Sildenafil Supplementation for Women Undergoing Infertility Treatments: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Search Strategy
2.3. Inclusion Criteria
- Language: studies reported in English language
- Study designs: randomized controlled trials
- Population: infertile women undergoing MAR and ARTs procedures, including TI, IUI and IVF with fresh fresh-ET or Frozen-ET.
- Intervention: Sildenafil therapy
- Timing of intervention: during the monitored cycle for TI or IUI; during the course of controlled ovarian stimulation (COS) for IVF and fresh_ET and during the course of endometrial preparation for frozen_ET.
- Comparator: infertile women with unexplained infertility and with ovulatory or anovulatory cycles, with thin endometrium or with multiple failed ART cycles, undergoing TI or IUI or COS with fresh_ET embryo transfer or endometrial preparation for frozen-ET
- Outcomes and their definitions: endometrial thickness (ETh-transvaginal ultrasonography measurement of the endometrium at the maximal distance between each myometrial/endometrial interface in a prearranged moment of the menstrual cycle), clinical pregnancy rate (CPR-per woman randomized, defined as the presence of a gestational sac on transvaginal ultrasound), chemical pregnancy rate (ChPR-per woman randomized, defined as serum measurement of beta Human chorionic gonadotropin >5 mU/mL).
2.4. Study Selection and Data Extraction
2.5. Risk of Bias
2.6. Statistical Analysis
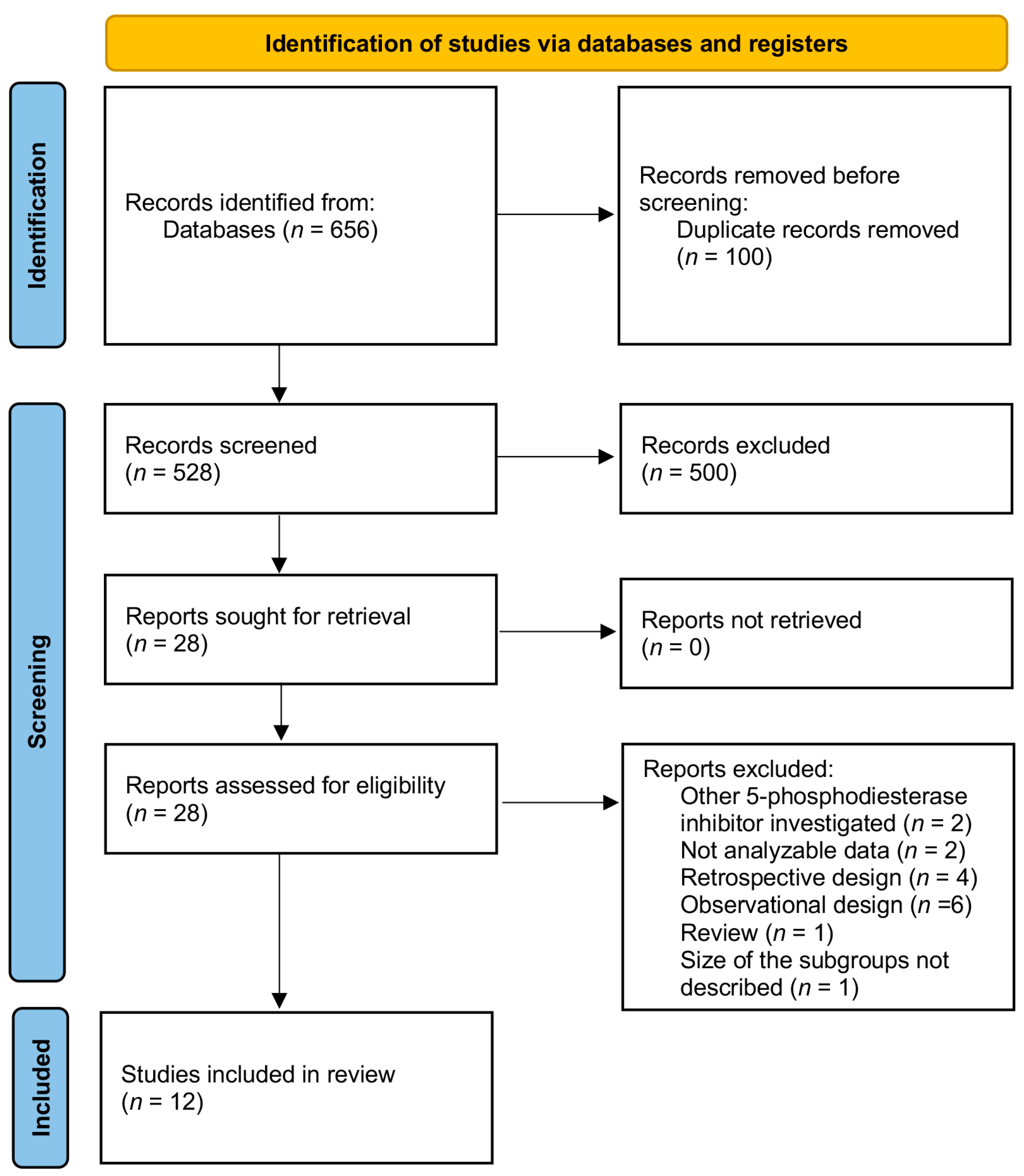
3. Results
3.1. Study Selection
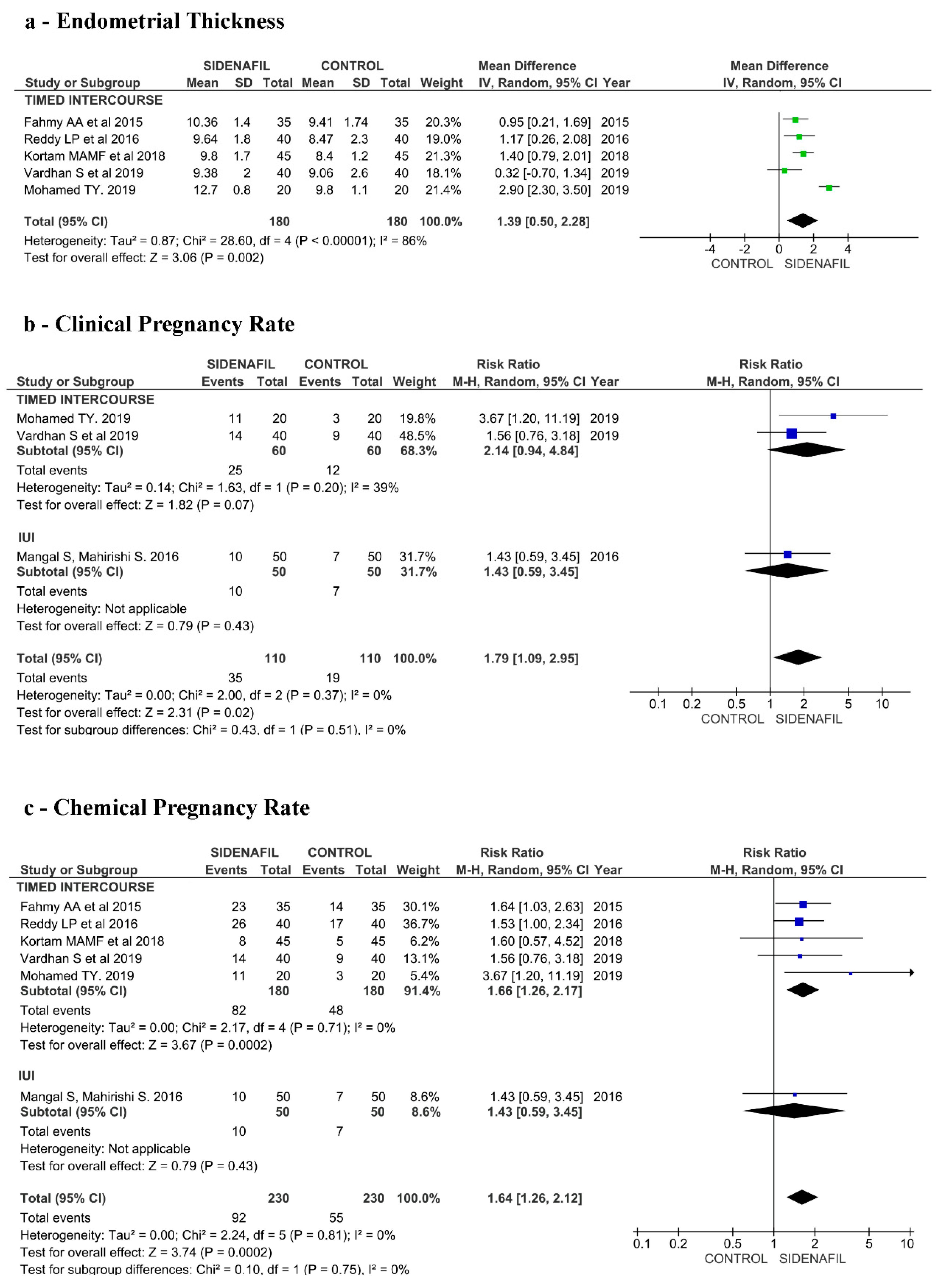
3.2. TI/IUI Section
3.2.1. Included Studies
3.2.2. Patients
3.2.3. Type, Dose and Duration of Intervention
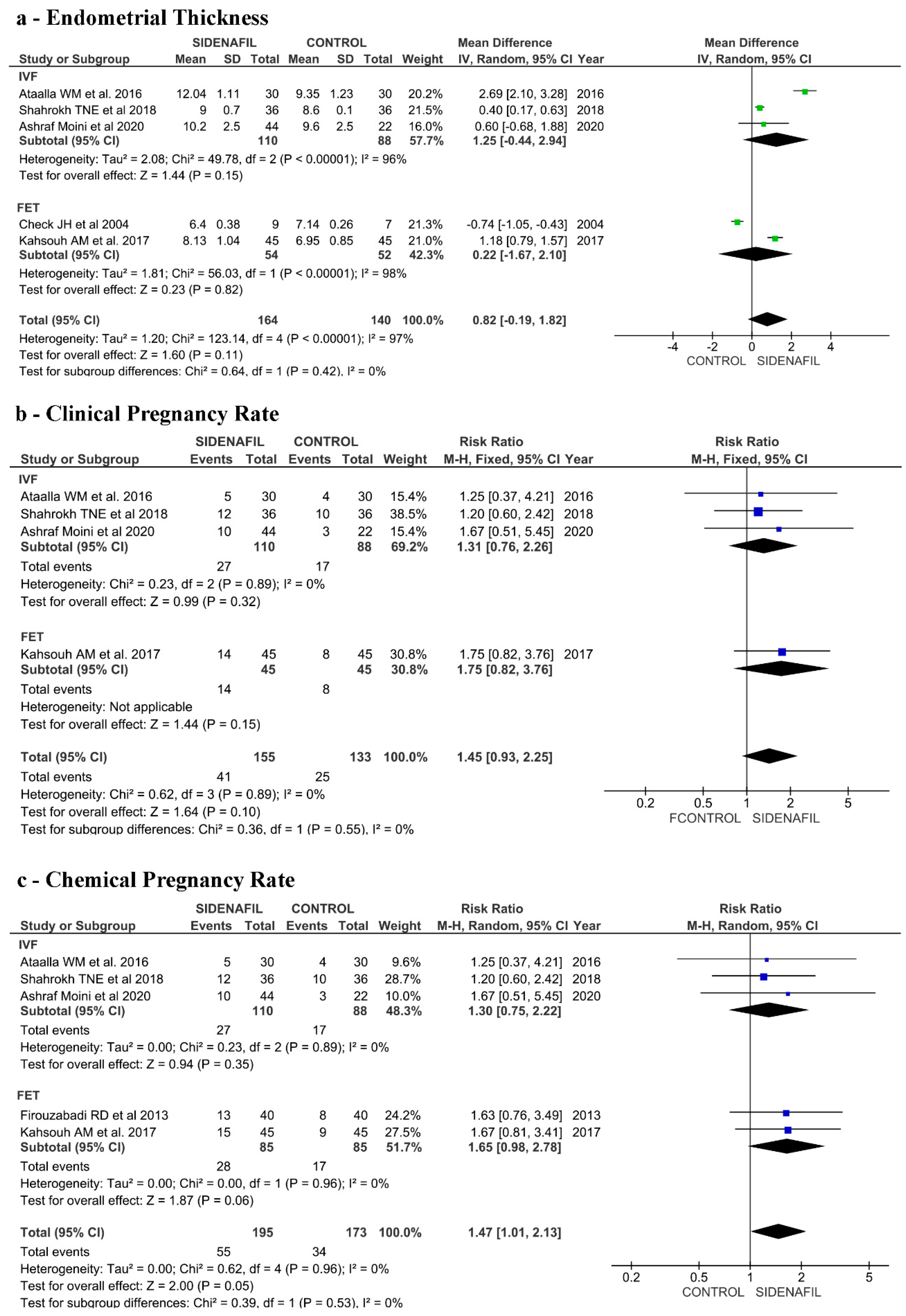
3.3. IVF Fresh-ET/Frozen-ET Section
3.3.1. Included Studies
3.3.2. Patients
3.3.3. COS Cycles
3.3.4. Type, Dose and Duration of Intervention
3.4. Risk of Bias
3.5. Adverse Effects
3.6. Effects of Intervention (TI/IUI Section)
3.7. Effects of Intervention (IVF Fresh-ET/Frozen-ET Section)
4. Discussion
4.1. General Considerations
4.2. Main Findings
4.3. Interpretation
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The European IVF-Monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology (ESHRE); Embryology; Wyns, C.; De Geyter, C.; Calhaz-Jorge, C.; Kupka, M.S.; Motrenko, T.; Smeenk, J.; Bergh, C.; Tandler-Schneider, A.; et al. ART in Europe, 2017: Results generated from European registries by ESHRE†. Hum. Reprod. Open 2021, 2021, hoab026. [Google Scholar] [PubMed]
- Zhang, T.; Li, Z.; Ren, X.; Huang, B.; Zhu, G.; Yang, W.; Jin, L. Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: A retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Med. Baltim. 2018, 97, e9689. [Google Scholar] [CrossRef] [PubMed]
- Blesa, D.; Ruiz-Alonso, M.; Simon, C. Clinical management of endometrial receptivity. Semin. Reprod. Med. 2014, 32, 410–413. [Google Scholar] [CrossRef]
- Liu, Y.; Ye, X.Y.; Chan, C. The association between endometrial thickness and pregnancy outcome in gonadotropin-stimulated intrauterine insemination cycles. Reprod. Biol. Endocrinol. 2019, 17, 14. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Qu, P.; Bai, H.; Shi, W.; Shi, J. Endometrial thickness as a predictor of ectopic pregnancy in 1125 in vitro fertilization-embryo transfer cycles: A matched case-control study. Arch. Gynecol. Obstet. 2019, 300, 1797–1803. [Google Scholar] [CrossRef]
- Groenewoud, E.R.; Cohlen, B.J.; Al-Oraiby, A.; Brinkhuis, E.A.; Broekmans, F.J.M.; de Bruin, J.P.; van Dool, G.; Fleisher, K.; Friederich, J.; Goddijn, M.; et al. Influence of endometrial thickness on pregnancy rates in modified natural cycle frozen-thawed embryo transfer. Acta Obstet. Gynecol. Scand. 2018, 97, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Zolghadri, J.; Haghbin, H.; Dadras, N.; Behdin, S. Vagifem is superior to vaginal Premarin in induction of endometrial thickness in the frozen-thawed cycle patients with refractory endometria: A randomized clinical trial. Iran. J. Reprod. Med. 2014, 12, 415–420. [Google Scholar]
- Noventa, M.; Vitagliano, A.; Andrisani, A.; Blaganje, M.; Viganò, P.; Papaelo, E.; Scioscia, M.; Cavallin, F.; Ambrosini, G.; Cozzolino, M. Testosterone therapy for women with poor ovarian response undergoing IVF: A meta-analysis of randomized controlled trials. J. Assist. Reprod. Genet. 2019, 36, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Davar, R.; Miraj, S.; Farid Mojtahedi, M. Effect of adding human chorionic gonadotropin to frozen thawed embryo transfer cycles with history of thin endometrium. Int. J. Reprod. Biomed. 2016, 14, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Qublan, H.; Amarin, Z.; Al-Qudah, M.; Diab, F.; Nawasreh, M.; Malkawi, S.; Balawneh, M. Luteal phase support with GnRH-a improves implantation and pregnancy rates in IVF cycles with endometrium of < or =7 mm on day of egg retrieval. Hum. Fertil. (Camb. Engl.) 2008, 11, 43–47. [Google Scholar]
- Weckstein, L.N.; Jacobson, A.; Galen, D.; Hampton, K.; Hammel, J. Low-dose aspirin for oocyte donation recipients with a thin endometrium: Prospective, randomized study. Fertil. Steril. 1997, 68, 927–930. [Google Scholar] [CrossRef]
- Firouzabadi, R.D.; Davar, R.; Hojjat, F.; Mahdavi, M. Effect of sildenafil citrate on endometrial preparation and outcome of frozen-thawed embryo transfer cycles: A randomized clinical trial. Iran. J. Reprod. Med. 2013, 11, 151–158. [Google Scholar]
- Acharya, S.; Yasmin, E.; Balen, A.H. The use of a combination of pentoxifylline and tocopherol in women with a thin endometrium undergoing assisted conception therapies—A report of 20 cases. Hum. Fertil. (Camb. Engl.) 2009, 12, 198–203. [Google Scholar] [CrossRef]
- Bodombossou-Djobo, M.M.; Zheng, C.; Chen, S.; Yang, D. Neuromuscular electrical stimulation and biofeedback therapy may improve endometrial growth for patients with thin endometrium during frozen-thawed embryo transfer: A preliminary report. Reprod. Biol. Endocrinol. 2011, 9, 122. [Google Scholar] [CrossRef] [Green Version]
- Sarvi, F.; Arabahmadi, M.; Alleyassin, A.; Aghahosseini, M.; Ghasemi, M. Effect of increased endometrial thickness and implantation rate by granulocyte colony-stimulating factor on unresponsive thin endometrium in fresh in vitro fertilization cycles: A randomized clinical trial. Obstet. Gynecol. Int. 2017, 2017, 3596079. [Google Scholar] [CrossRef] [Green Version]
- Kunicki, M.; Lukaszuk, K.; Liss, J.; Skowronska, P.; Szczyptanska, J. Granulocyte colony stimulating factor treatment of resistant thin endometrium in women with frozen-thawed blastocyst transfer. Syst. Biol. Reprod. Med. 2017, 63, 49–57. [Google Scholar] [CrossRef]
- Wang, X.; Liu, L.; Mou, S.; Zhao, H.; Fang, J.; Xiang, Y.; Zhao, T.; Sha, T.; Ding, J.; Hao, C. Investigation of platelet-rich plasma in increasing proliferation and migration of endometrial mesenchymal stem cells and improving pregnancy outcome of patients with thin endometrium. J. Cell. Biochem. 2019, 120, 7403–7411. [Google Scholar] [CrossRef] [PubMed]
- Scaglione, F.; Donde, S.; Hassan, T.A.; Jannini, E.A. Phosphodiesterase type 5 inhibitors for the treatment of erectile dysfunction: Pharmacology and clinical impact of the sildenafil citrate orodispersible tablet formulation. Clin. Ther. 2017, 39, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Kortam, M.F.; Mohammad, H.F.; Mobarak, M.H.; Bazazo, A.I. The effect of estradiol valerate with and without oral sildenafil on endometrial thickness and pregnancy rates in infertile women: A R.C.T. Evid. Based Womens Health J. 2018, 8, 5. [Google Scholar] [CrossRef]
- Jerzak, M.; Kniotek, M.; Mrozek, J.; Gorski, A.; Baranowski, W. Sildenafil citrate decreased natural killer cell activity and enhanced chance of successful pregnancy in women with a history of recurrent miscarriage. Fertil. Steril. 2008, 90, 1848–1853. [Google Scholar] [CrossRef]
- Forman, E.J.; Hong, K.H.; Ferry, K.M.; Tao, X.; Taylor, D.; Levy, B.; Treff, N.R.; Scott, R.T., Jr. In vitro fertilization with single euploid blastocyst transfer: A randomized controlled trial. Fertil. Steril. 2013, 100, 100–107.e101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammar, I.M.M.; Salem, M.A.A. Effect of oral Tadalafil on endometrial thickness in patients receiving Clomiphene citrate for ovulation induction. Middle East Fertil. Soc. J. 2018, 23, 121–125. [Google Scholar] [CrossRef]
- Mendez Lozano, D.H.; Lenero, M.V.; Gonzalez, R.L.; Scheffer, J.B.; Gonzalez, M.T.; Barron, Y.; Frydman, R. Tadalafil for Endometrial Growth in Clomiphene Citrate stimulated cycles in an IUI programma: A pilot study. Facts Views Vis. ObGyn 2015, 7, 231–237. [Google Scholar]
- Kim, K.R.; Lee, H.S.; Ryu, H.E.; Park, C.Y.; Min, S.H.; Park, C.; Jee, B.C. Efficacy of luteal supplementation of vaginal sildenafil and oral estrogen on pregnancy rate following IVF-ET in women with a history of thin endometria: A pilot study. Medicine 2010, 3, 155–158. [Google Scholar] [CrossRef]
- Das, V.; Agarwal, A.; Pandey, A.; Jain, V.; Agarwal, S.; Ara, A. P793 Evaluation of role of sildenafil in improving IUI success rates. Int. J. Gynecol. Obstet. 2009, 107, S638. [Google Scholar] [CrossRef]
- Chanona, J.; Garcia, M.; Ruvalcaba, L.; Bermudez, A.; Muniz, M.; Beltran, M.; Cuneo, S. The Mexican experience in the use of vaginal sildenafil in patients with poor endometrial response. Int. Congr. Ser. 2004, 1271, 19–21. [Google Scholar] [CrossRef]
- Margreiter, M.; Weghofer, A.; Feichtinger, W. Vaginal sildenafil in patients with poor endometrial development undergoing in vitro fertilization. Fertil. Steril. 2004, 82, S140. [Google Scholar] [CrossRef]
- Sher, G.; Fisch, J.D. Vaginal sildenafil (Viagra): A preliminary report of a novel method to improve uterine artery blood flow and endometrial development in patients undergoing IVF. Hum. Reprod. (Oxf. Engl.) 2000, 15, 806–809. [Google Scholar] [CrossRef] [Green Version]
- Sher, G.; Fisch, J.D. Effect of vaginal sildenafil on the outcome of in vitro fertilization (IVF) after multiple IVF failures attributed to poor endometrial development. Fertil. Steril. 2002, 78, 1073–1076. [Google Scholar] [CrossRef]
- Takasaki, A.; Tamura, H.; Miwa, I.; Taketani, T.; Shimamura, K.; Sugino, N. Endometrial growth and uterine blood flow: A pilot study for improving endometrial thickness in the patients with a thin endometrium. Fertil. Steril. 2010, 93, 1851–1858. [Google Scholar] [CrossRef]
- Paulus, W.E.; Strehler, E.; Zhang, M.; Jelinkova, L.; El-Danasouri, I.; Sterzik, K. Benefit of vaginal sildenafil citrate in assisted reproduction therapy. Fertil. Steril. 2002, 77, 846–847. [Google Scholar] [CrossRef]
- Fetih, A.N.; Habib, D.M.; Abdelaal, I.I.; Hussein, M.; Fetih, G.N.; Othman, E.R. Adding sildenafil vaginal gel to clomiphene citrate in infertile women with prior clomiphene citrate failure due to thin endometrium: A prospective self-controlled clinical trial. Facts Views Vis. ObGyn 2017, 9, 21–27. [Google Scholar] [PubMed]
- Al-Assadi, A.F.; Al-Rubaye, S.A.; Laaiby, Z. The effect of Sildenafil on endometrial characters in patients with infertility. Tikrit Med. J. 2012, 18, 9. [Google Scholar]
- Ranisavljevic, N.; Raad, J.; Anahory, T.; Grynberg, M.; Sonigo, C. Embryo transfer strategy and therapeutic options in infertile patients with thin endometrium: A systematic review. J. Assist. Reprod. Genet. 2019, 36, 2217–2231. [Google Scholar] [CrossRef] [PubMed]
- Aboelroose, A.A.; Ibrahim, Z.M.; Madny, E.H.; Elmazzahy, A.M.; Taha, O.T. A randomized clinical trial of sildenafil plus clomiphene citrate to improve the success rate of ovulation induction in patients with unexplained infertility. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2020, 150, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Fahmy, A.A.; El Sokkary, M.; Sayed, S. The value of oral sildenafil in the treatment of female infertility: A randomized clinical trial. Life Sci. J. 2015, 12, 5. [Google Scholar]
- Mangal, S.; Mehirishi, S. To study and compare the effect of vaginal sildenafil and estradiol valerate on endometrial thickness, blood flow and pregnancy rates in infertile women undergoing intrauterine insemination. Int. J. Reprod. Contracept. Obstet. Gynecol. 2016, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, T.Y. Oral sildenafil for treatment of female infertility among pco patients: Randomized comparative study. Austin J. Obstet. Gynecol. 2019, 6, 3. [Google Scholar]
- Reddy, L.P.; Madhavi, Y.; Khan, M.I. Role of Sildenafil in ovulation induction—A comparative study of outcomes with Sildenafil in ovulation induction cycles with Clomiphene Citrate. IAIM 2016, 3, 7. [Google Scholar]
- Vardhan, S.; Yadav, P.; Agarwal, R.; Garg, R.; Verma, U.; Pengoria, M. Effect of sildenafil citrate and estradiol valerate on endometrial characteristics in ovulation-induced cycle in women with dysovulatory infertility. J. South Asian Fed. Obstet. Gynaecol. 2019, 11, 3. [Google Scholar]
- Ataalla, W.M.; abd Elhamid, T.; Elhalwagy, A.E.E. Adjuvant sildenafil therapy in poor responders undergoing in vitro fertilization: A prospective, randomized, double-blind, placebo-controlled trial. Middle East Fertil. Soc. J. 2016, 21, 5. [Google Scholar] [CrossRef]
- Check, J.H.; Graziano, V.; Lee, G.; Nazari, A.; Choe, J.K.; Dietterich, C. Neither sildenafil nor vaginal estradiol improves endometrial thickness in women with thin endometria after taking oral estradiol in graduating dosages. Clin. Exp. Obstet. Gynecol. 2004, 31, 99–102. [Google Scholar] [PubMed]
- Kansouh, A.M.; El-Naggar, M.A. Value of vaginal sildenafil citrate for endometrial preparation and outcome in frozen thawed embryo transfer cycles. Med. J. Cairo Univ. 2017, 85, 7. [Google Scholar]
- Moini, A.; Zafarani, F.; Jahangiri, N.; Jahanian Sadatmahalleh, S.H.; Sadeghi, M.; Chehrazi, M.; Ahmadi, F. The effect of vaginal sildenafil on the outcome of assisted reproductive technology cycles in patients with repeated implantation failures: A randomized placebo-controlled trial. Int. J. Fertil. Steril. 2020, 13, 289–295. [Google Scholar] [PubMed]
- Tehraninejad, E.S.; Khazei, N.; Ayati, E.; Movafegh, A.; Azimaraghi, O. Effect of vaginal sildenafil on in vitro fertilization success rates in women with previous failed in vitro fertilization attempts. Asian J. Pharm. Clin. Res. 2018, 11, 486–488. [Google Scholar] [CrossRef]
- Alteri, A.; Corti, L.; Cermisoni, G.C.; Papaleo, E.; Viganò, P.; Noventa, M. Busting the myth of extended blastocyst culture until Day 7: Protocol for systematic review and meta-analysis. Medicine (Baltim.) 2020, 99, e18909. [Google Scholar] [CrossRef]
- Hromadová, L.; Tokareva, I.; Veselá, K.; Trávník, P.; Veselý, J. Endometrial Receptivity Analysis—A tool to increase an implantation rate in assisted reproduction. Ceska Gynekol. 2019, 84, 177–183. [Google Scholar]
- Mahajan, N.; Sharma, S. The endometrium in assisted reproductive technology: How thin is thin? J. Hum. Reprod. Sci. 2016, 9, 3–8. [Google Scholar] [CrossRef]
- Katzorke, N.; Vilella, F.; Ruiz, M.; Krüssel, J.S.; Simón, C. Diagnosis of endometrial-factor infertility: Current approaches and new avenues for research. Geburtshilfe Frauenheilkd. 2016, 76, 699–703. [Google Scholar] [CrossRef] [Green Version]
- Mahajan, N. Endometrial receptivity array: Clinical application. J. Hum. Reprod. Sci. 2015, 8, 121–129. [Google Scholar] [CrossRef]
- Craciunas, L.; Gallos, I.; Chu, J.; Bourne, T.; Quenby, S.; Brosens, J.J.; Coomarasamy, A. Conventional and modern markers of endometrial receptivity: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 202–223. [Google Scholar] [CrossRef]
- Tomic, V.; Kasum, M.; Vucic, K. Impact of embryo quality and endometrial thickness on implantation in natural cycle IVF. Arch. Gynecol. Obstet. 2020, 301, 1325–1330. [Google Scholar] [CrossRef] [Green Version]
- Cozzolino, M.; Vitagliano, A.; Di Giovanni, M.V.; Laganà, A.S.; Vitale, S.G.; Blaganje, M.; Drusany Starič, K.; Borut, K.; Patrelli, T.S.; Noventa, M. Ultrasound-guided embryo transfer: Summary of the evidence and new perspectives. A systematic review and meta-analysis. Reprod. Biomed. Online 2018, 36, 524–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balmaceda, J.P.; Ciuffardi, I. Hysteroscopy and assisted reproductive technology. Obstet. Gynecol. Clin. N. Am. 1995, 22, 507–518. [Google Scholar] [CrossRef]
- De Sá Rosa e de Silva, A.C.; Rosa e Silva, J.C.; Cândido dos Reis, F.J.; Nogueira, A.A.; Ferriani, R.A. Routine office hysteroscopy in the investigation of infertile couples before assisted reproduction. J. Reprod. Med. 2005, 50, 501–506. [Google Scholar]
- Cao, H.; You, D.; Yuan, M.; Xi, M. Hysteroscopy after repeated implantation failure of assisted reproductive technology: A meta-analysis. J. Obstet. Gynaecol. Res. 2018, 44, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Dreisler, E.; Kjer, J.J. Asherman’s syndrome: Current perspectives on diagnosis and management. Int. J. Womens Health 2019, 11, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Cicinelli, E.; Matteo, M.; Trojano, G.; Mitola, P.C.; Tinelli, R.; Vitagliano, A.; Crupano, F.M.; Lepera, A.; Miragliotta, G.; Resta, L. Chronic endometritis in patients with unexplained infertility: Prevalence and effects of antibiotic treatment on spontaneous conception. Am. J. Reprod. Immunol. 2018, 79, e12782. [Google Scholar] [CrossRef]
- Cicinelli, E.; Vitagliano, A.; Kumar, A.; Lasmar, R.B.; Bettocchi, S.; Haimovich, S. Unified diagnostic criteria for chronic endometritis at fluid hysteroscopy: Proposal and reliability evaluation through an international randomized-controlled observer study. Fertil. Steril. 2019, 112, 162–173.e162. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, A.; Saccardi, C.; Litta, P.S.; Noventa, M. Chronic endometritis: Really so relevant in repeated IVF failure? Am. J. Reprod. Immunol. 2017, 78, 28921706. [Google Scholar] [CrossRef] [PubMed]
- Vitagliano, A.; Saccardi, C.; Noventa, M.; Di Spiezio Sardo, A.; Saccone, G.; Cicinelli, E.; Pizzi, S.; Andrisani, A.; Litta, P.S. Effects of chronic endometritis therapy on in vitro fertilization outcome in women with repeated implantation failure: A systematic review and meta-analysis. Fertil. Steril. 2018, 110, 103–112.e101. [Google Scholar] [CrossRef]
- Griesinger, G.; Trevisan, S.; Cometti, B. Endometrial thickness on the day of embryo transfer is a poor predictor of IVF treatment outcome. Hum. Reprod. Open 2018, 2018, hox031. [Google Scholar] [CrossRef]
- Kasius, A.; Smit, J.G.; Torrance, H.L.; Eijkemans, M.J.; Mol, B.W.; Opmeer, B.C.; Broekmans, F.J. Endometrial thickness and pregnancy rates after IVF: A systematic review and meta-analysis. Hum. Reprod. Update 2014, 20, 530–541. [Google Scholar] [CrossRef]
- Oron, G.; Hiersch, L.; Rona, S.; Prag-Rosenberg, R.; Sapir, O.; Tuttnauer-Hamburger, M.; Shufaro, Y.; Fisch, B.; Ben-Haroush, A. Endometrial thickness of less than 7.5 mm is associated with obstetric complications in fresh IVF cycles: A retrospective cohort study. Reprod. Biomed. Online 2018, 37, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Lebovitz, O.; Orvieto, R. Treating patients with “thin” endometrium—An ongoing challenge. Gynecol. Endocrinol. Off. J. Int. Soc. Gynecol. Endocrinol. 2014, 30, 409–414. [Google Scholar] [CrossRef]
- Benni, J.M.; Patil, P.A. An overview on sildenafil and female infertility. Indian J. Health Sci. 2016, 9, 6. [Google Scholar] [CrossRef]
- Madeira, C.R.; Tonin, F.S.; Fachi, M.M.; Borba, H.H.; Ferreira, V.L.; Leonart, L.P.; Bonetti, A.F.; Moritz, R.P.; Trindade, A.; Gonçalves, A.G.; et al. Efficacy and safety of oral phosphodiesterase 5 inhibitors for erectile dysfunction: A network meta-analysis and multicriteria decision analysis. World J. Urol. 2020, 39, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Gambino, L.S.; Wreford, N.G.; Bertram, J.F.; Dockery, P.; Lederman, F.; Rogers, P.A. Angiogenesis occurs by vessel elongation in proliferative phase human endometrium. Hum. Reprod. (Oxf. Engl.) 2002, 17, 1199–1206. [Google Scholar] [CrossRef]
- Girling, J.E.; Rogers, P.A. Recent advances in endometrial angiogenesis research. Angiogenesis 2005, 8, 89–99. [Google Scholar] [CrossRef]
- Okada, H.; Tsuzuki, T.; Shindoh, H.; Nishigaki, A.; Yasuda, K.; Kanzaki, H. Regulation of decidualization and angiogenesis in the human endometrium: Mini review. J. Obstet. Gynaecol. Res. 2014, 40, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Barroso, R.P.; Osuamkpe, C.; Nagamani, M.; Yallampalli, C. Nitric oxide inhibits development of embryos and implantation in mice. Mol. Hum. Reprod. 1998, 4, 503–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaughan, D.A.; Harrity, C.; Sills, E.S.; Mocanu, E.V. Serum estradiol:oocyte ratio as a predictor of reproductive outcome: An analysis of data from >9000 IVF cycles in the Republic of Ireland. J. Assist. Reprod. Genet. 2016, 33, 481–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickey, R.P.; Taylor, S.N.; Lu, P.Y.; Sartor, B.M.; Rye, P.H.; Pyrzak, R. Relationship of follicle numbers and estradiol levels to multiple implantation in 3608 intrauterine insemination cycles. Fertil. Steril. 2001, 75, 69–78. [Google Scholar] [CrossRef]
- Hughes, E.G.; Robertson, D.M.; Handelsman, D.J.; Hayward, S.; Healy, D.L.; de Kretser, D.M. Inhibin and estradiol responses to ovarian hyperstimulation: Effects of age and predictive value for in vitro fertilization outcome. J. Clin. Endocrinol. Metab. 1990, 70, 358–364. [Google Scholar] [CrossRef] [PubMed]
| Author and Year | Study Design and Time of Realization | Participants and Main Inclusion Criteria (Number) | Enrollment Criteria | Controlled Ovarian Stimulation Protocols | Intervention Group | Main Finding |
|---|---|---|---|---|---|---|
| Kortam, M.A.M.F. et al., 2019 | - Type: Single-center RCT - Duration: from October 2017 to May 2018 - Randomization: closed envelope method - Blinding: not reported - Number of protocol: NCT03301233 | 90 patients undergoing timed intercourse Women: - Total: 90 - Treatment 45 - Control 45 | - unexplained infertility - Age 18–35 years - BMI < 30 - ovulatory cycles - No presence of any organic lesion of uterus, ovaries or tubes | - CC 100 mg/day starting from day 2 of the cycle for 5 days - oral E2 2 mg 2X/die from day 2 of the cycle till hCG - U-hCG (5000 UI) at leading follicle size of 18 mm - Luteal phase support with oral progesterone from day of ovulation (Dufaston 10 mg X2/daily) | Treatment group: - Treatment: Sildenafil oral, 75 mg/day - Timing: from cycle day 2 to HCG administration Control group: - Treatment: placebo - Timing: from cycle day 2 to HCG administration | Significant improvement of endometrial thickness and pattern in Sildenafil treated women No other significant beneficial effects |
| Mohamed, T.Y., 2019 | - Type: Single-center RCT - Randomization: simple - Blinding: not reported | 40 patients undergoing timed intercourse Women: - Total: 40 - Treatment 20 - Control 20 | - anovulatory infertility - Age: 18-40-years - polycystic ovary - normal baseline FSH and LH - normal uterus - no prior ovarian or adnexal surgery or organic pelvic pathology | - letrozole 5 mg from 3rd to 7th day of the cycle - U-hCG (10,000 UI) | Treatment group: - Treatment: Sildenafil oral, 50 mg/day - Timing: from cycle day 8 to hCG administration Control group: no intervention | In Sildenafil treated group: - Significant improvement of endometrial thickness - Significantly higher clinical pregnancy rate |
| Fahmy, A.A. et al., 2015 | - Type: Single-center RCT - Duration: from January to July 2012 - Randomization: sealed envelope - Blinding: not reported | 70 patients undergoing timed intercourse Women: - Total: 70 - Treatment 35 - Control 35 | - primary or secondary infertility - Age: 18-40 years - Regular menstrual cycles - No ovarian cysts, abnormal hormonal profile, history of any pelvic pathology | - clomiphene citrate 150 mg from 3rd to 7th day of the cycle - U-hCG (5000 UI) | Treatment group: - Treatment: Sildenafil oral, 75 mg/day - Timing: from cycle day 7 to day 11 of the cycle Control group: - Treatment: placebo - Timing: from cycle day 7 to day 11 of the cycle | In Sildenafil treated group: - Significant improvement of endometrial thickness - Significantly higher chemical pregnancy rate |
| Mangal, S., Mahirishi, S., 2016 | - Type: Single-center RCT - Duration: from July 2014 to April 2015 - Randomization: not reported - Blinding: not reported | 100 patients undergoing Intra Uterine Insemination Women: - Total: 100 - Treatment 50 - Control 50 | - thin endometrium (Day 8 ET <7mm) - Age: <40 years | clomiphene citrate or gon-adotropin | Treatment group: - Treatment: Sildenafil vaginal, 100 mg/day - Timing: from cycle day 8 of the cycle Control group: - Treatment: E2 2 mg 6–8 hourly | Significantly higher cumulative clinical pregnancy rate (3 intrauterine insemination cycles). Not significant improvement of endometrial thickness |
| Vardhan, S. et al., 2019 | - Type: Single-center RCT - Duration: 2 years - Randomization: not reported - Blinding not reported | 80 women undergoing timed intercourse Women: - Total: 80 - Treatment 40 - Control 40 | - Endometrial thickness of <7 mm on the day of ovulation - Age: 35 years - No organic pelvic pathology, congenital uterine anomaly, acquired deformities of uterus (Asherman syndrome) | clomiphene citrate 50 mg from 2nd to 6th day of the cycle estradiol valerate tablets orally by the step-up method: from the first day to the 4th day, 2 mg estradiol valerate tablets, and from the 5th to the 8th day, 4 mg estradiol tablets and from the 9th to the 12th day of the menstrual cycle, 6 mg estradiol valerate were given daily | Treatment group: - Treatment: Sildenafil oral, 25 mg/day - Timing: from cycle day 1 of the cycle to day 12 Control group: no intervention | In Sildenafil treated group: - Significant improvement of endometrial thickness - Significantly higher clinical pregnancy rate. |
| Reddy, L.P. et al., 2016 | - Type: Single-center RCT - Duration: 4 months - Randomization: not reported - Blinding not reported | 80 women undergoing timed intercourse Women: - Total: 80 - Treatment 40 - Control 40 | - Age: <40 years - Regular menstrual cycles - No organic pelvic pathology - No endocrine disorders except thyroid disorder | clomiphene citrate 100 mg from 3rd to 7th day of the cycle | Treatment group: - Treatment: Sildenafil oral, 50 mg/day - Timing: from cycle day 8 of the cycle Control group: no intervention | In Sildenafil treated group: - Significantly improvement of endometrial thickness - Significantly higher chemical pregnancy rate. |
| Author and Year | Study Design and Time of Realization | Participants and Main Inclusion Criteria (Number) | Enrollment Criteria | Controlled Ovarian Stimulation Protocols | Intervention Group | Main Finding |
|---|---|---|---|---|---|---|
| Moini, A. et al., 2020 | - Type: Single-center RCT - Duration: February 2014 to November 2016 - Randomization: random allocation sequence generated by a randomized block design - Blinding: double-blind - Number of protocol: NCT03192709 | 66 patients undergoing IVF fresh cycles Intention to treat: - Total: 66 - Treatment A: 22 - Treatment B: 22 - Control: 22 Per protocol: - Total: 66 - Treatment A: 21 - Treatment B: 18 - Control: 17 | - AMH >1.5, FSH < 10 - at least 2 consecutive failed IVF-ET cycles with at least a transfer of two good quality embryos - hCG day endometrial thickness <7 mm in all prior IVF/ICSI attempts - Age ≤ 38 years - No history of myomectomy or Asherman’s syndrome - normal endometrial appearance | - GnRH-ag long protocol - FSH/FSH +LH - U-hCG (10000 IU) at follicle size 18mm (≥2). - Oocyte retrieval 36 h after hCG - 2/3 days embryos transfer - Luteal phase support with progesterone (100 mg IM daily or 800 mg vaginal daily) | Treatment group A: - Treatment: Sildenafil vaginal, 100 mg/day - Timing: from cycle day 1 to OPU Treatment group B: - Treatment: Placebo then Sildenafil vaginal, 100 mg/day - Timing: placebo from cycle day 1 to 2 days before hCG administration, then Sildenafil to to OPU Control group: - Treatment: Placebo - Timing: from cycle day 1 to OPU | No significant beneficial effects |
| Shahrokh, T.N.E. et al., 2018 | - Type: Single-center RCT - Randomization: simple - Blinding: not reported | 72 patients undergoing IVF fresh cycles Women: - Total: 72 - Treatment 36 - Control 36 | - ≥2 failed IVF- ET cycles - Age <45 years (21–43) | - GnRH-ag long protocol Buserelin 1.5 mg daily - FSH (dose according to patients’ characteristics) | Treatment group: - Treatment: Sildenafil vaginal, 100 mg/day - Timing: from cycle day 2 to HCG administration Control group: no intervention | Significant improvement of endometrial thickness in Sildenafil treated women No other significant beneficial effects |
| Ataalla, W.M. et al., 2016 | - Type: Single-center RCT - Duration: from January 2012 to January 2014 - Randomization: women asked to choose a number from 1 to 60 - Blinding: double-blinded | 60 patients undergoing IVF fresh cycles Women: - Total: 60 - Treatment 30 - Control 30 | - Age ≤ 35 - No ovarian surgery - Low responders to COH: ≤ 3 follicles on the day of hCG administration or ≤ 3 oocytes - previous cycle cancellation due to poor follicular development | - GnRH-antagonist protocol - FSH 300 IU + 150 hMG from 2nd day of cycle, dose adjusted according patient characteristics - U-hCG (10,000 UI) at leading follicle(s) size of 17 mm - Oocyte retrieval 34–36 h after hCG - Day 3 embryos transferred - Luteal phase support with progesterone (150 mg IM daily) | Treatment group: - Treatment: Sildenafil oral, 50 mg/day - Timing: from cycle day 1 Control group: - Treatment: Placebo oral - Timing: from cycle day 1 | Significant improvement of endometrial thickness in Sildenafil treated women. No other significant beneficial effects. |
| Firouzabadi, R.D. et al. 2013 | - Type: Single-center RCT - Duration: from 2009 to 2011 - Randomization: random numbers tables - Blinding: not reported - Number of protocol: IRCT201210232575N3 NCT01668446 | 80 patients undergoing FET cycles Women: - Total: 80 - Treatment 40 - Control 40 | - an antecedent of poor endometrial response - high-grade embryos - Age < 40 years - No history of endocrine diseases -No hysteroscopic surgeries. | - estradiol by a step-up method while in menstruation - 3 days high-grade embryos transferred - Luteal phase support with progesterone (100 mg IM daily) - E2 and P4 were measured in an hour after first P4 injection | Treatment group: - Treatment: Sildenafil vaginal, 50 mg/day - Timing: during endometrial preparation until the start P4 administration Control group: no intervention | In Sildenafil treated group: - Significant improvement of endometrial thickness and pattern - Significantly higher chemical pregnancy rate |
| Check, J.H. et al., 2004 | Type: Single-center RCT - Randomization: random numbers table - Blinding: not reported | 20 patients undergoing FET cycles Intention to treat: - Total: 20 - Treatment 10 - Control 10 Per protocol: - Total: 16 - Treatment 9 - Control 7 | - failed to attain an 8 mm endometrial thickness with oral E2 | - E2 oral regimen Step up method: E2 2 mg × 5 days, 4 mg × 4 days, 6 mg × 5 days - Luteal phase support with progesterone: 200 mg twice daily vaginal suppositories and 100 mg in oil daily | Treatment group: - Treatment: Sildenafil vaginal, 100 mg/day - Timing: from day 3 to day 9 of endometrial preparation Control group: Vaginal E2 2 mg 2× per day Timing: from day 2 to peak thickness | No significant beneficial effects |
| Kahsouh, A.M. et al., 2017 | Type: Single-center RCT - Duration: Jun 2015-Dec. 2016 - Randomization: simple - Blinding: not reported | 90 patients undergoing FET cycles Women: - Total: 90 - Treatment 45 - Control 45 | - an antecedent of poor endometrial response and frozen embryos 2 mg of estradiol valerate 6–8 hourly from the day 2–14 of the menstrual cycle | - estradiol valerate 2 mg, every 6–8 h from the day 2 to day 14 of the menstrual cycle - Luteal phase support with progesterone: 400 mg pessaries vaginal 3 days prior embryo transfer | Treatment group: - Treatment: Sildenafil vaginal, 100 mg/day - Timing: from day 2 of menstrual cycle and discontinued 48–72 hours prior to the embryo transfer. Control group: E2: 2 mg every 6–8 h | Significant improvement of endometrial thickness and patternNo other significant beneficial effects |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marin, L.; Andrisani, A.; Bordin, L.; Dessole, F.; Noventa, M.; Vitagliano, A.; Capobianco, G.; Ambrosini, G. Sildenafil Supplementation for Women Undergoing Infertility Treatments: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 4346. https://doi.org/10.3390/jcm10194346
Marin L, Andrisani A, Bordin L, Dessole F, Noventa M, Vitagliano A, Capobianco G, Ambrosini G. Sildenafil Supplementation for Women Undergoing Infertility Treatments: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine. 2021; 10(19):4346. https://doi.org/10.3390/jcm10194346
Chicago/Turabian StyleMarin, Loris, Alessandra Andrisani, Luciana Bordin, Francesco Dessole, Marco Noventa, Amerigo Vitagliano, Giampiero Capobianco, and Guido Ambrosini. 2021. "Sildenafil Supplementation for Women Undergoing Infertility Treatments: A Systematic Review and Meta-Analysis of Randomized Controlled Trials" Journal of Clinical Medicine 10, no. 19: 4346. https://doi.org/10.3390/jcm10194346
APA StyleMarin, L., Andrisani, A., Bordin, L., Dessole, F., Noventa, M., Vitagliano, A., Capobianco, G., & Ambrosini, G. (2021). Sildenafil Supplementation for Women Undergoing Infertility Treatments: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Journal of Clinical Medicine, 10(19), 4346. https://doi.org/10.3390/jcm10194346









