Vascular Protective Effects of New Oral Anticoagulants in Patients with Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
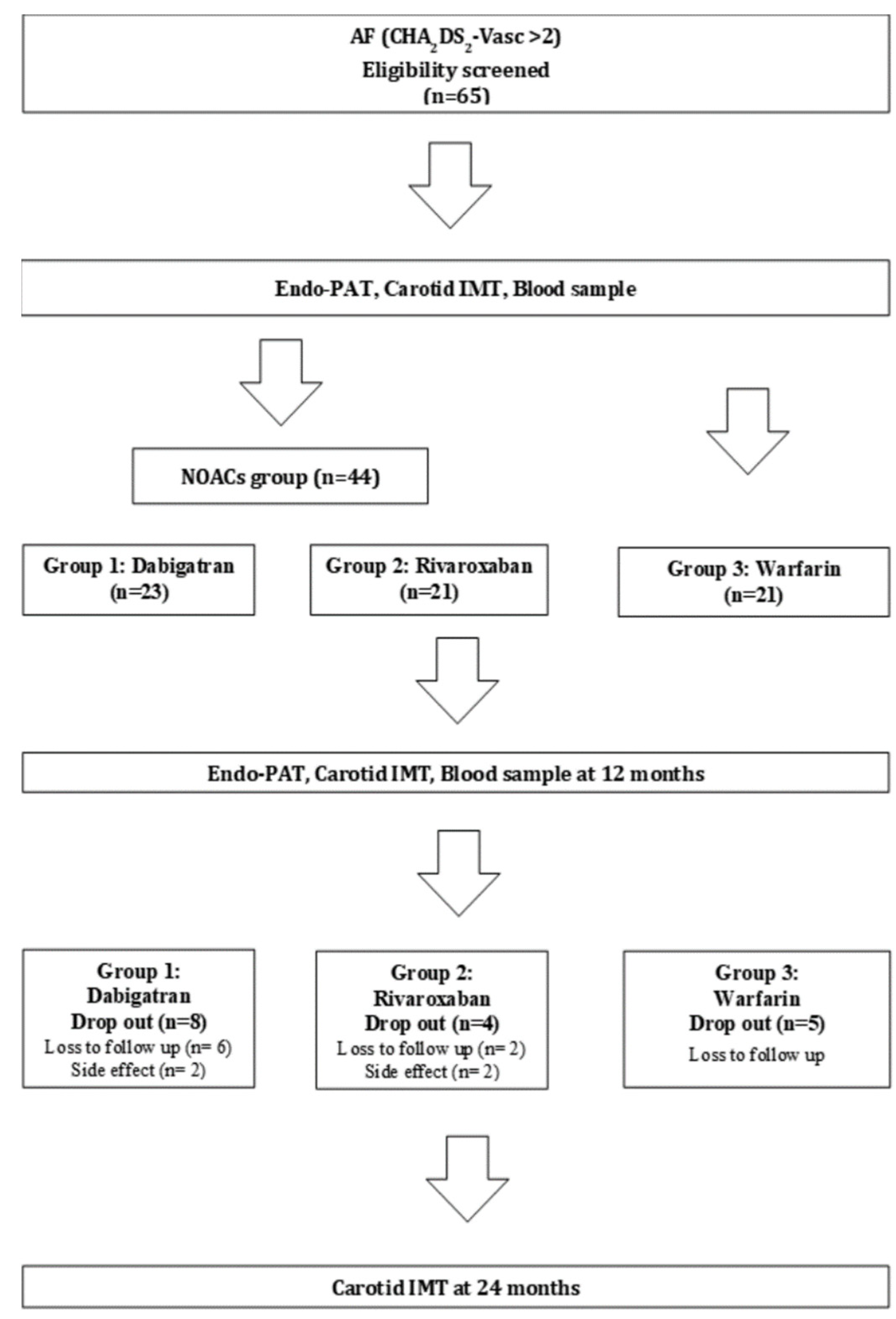
2.1. Study Subjects
2.2. Randomization
2.3. Primary Outcome
2.4. RHI Measurement
2.5. Carotid IMT
2.6. Statistical Analyses
2.7. Biomarkers of Atherosclerotic Plaque
3. Results
3.1. Patient Characteristics
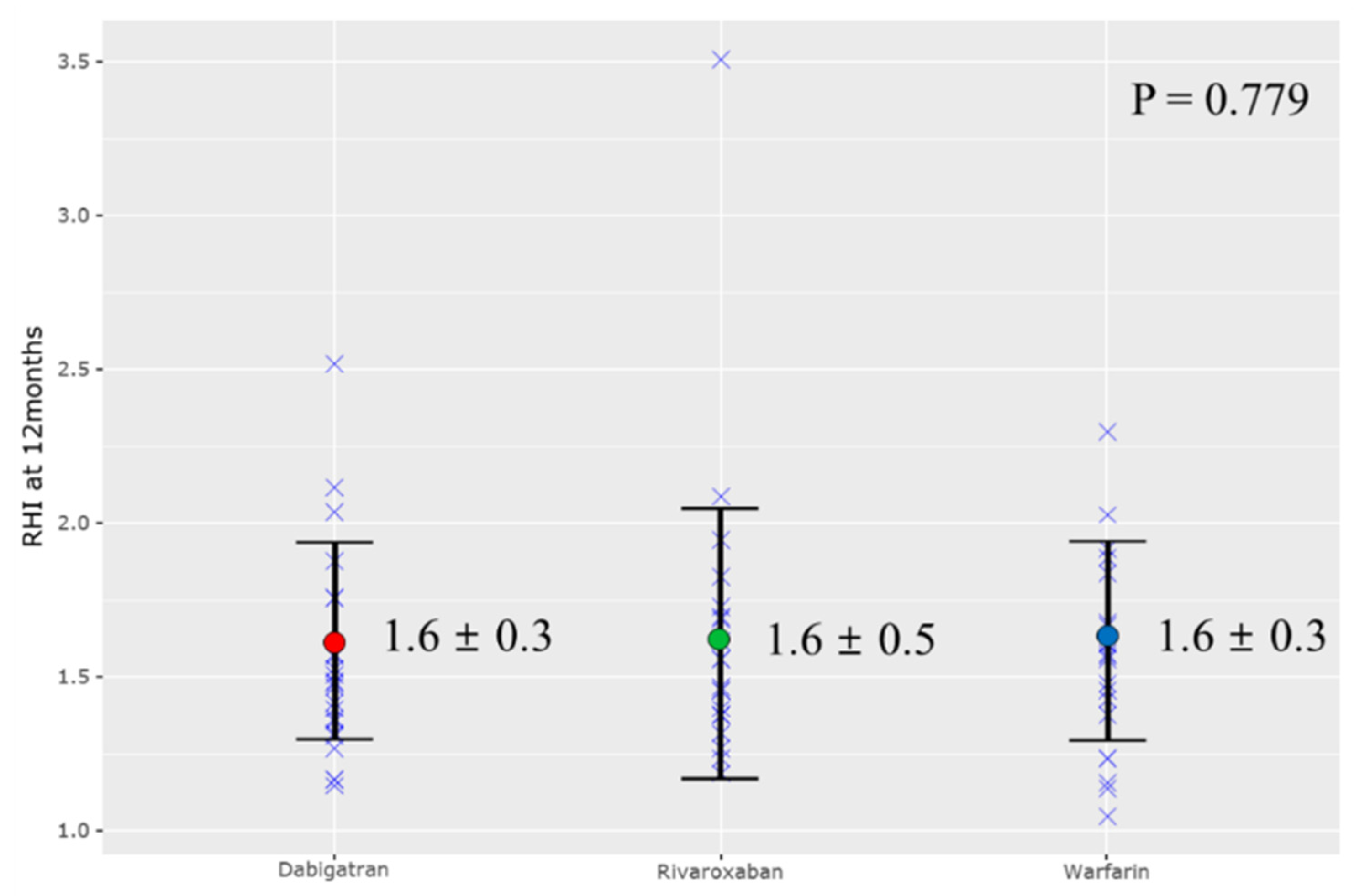
3.2. Reactive Hyperemia Index (RHI) and Carotid IMT
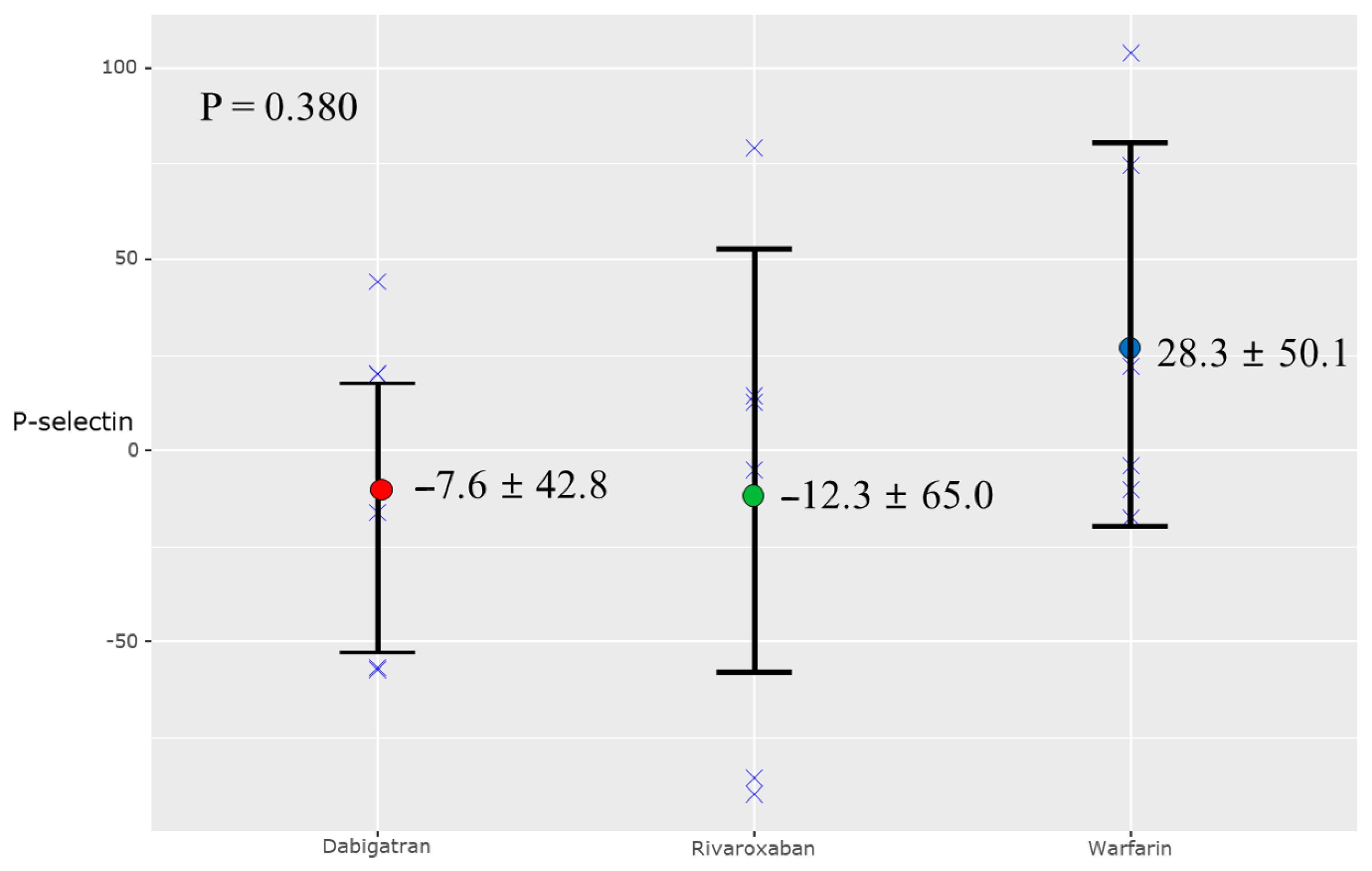
3.3. Biomarkers of Atherosclerotic Plaque
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esmon, C.T. Targeting factor Xa and thrombin: Impact on coagulation and beyond. Thromb. Haemost. 2014, 111, 625–633. [Google Scholar] [CrossRef]
- Borissoff, J.I.; Spronk, H.M.H.; ten Cate, H. The Hemostatic System as a Modulator of atherosclerosis. N. Engl. J. Med. 2011, 364, 1746–1760. [Google Scholar] [CrossRef]
- Lee, I.O.; Kratz, M.T.; Schirmer, S.H.; Baumhakel, M.; Bohm, M. The effects of direct thrombin inhibition with dabigatran on plaque formation and endothelial function in apolipoprotein E-deficient mice. J. Pharmacol. Exp. Ther. 2012, 343, 253–257. [Google Scholar] [CrossRef] [Green Version]
- Borissoff, J.I.; Otten, J.J.; Heeneman, S.; Leenders, P.; van Oerle, R.; Soehnlein, O.; Loubele, S.T.; Hamulyak, K.; Hackeng, T.M.; Daemen, M.J.; et al. Genetic and pharmacological modifications of thrombin formation in apolipoprotein e-deficient mice determine atherosclerosis severity and atherothrombosis onset in a neutrophil-dependent manner. PLoS ONE 2013, 8, e55784. [Google Scholar] [CrossRef]
- Musz, P.; Podhajski, P.; Grzelakowska, K.; Umińska, J.M. Non-invasive assessment of endothelial function–a review of available methods. Med. Res. J. 2021, 6, 53–58. [Google Scholar] [CrossRef]
- Bonetti, P.O.; Pumper, G.M.; Higano, S.T.; Holmes, D.R., Jr.; Kuvin, J.T.; Lerman, A. Noninvasive identification of patients with early coronary atherosclerosis by assessment of digital reactive hyperemia. J. Am. Coll. Cardiol. 2004, 44, 2137–2141. [Google Scholar] [CrossRef] [Green Version]
- Rubinshtein, R.; Kuvin, J.T.; Soffler, M.; Lennon, R.J.; Lavi, S.; Nelson, R.E.; Pumper, G.M.; Lerman, L.O.; Lerman, A. Assessment of endothelial function by non-invasive peripheral arterial tonometry predicts late cardiovascular adverse events. Eur. Heart J. 2010, 31, 1142–1148. [Google Scholar] [CrossRef] [Green Version]
- Simon, A.; Gariepy, J.; Chironi, G.; Megnien, J.-L.; Levenson, J. Intima–media thickness: A new tool for diagnosis and treatment of cardiovascular risk. J. Hypertens. 2002, 20, 159–169. [Google Scholar] [CrossRef]
- Halcox, J.P.; Donald, A.E.; Ellins, E.; Witte, D.R.; Shipley, M.J.; Brunner, E.J.; Marmot, M.G.; Deanfield, J.E. Endothelial function predicts progression of carotid intima-media thickness. Circulation 2009, 119, 1005–1012. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.J.; Jeong, M.H.; Cho, S.H.; Kim, K.H.; Lee, M.G.; Park, K.H.; Sim, D.S.; Yoon, N.S.; Hong, Y.J.; Kim, J.H.; et al. Endothelial dysfunction and increased carotid intima-media thickness in the patients with slow coronary flow. J. Korean Med. Sci. 2012, 27, 614–618. [Google Scholar] [CrossRef]
- Kim, J.B.; Joung, H.J.; Lee, J.M.; Woo, J.S.; Kim, W.S.; Kim, K.S.; Lee, K.H.; Kim, W. Evaluation of the vascular protective effects of new oral anticoagulants in high-risk patients with atrial fibrillation (PREFER-AF): Study protocol for a randomized controlled trial. Trials 2016, 17, 422. [Google Scholar] [CrossRef] [Green Version]
- Clarkson, P.; Montgomery, H.E.; Mullen, M.J.; Donald, A.E.; Powe, A.J.; Bull, T.; Jubb, M.; World, M.; Deanfield, J.E. Exercise training enhances endothelial function in young men. J. Am. Coll. Cardiol. 1999, 33, 1379–1385. [Google Scholar] [CrossRef] [Green Version]
- Flammer, A.J.; Hermann, F.; Wiesli, P.; Schwegler, B.; Chenevard, R.; Hürlimann, D. Effect of losartan, compared with atenolol, on endothelial function and oxidative stress in patients with type 2 diabetes and hypertension. J. Hypertens 2007, 24, 785–791. [Google Scholar] [CrossRef]
- Reriani, M.K.; Dunlay, S.M.; Gupta, B.; West, C.P.; Rihal, C.S.; Lerman, L.O.; Lerman, A. Effects of statins on coronary and peripheral endothelial function in humans: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Cardiovasc. Prev. Rehabil. 2011, 18, 704–716. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.X.; Lim, H.S.; Schultz, C.D.; Sanders, P.; Worthley, M.I.; Willoughby, S.R. Assessment of endothelial function in atrial fibrillation: Utility of peripheral arterial tonometry. Clin. Exp. Pharmacol. Physiol. 2012, 39, 141–144. [Google Scholar] [CrossRef]
- Woo, J.S.; Jang, W.S.; Kim, H.S.; Lee, J.H.; Choi, E.Y.; Kim, J.B.; Kim, W.S.; Kim, K.S.; Kim, W. Comparison of peripheral arterial tonometry and flow-mediated vasodilation for assessment of the severity and complexity of coronary artery disease. Coron. Artery Dis. 2014, 25, 421–426. [Google Scholar] [CrossRef]
- Skalidis, E.I.; Zacharis, E.A.; Tsetis, D.K.; Pagonidis, K.; Chlouverakis, G.; Yarmenitis, S.; Hamilos, M.; Manios, E.G.; Vardas, P.E. Endothelial cell function during atrial fibrillation and after restoration of sinus rhythm. Am. J. Cardiol. 2007, 99, 1258–1262. [Google Scholar] [CrossRef]
- Yoshino, S.; Yoshikawa, A.; Hamasaki, S.; Ishida, S.; Oketani, N.; Saihara, K.; Okui, H.; Kuwahata, S.; Fujita, S.; Ichiki, H.; et al. Atrial fibrillation-induced endothelial dysfunction improves after restoration of sinus rhythm. Int. J. Cardiol. 2013, 168, 1280–1285. [Google Scholar] [CrossRef]
- Borissoff, J.I.; Heeneman, S.; Kilinc, E.; Kassak, P.; Van Oerle, R.; Winckers, K.; Govers-Riemslag, J.W.; Hamulyak, K.; Hackeng, T.M.; Daemen, M.J.; et al. Early atherosclerosis exhibits an enhanced procoagulant state. Circulation 2010, 122, 821–830. [Google Scholar] [CrossRef] [Green Version]
- Ragosta, M.; Gimple, L.W.; Gertz, S.; Dunwiddie, C.T.; Vlasuk, G.P.; Haber, H.; Powers, E.R.; Roberts, W.C.; Sarembock, I. Specific factor Xa inhibition reduces restenosis after balloon angioplasty of atherosclerotic femoral arteries in rabbits. Circulation 1994, 89, 1262–1271. [Google Scholar] [CrossRef] [Green Version]
- Antoniak, S.; Sparkenbaugh, E.; Pawlinski, R. Tissue factor, protease activated receptors and pathologic heart remodelling. Thromb. Haemost. 2014, 112, 893–900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posthuma, J.J.; Posma, J.J.N.; van Oerle, R.; Leenders, P.; van Gorp, R.H.; Jaminon, A.M.G.; Mackman, N.; Heitmeier, S.; Schurgers, L.J.; Ten Cate, H.; et al. Targeting Coagulation Factor Xa Promotes Regression of Advanced Atherosclerosis in Apolipoprotein-E Deficient Mice. Sci. Rep. 2019, 9, 3909. [Google Scholar] [CrossRef] [Green Version]
- Win, T.T.; Nakanishi, R.; Osawa, K.; Li, D.; Susaria, S.S.; Jayawardena, E.; Hamal, S.; Kim, M.; Broersen, A.; Kitslaar, P.H.; et al. Apixaban versus warfarin in evaluation of progression of atherosclerotic and calcified plaques (prospective randomized trial). Am. Heart J. 2019, 212, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Pingel, S.; Tiyerili, V.; Mueller, J.; Werner, N.; Nickenig, G.; Mueller, C. Thrombin inhibition by dabigatran attenuates atherosclerosis in ApoE deficient mice. Arch. Med. Sci. 2014, 10, 154–160. [Google Scholar] [CrossRef] [Green Version]
- Kirchhof, P.; Ezekowitz, M.D.; Purmah, Y.; Schiffer, S.; Meng, I.L.; Camm, A.J.; Hohnloser, S.H.; Schulz, A.; Wosnitza, M.; Cappato, R. Effects of Rivaroxaban on Biomarkers of Coagulation and Inflammation: A Post Hoc Analysis of the X-VeRT Trial. TH Open 2020, 4, e20–e32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, N.H.; Holme, P.A.; Bjornsen, S.; Henriksson, C.E.; Sandset, P.M.; Jacobsen, E.M. The impact of rivaroxaban on primary hemostasis in patients with venous thrombosis. Platelets 2020, 31, 43–47. [Google Scholar] [CrossRef]
| Dabigatran | Rivaroxaban | Warfarin | p-Value | |
|---|---|---|---|---|
| (n = 23) | (n = 21) | (n = 21) | ||
| Age (years) | 67.1 ± 9.4 | 64.3 ± 7.7 | 67.7 ± 7.1 | 0.360 |
| Sex | 0.083 | |||
| Female | 11 (47.8%) | 5 (23.8%) | 4 (19.0%) | |
| Male | 12 (52.2%) | 16 (76.2%) | 17 (81.0%) | |
| BMI (kg/m2) | 25.3 ± 2.8 | 25.6 ± 3.1 | 25.3 ± 3.3 | 0.928 |
| Smoking | 0.586 | |||
| Current | 3 (13.0%) | 3 (14.3%) | 6 (28.6%) | |
| Former | 5 (21.7%) | 8 (38.1%) | 5 (23.8%) | |
| Never | 14 (60.9%) | 10 (47.6%) | 9 (42.9%) | |
| Medical history | ||||
| Congestive heart failure | 6 (26.1%) | 9 (42.9%) | 6 (28.6%) | 0.447 |
| Diabetes mellitus | 8 (34.8%) | 2 (9.5%) | 6 (28.6%) | 0.133 |
| Hypertension | 17 (73.9%) | 15 (71.4%) | 11 (52.4%) | 0.265 |
| Dyslipidemia | 12 (52.2%) | 12 (57.1%) | 8 (38.1%) | 0.385 |
| Current medication | ||||
| Aspirin | 2 (8.7%) | 4 (19.0%) | 2 (9.5%) | 0.519 |
| ACEi or ARB | 10 (43.5%) | 8 (38.1%) | 7 (33.3%) | 0.787 |
| Beta blocker | 15 (65.2%) | 12 (57.1%) | 9 (42.9%) | 0.323 |
| Calcium channel blocker | 8 (34.8%) | 8 (38.1%) | 9 (42.9%) | 0.859 |
| Statin | 11 (47.8%) | 11 (52.4%) | 7 (33.3%) | 0.430 |
| Atorvastatin | 4 (17.4%) | 4 (28.6%) | 4(14.3%) | 0.475 |
| Rosuvastatin | 4(17.4%) | 4((19.0%) | 2(9.5%) | 0.656 |
| Dabigatran | Rivaroxaban | Warfarin | p-Value | |
|---|---|---|---|---|
| (n = 23) | (n = 21) | (n = 21) | ||
| Baseline | ||||
| RHI | 1.5 ± 0.4 | 1.5 ± 0.4 | 1.6 ± 0.5 | 0.487 |
| Lt carotid IMT (mm) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.697 |
| Rt carotid IMT (mm) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.495 |
| Maximal plaque of Lt carotid IMT (mm) | 1.9 ± 0.7 | 1.6 ± 0.5 | 2.0 ± 0.4 | 0.349 |
| Maximal plaque of Rt carotid IMT (mm) | 2.2 ± 0.7 | 1.9 ± 0.8 | 1.9 ± 0.6 | 0.452 |
| 12 months | ||||
| RHI | 1.6 ± 0.3 | 1.6 ± 0.5 | 1.6 ± 0.3 | 0.779 |
| Lt carotid IMT (mm) | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.2 | 0.629 |
| Rt carotid IMT (mm) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.145 |
| Maximal plaque of Lt carotid IMT (mm) | 1.8 ± 0.4 | 1.8 ± 0.5 | 1.6 ± 0.3 | 0.562 |
| Maximal plaque of Rt carotid IMT (mm) | 1.9 ± 0.7 | 1.6 ± 0.4 | 2.0 ± 0.4 | 0.218 |
| 24 months | ||||
| Lt carotid IMT (mm) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.901 |
| Rt carotid IMT (mm) | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.850 |
| Maximal plaque of Lt carotid IMT (mm) | 1.7 ± 0.3 | 1.9 ± 0.9 | 1.9 ± 0.7 | 0.714 |
| Maximal plaque of Rt carotid IMT (mm) | 1.9 ± 0.6 | 1.6 ± 0.5 | 2.0 ± 0.4 | 0.113 |
| NOACs | Warfarin | p-Value | |
|---|---|---|---|
| Baseline | |||
| IL-6 (pg/mL) | 32.2 ± 8.9 | 28.3 ± 5.8 | 0.312 |
| TNF-α (pg/mL) | 1.8 ± 5.2 | 1.3 ± 3.7 | 0.800 |
| P-selectin (ng/mL) | 161.2 ± 51.8 | 166.0 ± 54.7 | 0.846 |
| vWF (μg/mL) | 6.7 ± 2.2 | 7.6 ± 2.6 | 0.416 |
| 12 months | |||
| IL-6 (pg/mL) | 35.6 ± 10.4 | 28.2 ± 5.0 | 0.094 |
| TNF-α (pg/mL) | 3.7 ± 5.1 | 2.8 ± 4.7 | 0.710 |
| P-selectin (ng/mL) | 155.8 ± 71.6 | 209.5 ± 94.3 | 0.161 |
| vWF (μg/mL) | 7.5 ± 2.9 | 6.8 ± 3.4 | 0.667 |
| Change in biomarkers at 12 months | |||
| IL-6 (pg/mL) | 3.5 ± 11.4 | −0.1 ± 1.6 | 0.279 |
| TNF-α (pg/mL) | 1.8 ± 5.6 | 1.5 ± 1.8 | 0.856 |
| P-selectin (ng/mL) | −5.4 ± 54.7 | 43.5 ± 60.8 | 0.078 |
| vWF (μg/mL) | 0.7 ± 2.7 | −0.8 ± 2.4 | 0.225 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, G.-W.; Lee, J.M.; Choi, S.W.; Kim, J.; Lee, Y.S.; Kim, H.O.; Chung, H.; Woo, J.S.; Kim, J.B.; Kim, W.-S.; et al. Vascular Protective Effects of New Oral Anticoagulants in Patients with Atrial Fibrillation. J. Clin. Med. 2021, 10, 4332. https://doi.org/10.3390/jcm10194332
Jang G-W, Lee JM, Choi SW, Kim J, Lee YS, Kim HO, Chung H, Woo JS, Kim JB, Kim W-S, et al. Vascular Protective Effects of New Oral Anticoagulants in Patients with Atrial Fibrillation. Journal of Clinical Medicine. 2021; 10(19):4332. https://doi.org/10.3390/jcm10194332
Chicago/Turabian StyleJang, Gyeong-Won, Jung Myung Lee, Seung Woo Choi, Joan Kim, Young Shin Lee, Hyung Oh Kim, Hyemoon Chung, Jong Shin Woo, Jin Bae Kim, Woo-Shik Kim, and et al. 2021. "Vascular Protective Effects of New Oral Anticoagulants in Patients with Atrial Fibrillation" Journal of Clinical Medicine 10, no. 19: 4332. https://doi.org/10.3390/jcm10194332
APA StyleJang, G.-W., Lee, J. M., Choi, S. W., Kim, J., Lee, Y. S., Kim, H. O., Chung, H., Woo, J. S., Kim, J. B., Kim, W.-S., & Kim, W. (2021). Vascular Protective Effects of New Oral Anticoagulants in Patients with Atrial Fibrillation. Journal of Clinical Medicine, 10(19), 4332. https://doi.org/10.3390/jcm10194332






