as resuscitation fluids; norephinephrine as first line vasopressor | Administer platelets (if < 20.000 × 109/L) and fibrinogen (if <1 g/L) if invasive procedures | Administer oxygen and ventilation with lung protective strategy | Administer lactulose and enemas for hepatic encephalopathy. | Administer broad spectrum high-dose antibiotics at ACLF diagnosis and frequently re-assess therapy |
| Consider RRT as bridge to LT | Consider 20% albumin if AKI (see Kidney), SBP, LVP; consider terlipressin if additional agent needed | Consider prophylaxis for DVT in patients without severe coagulopathy | Consider intubation if risk of aspiration (West Haven grade III or IV hepatic encephalopathy) | Consider short-acting sedative agents if necessary | Consider antifungal agents if risk factors for fungal infections |
| Avoid NSAIDs | Avoid starches | Avoid fresh frozen plasma to correct INR if no bleeding | Avoid delay in intubation even if normal blood oxygen level | Avoid deep sedation and benzodiazepines | Avoid delay in antibiotics administration |
5.2. Treating Organ Failures
6. Treating the Precipitating Event
6.1. Bacterial or Fungal Infection
6.2. Alcoholic Hepatitis
6.3. Acute Variceal Haemorrhage
6.4. Hepatitis B Virus Reactivation
6.5. Liver Transplantation
6.6. Extracorporeal Liver Support
6.7. Granulocyte-Colony Stimulating Factor
6.8. Human Allogeneic Liver-Derived Progenitor Cells
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- D’Amico, G.; Garcia-Tsao, G.; Pagliaro, L. Natural history and prognostic indicators of survival in cirrhosis: A systematic review of 118 studies. J. Hepatol. 2006, 44, 217–231. [Google Scholar] [CrossRef]
- Moreau, R.; Jalan, R.; Gines, P.; Pavesi, M.; Angeli, P.; Cordoba, J.; Durand, F.; Gustot, T.; Saliba, F.; Domenicali, M.; et al. Acute-on-chronic liver failure is a distinct syndrome that develops in patients with acute decompensation of cirrhosis. Gastroenterology 2013, 144, 1426–1437.e9. [Google Scholar] [CrossRef]
- Arroyo, V.; Moreau, R.; Jalan, R. Acute-on-chronic liver failure. N. Engl. J. Med. 2020, 382, 2137–2145. [Google Scholar] [CrossRef] [PubMed]
- Zaccherini, G.; Weiss, E.; Moreau, R. Acute-on-chronic liver failure: Definitions, pathophysiology and principles of treatment. JHEP Rep. 2020, 3, 100176. [Google Scholar] [CrossRef]
- Bajaj, J.S.; O’Leary, J.G.; Reddy, K.R.; Wong, F.; Biggins, S.W.; Patton, H.; Fallon, M.B.; Garcia-Tsao, G.; Maliakkal, B.; Malik, R.; et al. Survival in infection-related acute-on-chronic liver failure is defined by extrahepatic organ failures. Hepatology 2014, 60, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Li, J.; Shao, L.; Xin, J.; Jiang, L.; Zhou, Q.; Shi, D.; Jiang, J.; Sun, S.; Jin, L.; et al. Development of diagnostic criteria and a prognostic score for hepatitis B virus-related acute-on-chronic liver failure. Gut 2017, 67, 2181–2191. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, A.; Almeida, J.A.; Chawla, Y.K.; Fan, S.T.; Garg, H.; De Silva, H.J.; Hamid, S.S.; Jalan, R.; Komolmit, P.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the study of the liver (APASL). Hepatol. Int. 2009, 3, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Sarin, S.K.; Party, T.A.A.W.; Kedarisetty, C.K.; Abbas, Z.; Amarapurkar, D.; Bihari, C.; Chan, A.C.; Chawla, Y.K.; Dokmeci, A.K.; Garg, H.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific Association for the Study of the Liver (APASL) 2014. Hepatol. Int. 2014, 8, 453–471. [Google Scholar] [CrossRef]
- Sarin, S.K.; APASL ACLF Research Consortium (AARC) for APASL ACLF working Party; Choudhury, A.; Sharma, M.K.; Maiwall, R.; Al Mahtab, M.; Rahman, S.; Saigal, S.; Saraf, N.; Soin, A.S.; et al. Acute-on-chronic liver failure: Consensus recommendations of the Asian Pacific association for the study of the liver (APASL): An update. Hepatol. Int. 2019, 13, 353–390. [Google Scholar] [CrossRef] [Green Version]
- Hernaez, R.; Kramer, J.R.; Liu, Y.; Tansel, A.; Natarajan, Y.; Hussain, K.B.; Ginès, P.; Solà, E.; Moreau, R.; Gerbes, A.; et al. Prevalence and short-term mortality of acute-on-chronic liver failure: A national cohort study from the USA. J. Hepatol. 2019, 70, 639–647. [Google Scholar] [CrossRef]
- Piano, S.; Tonon, M.; Vettore, E.; Stanco, M.; Pilutti, C.; Romano, A.; Mareso, S.; Gambino, C.; Brocca, A.; Sticca, A.; et al. Incidence, predictors and outcomes of acute-on-chronic liver failure in outpatients with cirrhosis. J. Hepatol. 2017, 67, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Fernández, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Giovo, I.; Uschner, F.E.; Jimenez, C.; Mookerjee, R.; et al. The PREDICT study uncovers three clinical courses of acutely decompensated cirrhosis that have distinct pathophysiology. J. Hepatol. 2020, 73, 842–854. [Google Scholar] [CrossRef] [PubMed]
- Trebicka, J.; Fernandez, J.; Papp, M.; Caraceni, P.; Laleman, W.; Gambino, C.; Giovo, I.; Uschner, F.E.; Jansen, C.; Jimenez, C.; et al. PREDICT identifies precipitating events associated with the clinical course of acutely decompensated cirrhosis. J. Hepatol. 2020, 74, 1097–1108. [Google Scholar] [CrossRef]
- O’Leary, J.G.; Reddy, K.R.; Garcia-Tsao, G.; Biggins, S.W.; Wong, F.; Fallon, M.B.; Subramanian, R.M.; Kamath, P.S.; Thuluvath, P.; Vargas, H.E.; et al. NACSELD acute-on-chronic liver failure (NACSELD-ACLF) score predicts 30-day survival in hospitalized patients with cirrhosis. Hepatology 2018, 67, 2367–2374. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Thuluvath, P.J. EASL-CLIF criteria outperform NACSELD criteria for diagnosis and prognostication in ACLF. J. Hepatol. 2021, in press. [Google Scholar] [CrossRef]
- Moreau, R.; Gao, B.; Papp, M.; Bañares, R.; Kamath, P.S. Acute-on-chronic liver failure: A distinct clinical syndrome. J. Hepatol. 2021, 75, S27–S35. [Google Scholar] [CrossRef]
- Jalan, R.; Saliba, F.; Pavesi, M.; Amoros, A.; Moreau, R.; Ginès, P.; Levesque, E.; Durand, F.; Angeli, P.; Caraceni, P.; et al. Development and validation of a prognostic score to predict mortality in patients with acute-on-chronic liver failure. J. Hepatol. 2014, 61, 1038–1047. [Google Scholar] [CrossRef]
- Chen, T.; Yang, Z.; Choudhury, A.K.; Al Mahtab, M.; Li, J.; Chen, Y.; Tan, S.-S.; Han, T.; Hu, J.; Hamid, S.S.; et al. Complications constitute a major risk factor for mortality in hepatitis B virus-related acute-on-chronic liver failure patients: A multi-national study from the Asia–Pacific region. Hepatol. Int. 2019, 13, 695–705. [Google Scholar] [CrossRef]
- Arroyo, V.; Angeli, P.; Moreau, R.; Jalan, R.; Clària, J.; Trebicka, J.; Fernández, J.; Gustot, T.; Caraceni, P.; Bernardi, M. The systemic inflammation hypothesis: Towards a new paradigm of acute decompensation and multiorgan failure in cirrhosis. J. Hepatol. 2020, 74, 670–685. [Google Scholar] [CrossRef]
- Jalan, R.; D’Amico, G.; Trebicka, J.; Moreau, R.; Angeli, P.; Arroyo, V. New clinical and pathophysiological perspectives defining the trajectory of cirrhosis. J. Hepatol. 2021, 75, S14–S26. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Lucey, M.R.; Mathurin, P.; Morgan, T.R. Alcoholic hepatitis. N. Engl. J. Med. 2009, 360, 2758–2769. [Google Scholar] [CrossRef]
- Cárdenas, A.; Ginès, P.; Uriz, J.; Bessa, X.; Salmerón, J.M.; Mas, A.; Ortega, R.; Calahorra, B.; Heras, D.D.L.; Bosch, J.; et al. Renal failure after upper gastrointestinal bleeding in cirrhosis: Incidence, clinical course, predictive factors, and short-term prognosis. Hepatology 2001, 34, 671–676. [Google Scholar] [CrossRef] [PubMed]
- Moreau, R.; Clària, J.; Aguilar, F.; Fenaille, F.; Lozano, J.J.; Junot, C.; Colsch, B.; Caraceni, P.; Trebicka, J.; Pavesi, M.; et al. Blood metabolomics uncovers inflamma-tion-associated mitochondrial dysfunction as a potential mechanism underlying ACLF. J Hepatol. 2020, 72, 688–701. [Google Scholar] [CrossRef] [PubMed]
- Gustot, T.; Fernandez, J.; Garcia, E.; Morando, F.; Caraceni, P.; Alessandria, C.; Laleman, W.; Trebicka, J.; Elkrief, L.; Hopf, C.; et al. Clinical Course of acute-on-chronic liver failure syndrome and effects on prognosis. Hepatology 2015, 62, 243–252. [Google Scholar] [CrossRef]
- Choudhury, A.; Party, A.A.W.; Jindal, A.; Maiwall, R.; Sharma, M.K.; Sharma, B.C.; Pamecha, V.; Mahtab, M.; Rahman, S.; Chawla, Y.K.; et al. Liver failure determines the outcome in patients of acute-on-chronic liver failure (ACLF): Comparison of APASL ACLF research consortium (AARC) and CLIF-SOFA models. Hepatol. Int. 2017, 11, 461–471. [Google Scholar] [CrossRef]
- Li, J.; Liang, X.; You, S.; Feng, T.; Zhou, X.; Zhu, B.; Luo, J.; Xin, J.; Jiang, J.; Shi, D.; et al. Development and validation of a new prognostic score for hepatitis B virus-related acute-on-chronic liver failure. J. Hepatol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.; Dhiman, R.K.; Singh, V.; Duseja, A.; Taneja, S.; Choudhury, A.; Sharma, M.K.; Eapen, C.E.; Devarbhavi, H.; Al Mahtab, M.; et al. Comparative accuracy of prognostic models for short-term mortality in acute-on-chronic liver failure patients: CAP-ACLF. Hepatol. Int. 2021, 15, 753–765. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Liu, Y.; Cai, M.; Xu, Y.; Xiang, X.; Zhao, G.; Cai, W.; Wang, H.; Wang, W.; Xie, Q. The Use of NACSELD and EASL-CLIF Classification systems of ACLF in the prediction of prognosis in hospitalized patients with cirrhosis. Am. J. Gastroenterol. 2020, 115, 2026–2035. [Google Scholar] [CrossRef]
- Piano, S.; Favaretto, E.; Tonon, M.; Antonelli, G.; Brocca, A.; Sticca, A.; Mareso, S.; Gringeri, E.; Scaroni, C.; Plebani, M.; et al. Including relative adrenal insufficiency in definition and classification of acute-on-chronic liver failure. Clin. Gastroenterol. Hepatol. 2020, 18, 1188–1196.e3. [Google Scholar] [CrossRef]
- Karvellas, C.J.; Garcia-Lopez, E.; Fernandez, J.; Saliba, F.; Sy, E.; Jalan, R.; Pavesi, M.; Gustot, T.; Ronco, J.; Arroyo, V.; et al. Dynamic prognostication in critically ill cirrhotic pa-tients with multiorgan failure in ICUs in Europe and North America: A multicenter analysis. Crit. Care. Med. 2018, 46, 1783–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karvellas, C.; Bagshaw, S.M. Advances in management and prognostication in critically ill cirrhotic patients. Curr. Opin. Crit. Care 2014, 20, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Durand, F.; Roux, O.; Weiss, E.; Francoz, C. Acute-on-chronic liver failure: Where do we stand? Liver Int. 2021, 41, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Weil, D.; METAREACIR Group; Levesque, E.; McPhail, M.; Cavallazzi, R.; Theocharidou, E.; Cholongitas, E.; Galbois, A.; Pan, H.C.; Karvellas, C.J.; et al. Prognosis of cirrhotic patients admitted to intensive care unit: A meta-analysis. Ann. Intensiv. Care 2017, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL clinical practice guidelines for the management of patients with de-compensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [Green Version]
- Angeli, P.; Ginès, P.; Wong, F.; Bernardi, M.; Boyer, T.D.; Gerbes, A.; Moreau, R.; Jalan, R.; Sarin, S.K.; Piano, S.; et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: Revised consensus recommendations of the International Club of Ascites. J. Hepatol. 2015, 62, 968–974. [Google Scholar] [CrossRef] [Green Version]
- Cavallin, M.; Kamath, P.S.; Merli, M.; Fasolato, S.; Toniutto, P.; Salerno, F.; Bernardi, M.; Romanelli, R.G.; Colletta, C.; Salinas, F.; et al. Terlipressin plus albumin versus midodrine and oc-treotide plus albumin in the treatment of hepatorenal syndrome: A randomized trial. Hepatology 2015, 62, 567–574. [Google Scholar] [CrossRef]
- Piano, S.; Schmidt, H.H.; Ariza, X.; Amoros, A.; Romano, A.; Hüsing-Kabar, A.; Solà, E.; Gerbes, A.; Bernardi, M.; Alessandria, C.; et al. Association between grade of acute on chronic liver failure and response to terlipressin and albumin in patients with hepatorenal syndrome. Clin. Gastroenterol. Hepatol. 2018, 16, 1792–1800.e3. [Google Scholar] [CrossRef] [Green Version]
- Boyer, T.D.; Sanyal, A.J.; Garcia-Tsao, G.; Blei, A.; Carl, D.; Bexon, A.S.; Terlipressin Study Group. Predictors of response to terlipressin plus albumin in hepatorenal syndrome (HRS) type 1: Relationship of serum creatinine to hemodynamics. J Hepatol. 2011, 55, 315–321. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Maddukuri, G.; Jaipaul, N.; Cai, C.X. Role of renal replacement therapy in patients with type 1 hepatorenal syndrome receiving combination treatment of vasoconstrictor plus albumin. J. Crit. Care 2015, 30, 969–974. [Google Scholar] [CrossRef]
- Nadim, M.K.; Durand, F.; Kellum, J.A.; Levitsky, J.; O’Leary, J.G.; Karvellas, C.J.; Bajaj, J.S.; Davenport, A.; Jalan, R.; Angeli, P.; et al. Management of the critically ill patient with cirrhosis: A multidisciplinary perspective. J. Hepatol. 2015, 64, 717–735. [Google Scholar] [CrossRef] [Green Version]
- Choudhury, A.; Kedarisetty, C.K.; Vashishtha, C.; Saini, D.; Kumar, S.; Maiwall, R.; Sharma, M.K.; Bhadoria, A.S.; Kumar, G.; Joshi, Y.K.; et al. A randomized trial comparing terlipressin and noradrenaline in patients with cirrhosis and septic shock. Liver Int. 2016, 37, 552–561. [Google Scholar] [CrossRef]
- Fernández, J.; Acevedo, J.; Wiest, R.; Gustot, T.; Amoros, A.; Deulofeu, C.; Reverter, E.; Martínez, J.; Saliba, F.; Jalan, R.; et al. Bacterial and fungal infections in acute-on-chronic liver failure: Prevalence, characteristics and impact on prognosis. Gut 2017, 67, 1870–1880. [Google Scholar] [CrossRef]
- Piano, S.; Singh, V.; Caraceni, P.; Maiwall, R.; Alessandria, C.; Fernandez, J.; Soares, E.C.; Kim, D.J.; Kim, S.E.; Marino, M.; et al. Epidemiology and effects of bacterial infections in patients with cirrhosis worldwide. Gastroenterology 2019, 156, 1368–1380.e10. [Google Scholar] [CrossRef] [Green Version]
- Fernández, J.; Prado, V.; Trebicka, J.; Amoros, A.; Gustot, T.; Wiest, R.; Deulofeu, C.; Garcia, E.; Acevedo, J.; Fuhrmann, V.; et al. Multidrug-resistant bacterial infections in patients with decompensated cirrhosis and with acute-on-chronic liver failure in Europe. J. Hepatol. 2018, 70, 398–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, F.; Piano, S.; Singh, V.; Bartoletti, M.; Maiwall, R.; Alessandria, C.; Fernandez, J.; Soares, E.C.; Kim, D.J.; Kim, S.E.; et al. Clinical features and evolution of bacterial infection-related acute-on-chronic liver failure. J. Hepatol. 2020, 74, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Reddy, R.K.; Tandon, P.; Wong, F.; Kamath, P.S.; Biggins, S.W.; Garcia-Tsao, G.; Fallon, M.; Maliakkal, B.; Lai, J.; et al. Prediction of fungal infection development and their impact on survival using the NACSELD cohort. Am. J. Gastroenterol. 2018, 113, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Rinaldi, M.; Pasquini, Z.; Scudeller, L.; Piano, S.; Giacobbe, D.R.; Maraolo, A.E.; Bussini, L.; Del Puente, F.; Incicco, S.; et al. Risk factors for Candidaemia in hospitalized patients with liver cirrhosis: A multicentre case–control–control study. Clin. Microbiol. Infect. 2020, 27, 276–282. [Google Scholar] [CrossRef]
- Sersté, T.; Cornillie, A.; Njimi, H.; Pavesi, M.; Arroyo, V.; Putignano, A.; Weichselbaum, L.; Deltenre, P.; Degré, D.; Trépo, E.; et al. The prognostic value of acute-on-chronic liver failure during the course of severe alcoholic hepatitis. J. Hepatol. 2018, 69, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Tsao, G.; Bosch, J. Management of varices and variceal hemorrhage in cirrhosis. N. Engl. J. Med. 2010, 362, 823–832. [Google Scholar] [CrossRef] [Green Version]
- Trebicka, J.; Gu, W.; Ibáñez-Samaniego, L.; Hernández-Gea, V.; Pitarch, C.; Garcia, E.; Procopet, B.; Giráldez, Á.; Amitrano, L.; Villanueva, C.; et al. Rebleeding and mortality risk are increased by ACLF but reduced by pre-emptive TIPS. J. Hepatol. 2020, 73, 1082–1091. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, V.; Jalan, R.; Wu, T.; Volk, M.L.; Asrani, S.K.; Klein, A.S.; Wong, R.J. Factors associated with survival of patients with severe acute-on-chronic liver failure before and after liver transplantation. Gastroenterology 2019, 156, 1381–1391.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Artru, F.; Louvet, A.; Ruiz, I.; Levesque, E.; Labreuche, J.; Ursic-Bedoya, J.; Lassailly, G.; Dharancy, S.; Boleslawski, E.; Lebuffe, G.; et al. Liver transplantation in the most severely ill cirrhotic patients: A multicenter study in acute-on-chronic liver failure grade 3. J. Hepatol. 2017, 67, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Belli, L.S.; Duvoux, C.; Artzner, T.; Bernal, W.; Conti, S.; Cortesi, P.A.; Sacleux, S.-C.; Pageaux, G.-P.; Radenne, S.; Trebicka, J.; et al. Liver transplantation for patients with acute-on-chronic liver failure (ACLF) in Europe: Results of the ELITA/EF-CLIF collaborative study (ECLIS). J. Hepatol. 2021, 75, 610–622. [Google Scholar] [CrossRef] [PubMed]
- Hernaez, R.; Liu, Y.; Kramer, J.R.; Rana, A.; El-Serag, H.B.; Kanwal, F. Model for end-stage liver disease-sodium underestimates 90-day mortality risk in patients with acute-on-chronic liver failure. J. Hepatol. 2020, 73, 1425–1433. [Google Scholar] [CrossRef]
- Sundaram, V.; Shah, P.; Wong, R.J.; Karvellas, C.J.; Fortune, B.E.; Mahmud, N.; Kuo, A.; Jalan, R. Patients with acute on chronic liver failure grade 3 have greater 14-day waitlist mortality than status-1a patients. Hepatology 2019, 70, 334–345. [Google Scholar] [CrossRef]
- Abdallah, M.A.; Kuo, Y.-F.; Asrani, S.; Wong, R.J.; Ahmed, A.; Kwo, P.; Terrault, N.; Kamath, P.S.; Jalan, R.; Singal, A.K. Validating a novel score based on interaction between ACLF grade and MELD score to predict waitlist mortality. J. Hepatol. 2020, 74, 1355–1361. [Google Scholar] [CrossRef]
- Rodríguez-Perálvarez, M.; Gómez-Bravo, M.; Sánchez-Antolín, G.; De la Rosa, G.; Bilbao, I.; Colmenero, J. Expanding Indications of Liver Transplantation in Spain: Consensus Statement and Recommendations by the Spanish Society of Liver Transplantation. Transplantation 2020, 105, 602–607. [Google Scholar] [CrossRef]
- Jalan, R.; Gustot, T.; Fernandez, J.; Bernal, W. ‘Equity’ and ‘Justice’ for patients with acute-on chronic liver failure: A call to action. J. Hepatol. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Artzner, T.; Michard, B.; Weiss, E.; Barbier, L.; Noorah, Z.; Merle, J.; Paugam-Burtz, C.; Francoz, C.; Durand, F.; Soubrane, O.; et al. Liver transplantation for critically ill cirrhotic patients: Stratifying utility based on pretransplant factors. Arab. Archaeol. Epigr. 2020, 20, 2437–2448. [Google Scholar] [CrossRef]
- Bañares, R.; Nevens, F.; Larsen, F.S.; Jalan, R.; Albillos, A.; Dollinger, M.; Saliba, F.; Sauerbruch, T.; Klammt, S.; Ockenga, J.; et al. Extracorporeal albumin dialysis with the molecular adsorbent recirculating system in acute-on-chronic liver failure: The RELIEF trial. Hepatology 2012, 57, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Kribben, A.; Gerken, G.; Haag, S.; Herget–Rosenthal, S.; Treichel, U.; Betz, C.; Sarrazin, C.; Hoste, E.; Van Vlierberghe, H.; Escorsell, À.; et al. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology 2012, 142, 782–789.e3. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Garg, H.; Khan, A.; Trehanpati, N.; Kumar, A.; Sharma, B.C.; Sakhuja, P.; Sarin, S.K. Granulocyte colony–stimulating factor mobilizes CD34+ cells and improves survival of patients with acute-on-chronic liver failure. Gastroenterology 2012, 142, 505–512.e1. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.-Z.; Liu, F.-F.; Tong, J.-J.; Yang, H.-Z.; Chen, J.; Liu, X.-Y.; Mao, Y.-L.; Xin, S.-J.; Hu, J.-H. Granulocyte-colony stimulating factor therapy improves survival in patients with hepatitis B virus-associated acute-on-chronic liver failure. World J. Gastroenterol. 2013, 19, 1104–1110. [Google Scholar] [CrossRef]
- Engelmann, C.; Herber, A.; Franke, A.; Bruns, T.; Schiefke, I.; Zipprich, A.; Zeuzem, S.; Goeser, T.; Canbay, A.; Berg, C.; et al. Granulocyte-colony stimulating factor (G-CSF) to treat acuteon- chronic liver failure, a multicenter randomized trial (GRAFT study). J. Hepatol. 2021, 5. Online ahead of print. [Google Scholar]
- Nevens, F.; Gustot, T.; Laterre, P.-F.; Lasser, L.L.; Haralampiev, L.E.; Vargas, V.; Lyubomirova, D.; Albillos, A.; Najimi, M.; Michel, S.; et al. A phase II study of human allogeneic liver-derived progenitor cell therapy for acute-on-chronic liver failure and acute decompensation. JHEP Rep. 2021, 3, 100291. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | EASL-CLIF Consortium | NACSELD | COSSH | AARC |
|---|---|---|---|---|
| Population | Patients with AD of cirrhosis, independently from the absence/presence of previous AD | Patients with AD of cirrhosis, independently from the absence/presence of previous AD | Patients with AD of HBV-related chronic liver disease, with or without cirrhosis | Patients with CLD or compensated cirrhosis and acute liver insult that causes acute liver deterioration |
| Precipitating events | Intrahepatic (alcoholic hepatitis), extrahepatic (infection, gastrointestinal bleeding), or both | Intrahepatic, extrahepatic, or both | Intrahepatic (HBV flare), extrahepatic (bacterial infection) or both | Intrahepatic |
| Criteria of organ system failures used to define ACLF | Liver: Total bilirubin ≥ 12 mg/dL; Kidney: Creatinine ≥ 2 mg/dL or use of RRT; Coagulation: INR ≥ 2.5; Brain: HE Grade 3–4 in West Haven classification or use of mechanical ventilation because of HE; Circulation: Use of vasopressors including terlipressin; Lung: PaO2/FiO2 ≤ 200 or SpO2/FiO2 ≤ 214, or use of mechanical ventilation for reason other than HE | Kidney: Use of dialysis or other form of RRT; Brain: HE Grade 3–4 in West Haven classification; Circulation: MAP <60 mmHg or reduction of 40 mmHg in SBP from baseline, in spite of fluid resuscitation and adequate cardiac output; Lung: Use of mechanical ventilation | Same criteria as those used by the EASL-CLIF Consortium | Liver: Total bilirubin levels ≥ 5 mg/dL Brain: clinical HE |
| Criteria for the presence of ACLF and ACLF stratification | ACLF is stratified into 3 grades of increasing severity.
(2) single liver, coagulation, circulatory or lung failure that is associated with either kidney dysfunction, brain dysfunction, a or both; (3) single brain failure and kidney dysfunction a;
| Patients are stratified according to the number of organ failures (2, 3, or 4 organ failures) | ACLF is stratified into 3 grades of increasing severity.
(2) single liver failure and either INR ≥ 1.5, kidney dysfunction, brain dysfunction, a or any combination of these; (3) single coagulation, circulatory or respiratory failure and either kidney dysfunction, brain dysfunction, a or both; (4) cerebral failure alone and kidney dysfunction;
| Total bilirubin levels of 5 mg/dL or more and INR ≥ 1.5 or prothrombin activity <40% complicated within 4 weeks byclinical ascites, HE, or both. The severity of ACLF is assessed using the AARC score #: Grade 1 by scores 5–7, Grade 2 by scores 8–10 and Grade 3 for 11–15. |
| Short-term mortality rate of ACLF | By 28 days: Grade 1: 22% Grade 2: 32% Grade 3: 77% | By 30 days: 2 organ failures: 49% 3 organ failures: 64% 4 organ failures: 77% | By 28 days: Grade 1: 23% Grade 2: 61% Grade 3: 93% | By 30 days: Grade 1: 13% Grade 2: 45% Grade 3: 86% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambino, C.; Piano, S.; Angeli, P. Acute-on-Chronic Liver Failure in Cirrhosis. J. Clin. Med. 2021, 10, 4406. https://doi.org/10.3390/jcm10194406
Gambino C, Piano S, Angeli P. Acute-on-Chronic Liver Failure in Cirrhosis. Journal of Clinical Medicine. 2021; 10(19):4406. https://doi.org/10.3390/jcm10194406
Chicago/Turabian StyleGambino, Carmine, Salvatore Piano, and Paolo Angeli. 2021. "Acute-on-Chronic Liver Failure in Cirrhosis" Journal of Clinical Medicine 10, no. 19: 4406. https://doi.org/10.3390/jcm10194406
APA StyleGambino, C., Piano, S., & Angeli, P. (2021). Acute-on-Chronic Liver Failure in Cirrhosis. Journal of Clinical Medicine, 10(19), 4406. https://doi.org/10.3390/jcm10194406






