Different Impact of Beta-Blockers on Long-Term Mortality in Heart Failure Patients with and without Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Inclusion and Exclusion Criteria
2.3. Definitions
2.4. Other Variables
2.5. Study Endpoints
2.6. Data Robustness
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Predictors for Prescription of Beta-Blockers
3.3. Long-Term Outcomes and Clinical Impact of COPD in All the Patients
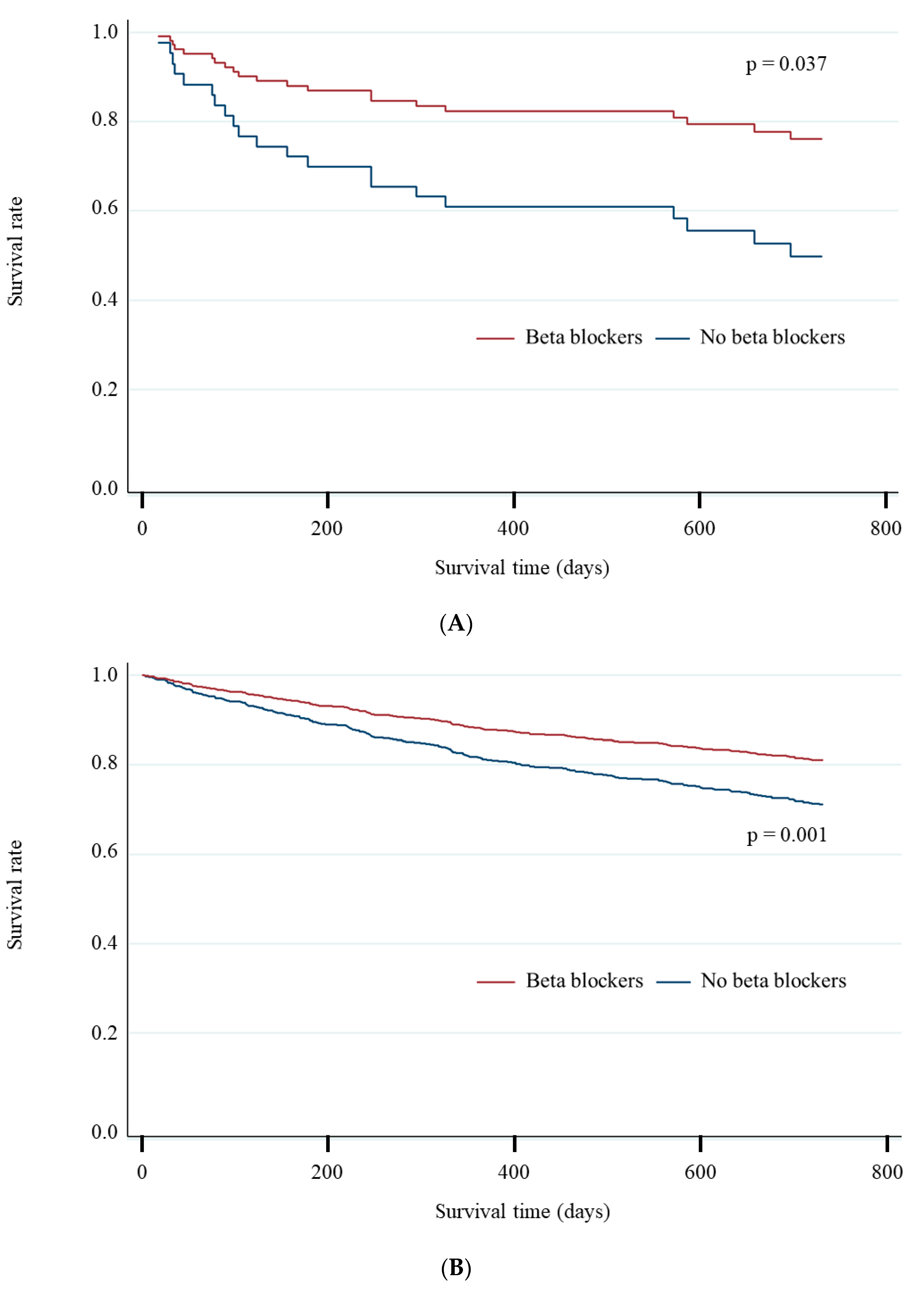
3.4. Different Impact of Beta-Blockers between Patients with and without COPD
3.5. Impact of Types and Doses of Beta-Blockers on Prognosis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.A.; Stefan, M.S.; Darling, C.; Lessard, D.; Goldberg, R.J. Impact of COPD on the mortality and treatment of patients hospitalized with acute decompensated heart failure: The Worcester Heart Failure Study. Chest 2015, 147, 637–645. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mentz, R.J.; Fiuzat, M.; Wojdyla, D.M.; Chiswell, K.; Gheorghiade, M.; Fonarow, G.C.; O’Connor, C.M. Clinical characteristics and outcomes of hospitalized heart failure patients with systolic dysfunction and chronic obstructive pulmonary disease: Findings from OPTIMIZE-HF. Eur. J. Heart Fail. 2012, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Tay, W.T.; Asai, K.; Murai, K.; Nakajima, I.; Hagiwara, N.; Ikeda, T.; Kurita, T.; Teng, T.K.; Anand, I.; et al. Chronic obstructive pulmonary disease and beta-blocker treatment in Asian patients with heart failure. ESC Heart Fail. 2018, 5, 297–305. [Google Scholar] [CrossRef]
- Dransfield, M.T.; Rowe, S.M.; Johnson, J.E.; Bailey, W.C.; Gerald, L.B. Use of beta blockers and the risk of death in hospitalised patients with acute exacerbations of COPD. Thorax 2008, 63, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Van Gestel, Y.R.; Hoeks, S.E.; Sin, D.D.; Welten, G.M.; Schouten, O.; Witteveen, H.J.; Simsek, C.; Stam, H.; Mertens, F.W.; Bax, J.J.; et al. Impact of cardioselective beta-blockers on mortality in patients with chronic obstructive pulmonary disease and atherosclerosis. Am. J. Respir. Crit. Care Med. 2008, 178, 695–700. [Google Scholar] [CrossRef]
- Vogelmeier, C.F.; Criner, G.J.; Martinez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Eur. Respir. J. 2017, 49, 1700214. [Google Scholar] [CrossRef]
- Dransfield, M.T.; Voelker, H.; Bhatt, S.P.; Brenner, K.; Casaburi, R.; Come, C.E.; Cooper, J.A.D.; Criner, G.J.; Curtis, J.L.; Han, M.K.; et al. Metoprolol for the Prevention of Acute Exacerbations of COPD. N. Engl. J. Med. 2019, 381, 2304–2314. [Google Scholar] [CrossRef]
- Janson, C.; Malinovschi, A.; Amaral, A.F.S.; Accordini, S.; Bousquet, J.; Buist, A.S.; Canonica, G.W.; Dahlen, B.; Garcia-Aymerich, J.; Gnatiuc, L.; et al. Bronchodilator reversibility in asthma and COPD: Findings from three large population studies. Eur. Respir. J. 2019, 54, 1900561. [Google Scholar] [CrossRef]
- Leung, J.M.; Sin, D.D. Asthma-COPD overlap syndrome: Pathogenesis, clinical features, and therapeutic targets. BMJ 2017, 358, j3772. [Google Scholar] [CrossRef]
- Lozano, R.; Naghavi, M.; Foreman, K.; Lim, S.; Shibuya, K.; Aboyans, V.; Abraham, J.; Adair, T.; Aggarwal, R.; Ahn, S.Y.; et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2095–2128. [Google Scholar] [CrossRef]
- Hawkins, N.M.; Petrie, M.C.; Jhund, P.S.; Chalmers, G.W.; Dunn, F.G.; McMurray, J.J. Heart failure and chronic obstructive pulmonary disease: Diagnostic pitfalls and epidemiology. Eur. J. Heart Fail. 2009, 11, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, K.; Harada, K.; Miyazaki, T.; Miyamoto, T.; Kohsaka, S.; Iida, K.; Yamamoto, Y.; Nagatomo, Y.; Yoshino, H.; Yamamoto, T.; et al. Younger- vs Older-Old Patients with Heart Failure with Preserved Ejection Fraction. J. Am. Geriatr. Soc. 2019, 67, 2123–2128. [Google Scholar] [CrossRef]
- Johnson, J.A. Ethnic differences in cardiovascular drug response: Potential contribution of pharmacogenetics. Circulation 2008, 118, 1383–1393. [Google Scholar] [CrossRef]
- Zhou, H.H.; Koshakji, R.P.; Silberstein, D.J.; Wilkinson, G.R.; Wood, A.J. Racial differences in drug response. Altered sensitivity to and clearance of propranolol in men of Chinese descent as compared with American whites. N. Engl. J. Med. 1989, 320, 565–570. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Kohsaka, S.; Abe, T.; Mizuno, A.; Goda, A.; Izumi, Y.; Yagawa, M.; Akita, K.; Sawano, M.; Inohara, T.; et al. Validation of the Get With The Guideline-Heart Failure risk score in Japanese patients and the potential improvement of its discrimination ability by the inclusion of B-type natriuretic peptide level. Am. Heart J. 2016, 171, 33–39. [Google Scholar] [CrossRef]
- Ho, K.K.; Anderson, K.M.; Kannel, W.B.; Grossman, W.; Levy, D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation 1993, 88, 107–115. [Google Scholar] [CrossRef]
- Shephard, D.A. The 1975 Declaration of Helsinki and consent. Can. Med. Assoc. J. 1976, 115, 1191–1192. [Google Scholar]
- Canepa, M.; Straburzynska-Migaj, E.; Drozdz, J.; Fernandez-Vivancos, C.; Pinilla, J.M.G.; Nyolczas, N.; Temporelli, P.L.; Mebazaa, A.; Lainscak, M.; Laroche, C.; et al. Characteristics, treatments and 1-year prognosis of hospitalized and ambulatory heart failure patients with chronic obstructive pulmonary disease in the European Society of Cardiology Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2018, 20, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Melenovsky, V.; Kotrc, M.; Borlaug, B.A.; Marek, T.; Kovar, J.; Malek, I.; Kautzner, J. Relationships between right ventricular function, body composition, and prognosis in advanced heart failure. J. Am. Coll. Cardiol. 2013, 62, 1660–1670. [Google Scholar] [CrossRef]
- Sato, Y.; Yoshihisa, A.; Kimishima, Y.; Yokokawa, T.; Abe, S.; Shimizu, T.; Misaka, T.; Yamada, S.; Sato, T.; Kaneshiro, T.; et al. Prognostic factors in heart failure patients with cardiac cachexia. J. Geriatr. Cardiol. 2020, 17, 26–34. [Google Scholar] [PubMed]
- Cohen-Solal, A.; Jacobson, A.F.; Piña, I.L. Beta blocker dose and markers of sympathetic activation in heart failure patients: Interrelationships and prognostic significance. ESC Heart Fail. 2017, 4, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–270. [Google Scholar] [CrossRef] [PubMed]
- Hicks, K.A.; Tcheng, J.E.; Bozkurt, B.; Chaitman, B.R.; Cutlip, D.E.; Farb, A.; Fonarow, G.C.; Jacobs, J.P.; Jaff, M.R.; Lichtman, J.H.; et al. 2014 ACC/AHA Key Data Elements and Definitions for Cardiovascular Endpoint Events in Clinical Trials: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). J. Am. Coll. Cardiol. 2015, 66, 403–469. [Google Scholar] [PubMed]
- Higuchi, S.; Kabeya, Y.; Matsushita, K.; Taguchi, H.; Ishiguro, H.; Kohshoh, H.; Yoshino, H. Barthel Index as a Predictor of 1-Year Mortality in Very Elderly Patients Who Underwent Percutaneous Coronary Intervention for Acute Coronary Syndrome: Better Activities of Daily Living, Longer Life. Clin. Cardiol. 2016, 39, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Akita, K.; Kohno, T.; Kohsaka, S.; Shiraishi, Y.; Nagatomo, Y.; Izumi, Y.; Goda, A.; Mizuno, A.; Sawano, M.; Inohara, T.; et al. Current use of guideline-based medical therapy in elderly patients admitted with acute heart failure with reduced ejection fraction and its impact on event-free survival. Int. J. Cardiol. 2017, 235, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Kohsaka, S.; Shiraishi, Y.; Katsuki, T.; Nagatomo, Y.; Mizuno, A.; Sujino, Y.; Kohno, T.; Goda, A.; Yoshikawa, T. Association of renin-angiotensin system inhibitors with long-term outcomes in patients with systolic heart failure and moderate-to-severe kidney function impairment. Eur. J. Intern. Med. 2019, 62, 58–66. [Google Scholar] [CrossRef]
- Al-Bawardy, R.; Vemulapalli, S.; Thourani, V.H.; Mack, M.; Dai, D.; Stebbins, A.; Palacios, I.; Inglessis, I.; Sakhuja, R.; Ben-Assa, E.; et al. Association of Pulmonary Hypertension with Clinical Outcomes of Transcatheter Mitral Valve Repair. JAMA Cardiol. 2020, 5, 47–56. [Google Scholar] [CrossRef]
- Higuchi, S.; Orban, M.; Stolz, L.; Karam, N.; Praz, F.; Kalbacher, D.; Ludwig, S.; Braun, D.; Näbauer, M.; Wild, M.G.; et al. Impact of Residual Mitral Regurgitation on Survival after Transcatheter Edge-to-Edge Repair for Secondary Mitral Regurgitation. JACC Cardiovasc. Interv. 2021, 14, 1243–1253. [Google Scholar] [CrossRef]
- Short, P.M.; Lipworth, S.I.; Elder, D.H.; Schembri, S.; Lipworth, B.J. Effect of beta blockers in treatment of chronic obstructive pulmonary disease: A retrospective cohort study. BMJ 2011, 342, d2549. [Google Scholar] [CrossRef] [PubMed]
- Quint, J.K.; Herrett, E.; Bhaskaran, K.; Timmis, A.; Hemingway, H.; Wedzicha, J.A.; Smeeth, L. Effect of beta blockers on mortality after myocardial infarction in adults with COPD: Population based cohort study of UK electronic healthcare records. BMJ 2013, 347, f6650. [Google Scholar] [CrossRef] [PubMed]
- Bristow, M.R. beta-adrenergic receptor blockade in chronic heart failure. Circulation 2000, 101, 558–569. [Google Scholar] [CrossRef] [PubMed]
- Andreas, S.; Haarmann, H.; Klarner, S.; Hasenfuss, G.; Raupach, T. Increased sympathetic nerve activity in COPD is associated with morbidity and mortality. Lung 2014, 192, 235–241. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.N.; Wouters, E.F.M.; Rutten, E.; Casaburi, R.; Rennard, S.I.; Lomas, D.A.; Bamman, M.; Celli, B.; Agusti, A.; Tal-Singer, R.; et al. It’s more than low BMI: Prevalence of cachexia and associated mortality in COPD. Respir Res. 2019, 20, 100. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Chamberlain, A.M. Multimorbidity in Older Patients with Cardiovascular Disease. Curr. Cardiovasc. Risk Rep. 2016, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Tinetti, M.E.; Fried, T.R.; Boyd, C.M. Designing health care for the most common chronic condition--multimorbidity. JAMA 2012, 307, 2493–2494. [Google Scholar] [CrossRef]
- Forman, D.E.; Maurer, M.S.; Boyd, C.; Brindis, R.; Salive, M.E.; Horne, F.M.; Bell, S.P.; Fulmer, T.; Reuben, D.B.; Zieman, S.; et al. Multimorbidity in Older Adults With Cardiovascular Disease. J. Am. Coll. Cardiol. 2018, 71, 2149–2161. [Google Scholar] [CrossRef]
- Sin, D.D.; Anthonisen, N.R.; Soriano, J.B.; Agusti, A.G. Mortality in COPD: Role of comorbidities. Eur. Respir. J. 2006, 28, 1245–1257. [Google Scholar] [CrossRef]
- Greene, S.J.; Fonarow, G.C.; DeVore, A.D.; Sharma, P.P.; Vaduganathan, M.; Albert, N.M.; Duffy, C.I.; Hill, C.L.; McCague, K.; Patterson, J.H.; et al. Titration of Medical Therapy for Heart Failure With Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2019, 73, 2365–2383. [Google Scholar] [CrossRef]
| All | Patients with COPD | Patients without COPD | |||||||
|---|---|---|---|---|---|---|---|---|---|
| All | Beta-Blocker | No Beta- Blocker | p Value | All | Beta-Blocker | No Beta- Blocker | p Value | ||
| n = 1843 | n = 83 | n = 67 | n = 16 | n = 1760 | n = 1509 | n = 251 | |||
| Age, years | 72 ± 14 | 75 ± 11 | 75 ± 10 | 75 ± 15 | 0.974 | 71 ± 14 | 71 ± 14 | 77 ± 14 | <0.001 |
| Male, n (%) | 1271 (69) | 72 (87) | 58 (87) | 14 (88) | 1.000 | 1199 (68) | 1034 (69) | 165 (66) | 0.381 |
| BMI, kg/m2 | 22 ± 4 | 21 ± 4 | 22 ± 4 | 20 ± 4 | 0.186 | 22 ± 4 | 22 ± 4 | 21 ± 4 | 0.004 |
| Hypertension, n (%) | 1224 (67) | 57 (70) | 46 (69) | 11 (69) | 1.000 | 1167 (67) | 1006 (67) | 161 (64) | 0.448 |
| Dyslipidemia, n (%) | 760 (42) | 32 (40) | 28 (43) | 4 (25) | 0.257 | 728 (42) | 642 (43) | 86 (34) | 0.011 |
| Diabetes mellitus, n (%) | 686 (37) | 23 (28) | 21 (31) | 2 (13) | 0.213 | 663 (38) | 580 (38) | 83 (33) | 0.101 |
| Atrial fibrillation, n (%) | 778 (42) | 34 (41) | 28 (42) | 6 (38) | 1.000 | 744 (42) | 645 (43) | 99 (39) | 0.315 |
| Previous heart failure admission, n (%) | 582 (32) | 31 (37) | 27 (40) | 4 (25) | 0.389 | 551 (32) | 467 (31) | 84 (33) | 0.440 |
| History of ischemic stroke, n (%) | 233 (13) | 7 (8) | 6 (9) | 1 (6) | 1.000 | 226 (13) | 182 (12) | 44 (18) | 0.016 |
| Hemodialysis, n (%) | 60 (3) | 2 (2) | 1 (1) | 1 (6) | 0.350 | 58 (3) | 48 (3) | 10 (4) | 0.565 |
| Etiologies of heart failure | |||||||||
| Ischemic heart disease, n (%) | 688 (37) | 30 (36) | 27 (40) | 3 (19) | 0.107 | 658 (37) | 574 (38) | 84 (33) | 0.166 |
| Diastolic cardiomyopathy, n (%) | 432 (23) | 21 (25) | 17 (25) | 4 (25) | 0.975 | 411 (23) | 378 (25) | 33 (13) | <0.001 |
| Valvular heart disease, n (%) | 266 (14) | 10 (12) | 5 (7) | 5 (31) | 0.009 | 256 (15) | 198 (13) | 58 (23) | <0.001 |
| Others, n (%) | 457 (25) | 22 (27) | 18 (27) | 4 (25) | 0.879 | 435 (25) | 359 (24) | 76 (30) | 0.027 |
| NYHA classification at discharge ≥III, n (%) | 374 (20) | 21 (25) | 15 (22) | 6 (38) | 0.218 | 353 (20) | 278 (18) | 75 (30) | <0.001 |
| Cachexia, n (%) | 376 (20) | 19 (23) | 12 (18) | 7 (44) | 0.044 | 357 (20) | 287 (19) | 70 (28) | 0.001 |
| Laboratory data at discharge | |||||||||
| Sodium, mmol/L | 138 ± 3 | 138 ± 4 | 138 ± 3 | 137 ± 6 | 0.519 | 139 ± 3 | 139 ± 3 | 138 ± 4 | 0.042 |
| Potassium, mmol/L | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.3 ± 0.5 | 0.391 | 4.4 ± 0.5 | 4.4 ± 0.5 | 4.3 ± 0.5 | 0.041 |
| Creatinine, mg/dl | 1.1 (0.8–1.5) | 1.2 (0.9–1.5) | 1.2 (0.9–1.5) | 1.0 (0.9–1.6) | 0.695 | 1.1 (0.8–1.4) | 1.1 (0.8–1.4) | 1.1 (0.9–1.5) | 0.519 |
| eGFR, ml/min/m2 | 50 (35–64) | 44 (36–59) | 44 (36–60) | 54 (34–58) | 0.890 | 50 (35–64) | 51 (35–65) | 46 (32–62) | 0.047 |
| Hemoglobin, g/L | 12.4 ± 2.3 | 12.1 ± 2.0 | 12.2 ± 1.9 | 11.5 ± 2.0 | 0.151 | 12.5 ± 2.3 | 12.6 ± 2.3 | 11.8 ± 2.2 | <0.001 |
| Albumin, g/L | 3.5 ± 0.6 | 3.4 ± 0.5 | 3.4 ± 0.5 | 3.4 ± 0.3 | 0.985 | 3.5 ± 0.6 | 3.5 ± 0.5 | 3.4 ± 0.6 | <0.001 |
| Echocardiography | |||||||||
| LVEF, % | 34 ± 9 | 33 ± 9 | 33 ± 9 | 33 ± 9 | 0.949 | 34 ± 9 | 33 ± 9 | 36 ± 9 | <0.001 |
| Medication at discharge | |||||||||
| Beta-blockers, n (%) | 1576 (86) | 67 (81) | 67 (100) | 0 (0) | <0.001 | 1509 (86) | 1509 (100) | 0 (0) | <0.001 |
| Carvedilol, n (%) | 1143 (62) | 34 (41) | 1109 (63) | ||||||
| Bisoprolol, n (%) | 415 (23) | 32 (39) | 383 (22) | ||||||
| The others, n (%) | 18 (1) | 1 (1) | 17 (1) | ||||||
| RAS inhibitors, n (%) | 1253 (68) | 55 (66) | 47 (70) | 8 (50) | 0.148 | 1198 (68) | 1063 (70) | 135 (54) | <0.001 |
| MRA, n (%) | 806 (44) | 28 (34) | 25 (37) | 3 (19) | 0.240 | 778 (44) | 672 (45) | 106 (42) | 0.518 |
| Furosemide, n (%) | 1401 (76) | 66 (80) | 53 (79) | 13 (81) | 1.000 | 1335 (76) | 1156 (77) | 179 (72) | 0.083 |
| Tolvaptan, n (%) | 72 (5) | 3 (4) | 2 (3) | 1 (8) | 0.436 | 69 (5) | 59 (5) | 10 (4) | 1.000 |
| Statin, n (%) | 712 (39) | 33 (40) | 28 (42) | 5 (31) | 0.573 | 679 (39) | 612 (41) | 67 (27) | <0.001 |
| Devices at discharge | |||||||||
| Pacemaker implantation, n (%) | 167 (9) | 6 (7) | 5 (7) | 1 (7) | 1.000 | 161 (9) | 120 (8) | 41 (16) | <0.001 |
| ICD, n (%) | 136 (7) | 6 (7) | 5 (7) | 1 (7) | 1.000 | 130 (7) | 114 (8) | 16 (6) | 0.602 |
| CRT, n (%) | 73 (4) | 2 (2) | 2 (3) | 0 (0) | 1.000 | 71 (4) | 66 (4) | 5 (2) | 0.083 |
| All | Patients with COPD | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | |||||||
| OR | 95% CI | p Value | OR | 95% CI | p Value | OR | 95% CI | p Value | |
| Age (an increase of 1 year) | 0.97 | 0.96–0.98 | <0.001 | 0.98 | 0.97–0.99 | <0.001 | 1.00 | 0.95–1.05 | 0.974 |
| Male | 1.11 | 0.84–1.46 | 0.463 | NA | 0.92 | 0.18–4.74 | 0.921 | ||
| BMI (an increase of 1 kg/m2) | 1.06 | 1.02–1.10 | 0.002 | NA | 1.10 | 0.95–1.28 | 0.187 | ||
| Hypertension | 1.11 | 0.84–1.45 | 0.462 | NA | 1.05 | 0.32–3.40 | 0.941 | ||
| Dyslipidemia | 1.48 | 1.12–1.94 | 0.005 | NA | 2.27 | 0.66–7.79 | 0.193 | ||
| Diabetes mellitus | 1.32 | 1.00–1.74 | 0.048 | NA | 3.20 | 0.67–15.34 | 0.147 | ||
| COPD | 0.70 | 0.40–1.22 | 0.207 | NA | NA | ||||
| Atrial fibrillation | 1.15 | 0.89–1.50 | 0.290 | NA | 1.20 | 0.39–3.68 | 0.754 | ||
| Previous heart failure admission | 0.93 | 0.71–1.23 | 0.619 | NA | 2.03 | 0.59–6.94 | 0.262 | ||
| History of ischemic stroke | 0.67 | 0.47–0.95 | 0.025 | 0.63 | 0.43–0.92 | 0.016 | 1.48 | 0.16–13.20 | 0.728 |
| Hemodialysis | 0.75 | 0.38–1.46 | 0.393 | NA | 0.23 | 0.01–3.84 | 0.304 | ||
| Ischemic heart disease | 1.28 | 0.97–1.68 | 0.083 | NA | 2.93 | 0.76–11.25 | 0.118 | ||
| NYHA classification at discharge ≥III | 0.52 | 0.39–0.70 | <0.001 | 0.71 | 0.52–0.98 | 0.037 | 0.48 | 0.15–1.54 | 0.217 |
| Cachexia | 0.58 | 0.43–0.77 | <0.001 | 0.28 | 0.09–0.90 | 0.033 | |||
| Laboratory data at discharge | |||||||||
| Sodium (an increase of 1 mmol/L) | 1.04 | 1.00–1.08 | 0.030 | NA | 1.05 | 0.91–1.20 | 0.515 | ||
| Potassium (an increase of 1 mmol/L) | 1.35 | 1.03–1.76 | 0.031 | NA | 1.66 | 0.53–5.24 | 0.387 | ||
| Creatinine (an increase of 20 μmol/L) | 0.99 | 0.97–1.01 | 0.378 | NA | 1.01 | 0.87–1.17 | 0.886 | ||
| eGFR (an increase of 10 mL/min/m2) | 1.00 | 0.95–1.05 | 0.928 | NA | 1.04 | 0.82–1.32 | 0.744 | ||
| Hemoglobin (an increase of 10 g/L) | 1.18 | 1.11–1.25 | <0.001 | NA | 1.24 | 0.91–1.68 | 0.169 | ||
| Albumin (an increase of 10 g/L) | 1.78 | 1.39–2.26 | <0.001 | 1.35 | 1.04–1.76 | 0.025 | 0.99 | 0.29–3.32 | 0.985 |
| Echocardiography | |||||||||
| LVEF (an absolute increase of 10%) | 0.73 | 0.63–0.85 | <0.001 | 0.78 | 0.66–0.91 | 0.002 | 0.98 | 0.54–1.78 | 0.948 |
| Medication at discharge | |||||||||
| RAS inhibitors | 2.07 | 1.59–2.69 | <0.001 | 2.02 | 1.52–2.68 | <0.001 | 2.35 | 0.77–7.14 | 0.132 |
| MRA | 1.14 | 0.88–1.49 | 0.315 | NA | 2.58 | 0.67–9.94 | 0.169 | ||
| Furosemide | 1.27 | 0.95–1.71 | 0.106 | NA | 0.87 | 0.22–3.50 | 0.849 | ||
| Tolvaptan | 0.96 | 0.50–1.85 | 0.901 | NA | 0.39 | 0.03–4.71 | 0.461 | ||
| Statin | 1.85 | 1.39–2.47 | <0.001 | 2.02 | 1.48–2.76 | <0.001 | 1.58 | 0.49–5.05 | 0.441 |
| Devices at discharge | |||||||||
| Pacemaker implantation | 0.46 | 0.32–0.67 | <0.001 | NA | 1.13 | 0.12–10.44 | 0.915 | ||
| ICD | 1.20 | 0.71–2.03 | 0.501 | NA | 1.13 | 0.12–10.44 | 0.915 | ||
| CRT | 2.36 | 0.94–5.90 | 0.067 | NA | NA | ||||
| All | Patients with COPD | Patients without COPD | p Value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| All | Beta- Blockers | No Beta-Blockers | HR (95% CI) | p Value | All | Beta- Blockers | No Beta-Blockers | HR (95% CI) | |||
| (n = 1843) | (n = 83) | (n = 67) | (n = 16) | (n = 1760) | (n = 1509) | (n = 251) | |||||
| All-cause mortality, n (%) | 315 (17) | 21 (25) | 14 (21) | 7 (44) | 0.39 (0.16–0.98) | 0.044 | 294 (17) | 238 (16) | 56 (22) | 0.62 (0.46–0.83) | 0.001 |
| Cardiac mortality, n (%) | 149 (8) | 6 (7) | 4 (6) | 2 (13) | 0.37 (0.07–2.01) | 0.248 | 143 (8) | 108 (7) | 35 (14) | 0.45 (0.31–0.66) | <0.001 |
| Noncardiac mortality, n (%) | 166 (9) | 15 (18) | 10 (15) | 5 (31) | 0.40 (0.14–1.18) | 0.098 | 151 (9) | 130 (9) | 21 (8) | 0.90 (0.57–1.42) | 0.647 |
| Patients with COPD | Patients without COPD | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age (an increase of 1 year) | 1.07 | 1.01–1.12 | 0.017 | NA | 1.05 | 1.04–1.06 | <0.001 | 1.04 | 1.02–1.05 | <0.001 | ||
| Male | 0.65 | 0.22–1.93 | 0.435 | NA | 0.85 | 0.67–1.08 | 0.195 | NA | ||||
| BMI (an increase of 1 kg/m2) | 0.96 | 0.86–1.07 | 0.467 | NA | 0.88 | 0.85–0.91 | <0.001 | NA | ||||
| Hypertension | 0.47 | 0.20–1.11 | 0.086 | 0.33 | 0.13–0.85 | 0.021 | 1.03 | 0.81–1.32 | 0.791 | NA | ||
| Dyslipidemia | 0.32 | 0.11–0.94 | 0.039 | NA | 1.12 | 0.89–1.41 | 0.334 | NA | ||||
| Diabetes mellitus | 0.41 | 0.12–1.41 | 0.158 | NA | 1.26 | 1.00–1.58 | 0.053 | NA | ||||
| Atrial fibrillation | 0.53 | 0.20–1.36 | 0.184 | NA | 1.03 | 0.81–1.29 | 0.821 | NA | ||||
| Previous heart failure admission | 0.70 | 0.28–1.73 | 0.437 | NA | 1.75 | 1.39–2.21 | <0.001 | NA | ||||
| History of ischemic stroke | 0.48 | 0.06–3.58 | 0.474 | NA | 1.28 | 0.93–1.76 | 0.129 | NA | ||||
| Hemodialysis | 1.87 | 0.25–13.94 | 0.541 | NA | 3.01 | 1.93–4.69 | <0.001 | 1.86 | 1.09–3.18 | 0.023 | ||
| Ischemic heart failure | 0.71 | 0.27–1.82 | 0.472 | NA | 1.50 | 1.20–1.89 | <0.001 | NA | ||||
| NYHA classification at discharge ≥III | 3.72 | 1.57–8.80 | 0.003 | NA | 2.56 | 2.02–3.25 | <0.001 | 1.80 | 1.39–2.33 | <0.001 | ||
| Cachexia | 2.19 | 0.91–5.28 | 0.082 | NA | 2.67 | 2.10–3.39 | <0.001 | NA | ||||
| Laboratory data at discharge | ||||||||||||
| Sodium (an increase of 1 mmol/L) | 0.90 | 0.81–0.99 | 0.038 | NA | 0.92 | 0.89–0.94 | <0.001 | 0.95 | 0.92–0.98 | <0.001 | ||
| Potassium (an increase of 1 mmol/L) | 0.63 | 0.25–1.57 | 0.320 | NA | 0.91 | 0.72–1.16 | 0.448 | NA | ||||
| Creatinine (an increase of 20 μmol/L) | 1.01 | 0.90–1.12 | 0.921 | NA | 1.03 | 1.02–1.04 | <0.001 | NA | ||||
| eGFR (an increase of 10 mL/min/m2) | 1.21 | 0.99–1.48 | 0.058 | NA | 0.78 | 0.74–0.83 | <0.001 | 0.92 | 0.86–0.98 | 0.007 | ||
| Hemoglobin (an increase of 10 g/L) | 0.74 | 0.57–0.95 | 0.020 | NA | 0.75 | 0.71–0.79 | <0.001 | 0.88 | 0.81–0.95 | 0.002 | ||
| Albumin (an increase of 10 g/L) | 0.27 | 0.09–0.78 | 0.015 | 0.22 | 0.08-0.63 | 0.005 | 0.38 | 0.31–0.46 | <0.001 | 0.55 | 0.42–0.70 | <0.001 |
| Echocardiography | ||||||||||||
| LVEF (an absolute increase of 10%) | 1.19 | 0.71–1.99 | 0.503 | NA | 0.87 | 0.77–0.98 | 0.020 | 0.68 | 0.59–0.79 | <0.001 | ||
| Medication at discharge | ||||||||||||
| Beta-blockers | 0.39 | 0.16–0.98 | 0.044 | 0.36 | 0.14-0.92 | 0.033 | 0.62 | 0.46–0.83 | 0.001 | NA | ||
| RAS inhibitors | 0.63 | 0.26–1.49 | 0.289 | NA | 0.51 | 0.40–0.64 | <0.001 | 0.74 | 0.57–0.96 | 0.024 | ||
| MRA | 0.78 | 0.30–2.02 | 0.616 | NA | 0.86 | 0.68–1.08 | 0.189 | NA | ||||
| Furosemide | 1.50 | 0.44–5.10 | 0.516 | NA | 1.23 | 0.93–1.63 | 0.150 | NA | ||||
| Tolvaptan | NA | NA | 2.83 | 1.86–4.31 | <0.001 | 1.98 | 1.27–3.08 | 0.003 | ||||
| Statin | 1.02 | 0.81–1.28 | 0.861 | NA | 1.02 | 0.81–1.30 | 0.840 | NA | ||||
| Devices at discharge | ||||||||||||
| Pacemaker implantation | 0.59 | 0.08–4.42 | 0.609 | NA | 1.30 | 0.92–1.85 | 0.135 | NA | ||||
| ICD | 0.61 | 0.08–4.57 | 0.631 | NA | 1.24 | 0.85–1.82 | 0.270 | NA | ||||
| CRT | NA | NA | 1.29 | 0.78–2.14 | 0.321 | NA | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Higuchi, S.; Kohno, T.; Kohsaka, S.; Shiraishi, Y.; Takei, M.; Goda, A.; Shoji, S.; Nagatomo, Y.; Yoshikawa, T. Different Impact of Beta-Blockers on Long-Term Mortality in Heart Failure Patients with and without Chronic Obstructive Pulmonary Disease. J. Clin. Med. 2021, 10, 4378. https://doi.org/10.3390/jcm10194378
Higuchi S, Kohno T, Kohsaka S, Shiraishi Y, Takei M, Goda A, Shoji S, Nagatomo Y, Yoshikawa T. Different Impact of Beta-Blockers on Long-Term Mortality in Heart Failure Patients with and without Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine. 2021; 10(19):4378. https://doi.org/10.3390/jcm10194378
Chicago/Turabian StyleHiguchi, Satoshi, Takashi Kohno, Shun Kohsaka, Yasuyuki Shiraishi, Makoto Takei, Ayumi Goda, Satoshi Shoji, Yuji Nagatomo, and Tsutomu Yoshikawa. 2021. "Different Impact of Beta-Blockers on Long-Term Mortality in Heart Failure Patients with and without Chronic Obstructive Pulmonary Disease" Journal of Clinical Medicine 10, no. 19: 4378. https://doi.org/10.3390/jcm10194378
APA StyleHiguchi, S., Kohno, T., Kohsaka, S., Shiraishi, Y., Takei, M., Goda, A., Shoji, S., Nagatomo, Y., & Yoshikawa, T. (2021). Different Impact of Beta-Blockers on Long-Term Mortality in Heart Failure Patients with and without Chronic Obstructive Pulmonary Disease. Journal of Clinical Medicine, 10(19), 4378. https://doi.org/10.3390/jcm10194378







