Prevalence and Incidence of Osteoarthritis: A Population-Based Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Population
2.3. Data Collection
2.3.1. Estimation of Incidence and Prevalence
2.3.2. Ethics
2.4. Statistical Analysis
3. Results
3.1. Description of the OA Cohort
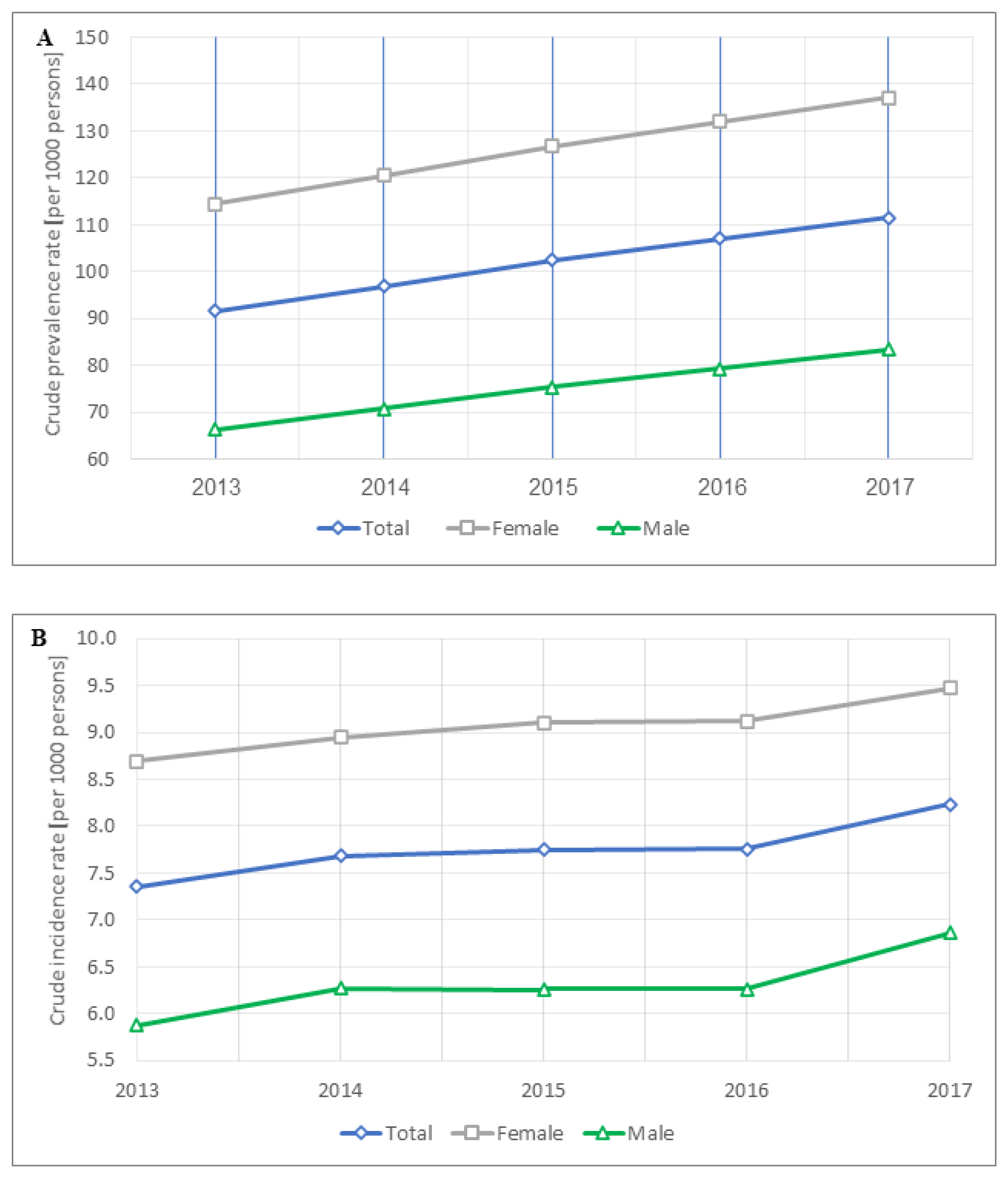
3.2. OA Prevalence Temporal Trends and Correlates
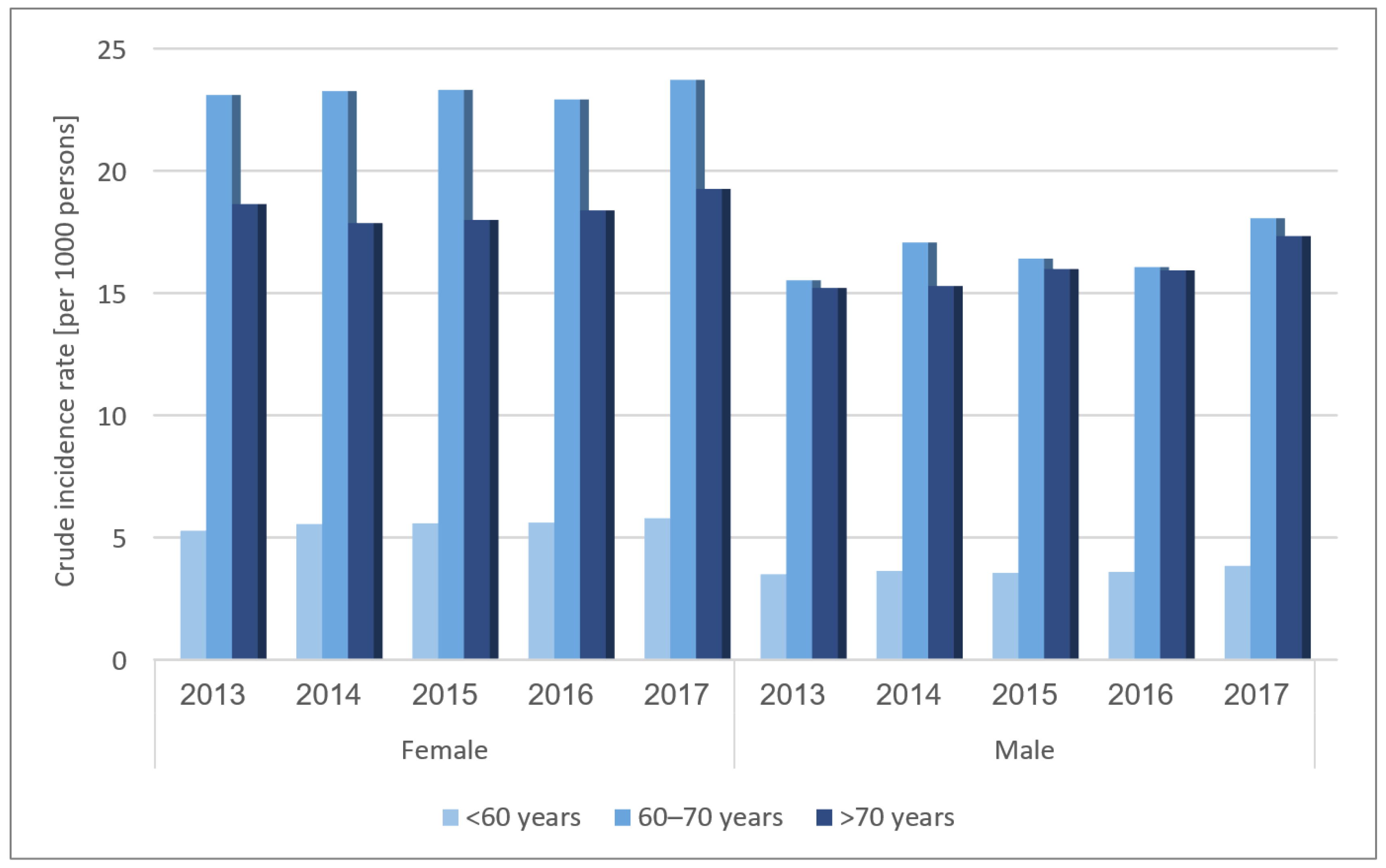
3.3. OA Incidence Temporal Trends
4. Discussion
4.1. OA Prevalence
4.2. OA Incidence
4.3. OA Correlates
4.4. Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cross, M.; Smith, E.; Hoy, D.; Nolte, S.; Ackerman, I.; Fransen, M.; Bridgett, L.; Williams, S.; Guillemin, F.; Hill, C.L.; et al. The global burden of hip and knee osteoarthritis: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Flaxman, A.D.; Naghavi, M.; Lozano, R.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; Aboyans, V.; et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2163–2196. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef] [Green Version]
- Safiri, S.; Kolahi, A.A.; Smith, E.; Hill, C.; Bettampadi, D.; Mansournia, M.A.; Hoy, D.; Ashrafi-Asgarabad, A.; Sepidarkish, M.; Almasi-Hashiani, A.; et al. Global, regional and national burden of osteoarthritis 1990–2017: A systematic analysis of the Global Burden of Disease Study 2017. Ann. Rheum. Dis. 2020, 79, 819–828. [Google Scholar] [CrossRef]
- Fallach, N.; Chodick, G.; Tirosh, M.; Eisenberg, E.; Lubovsky, O. Pain Pharmacotherapy in a Large Cohort of Patients with Osteoarthritis: A Real-World Data Analysis. Rheumatol. Ther. 2021. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- Al Saleh, J.; Sayed, M.E.; Monsef, N.; Darwish, E. The Prevalence and the Determinants of Musculoskeletal Diseases in Emiratis Attending Primary Health Care Clinics in Dubai. Oman Med. J. 2016, 31, 117–123. [Google Scholar] [CrossRef]
- Shiyovich, A.; Chodick, G.; Azani, L.; Tirosh, M.; Shuvy, M.; Pereg, D.; Katz, A.; Minha, S. Sex-specific contemporary trends in incidence, prevalence and survival of patients with non-valvular atrial fibrillation: A long-term real-world data analysis. PLoS ONE 2021, 16, e0247097. [Google Scholar] [CrossRef]
- Levkovitch-Verbin, H.; Goldshtein, I.; Chodick, G.; Zigman, N.; Shalev, V. The Maccabi Glaucoma Study: Prevalence and incidence of glaucoma in a large israeli health maintenance organization. Am. J. Ophthalmol. 2014, 158, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, A.; Olusi, S.; Moussa, M.; Al-Zaid, N.; Shehab, D.; Al-Herz, A.; Al-Jarallah, K.; Al-Salem, I.; Pedro, A. Validation of the Arabic version of the WHO-ILAR COPCORD Core Questionnaire for community screening of rheumatic diseases in Kuwaitis. World Health Organization. International League Against Rheumatism. Community Oriented Program for the Control of Rheumatic Diseases. J. Rheumatol. 2002, 29, 1754–1759. [Google Scholar] [PubMed]
- Abdel-Tawab, R.R.; Abdel-Nasser, A.M.; Darmawan, J.; Mahmoud, J.A.; Sammy, A.; Abdel-Fattah, M. The prevalence of rheumatic diseases in rural Egypt: COPCORD-Egypt: Abstract. In Proceedings of the 11th APLAR Congress, Jeju, Korea, 11–15 September 2004. [Google Scholar]
- Al-Arfaj, A.S.; Alballa, S.R.; Al-Saleh, S.S.; Al-Dalaan, A.M.; Bahabry, S.A.; Mousa, M.A.; Al-Sekeit, M.A. Knee osteoarthritis in Al-Qaseem, Saudi Arabia. Saudi Med. J. 2003, 24, 291–293. [Google Scholar]
- Cui, A.; Li, H.; Wang, D.; Zhong, J.; Chen, Y.; Lu, H. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine 2020, 29–30, 100587. [Google Scholar] [CrossRef] [PubMed]
- Davatchi, F.; Jamshidi, A.R.; Banihashemi, A.T.; Gholami, J.; Forouzanfar, M.H.; Akhlaghi, M.; Barghamdi, M.; Noorolahzadeh, E.; Khabazi, A.R.; Salesi, M.; et al. WHO-ILAR COPCORD Study (Stage 1, Urban Study) in Iran. J. Rheumatol. 2008, 35, 1384. [Google Scholar] [PubMed]
- Swain, S.; Sarmanova, A.; Mallen, C.; Kuo, C.F.; Coupland, C.; Doherty, M.; Zhang, W. Trends in incidence and prevalence of osteoarthritis in the United Kingdom: Findings from the Clinical Practice Research Datalink (CPRD). Osteoarthr. Cartil. 2020, 28, 792–801. [Google Scholar] [CrossRef]
- Eder, L.; Cohen, A.D.; Feldhamer, I.; Greenberg-Dotan, S.; Batat, E.; Zisman, D. The epidemiology of psoriatic arthritis in Israel—A population-based study. Arthritis Res. Ther. 2018, 20, 3. [Google Scholar] [CrossRef] [Green Version]
- Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [CrossRef] [Green Version]
- Neogi, T.; Zhang, Y. Epidemiology of osteoarthritis. Rheum. Dis. Clin. N. Am. 2013, 39, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Woolf, A.D.; Pfleger, B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003, 81, 646–656. [Google Scholar]
- Rahman, M.M.; Cibere, J.; Goldsmith, C.H.; Anis, A.H.; Kopec, J.A. Osteoarthritis incidence and trends in administrative health records from British Columbia, Canada. J. Rheumatol. 2014, 41, 1147–1154. [Google Scholar] [CrossRef]
- Morgan, O.J.; Hillstrom, H.J.; Ellis, S.J.; Golightly, Y.M.; Russell, R.; Hannan, M.T.; Deland, J.T., 3rd; Hillstrom, R. Osteoarthritis in England: Incidence Trends From National Health Service Hospital Episode Statistics. ACR Open Rheumatol. 2019, 1, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Yu, D.; Jordan, K.P.; Bedson, J.; Englund, M.; Blyth, F.; Turkiewicz, A.; Prieto-Alhambra, D.; Peat, G. Population trends in the incidence and initial management of osteoarthritis: Age-period-cohort analysis of the Clinical Practice Research Datalink, 1992–2013. Rheumatology 2017, 56, 1902–1917. [Google Scholar] [CrossRef] [Green Version]
- Spitaels, D.; Mamouris, P.; Vaes, B.; Smeets, M.; Luyten, F.; Hermens, R.; Vankrunkelsven, P. Epidemiology of knee osteoarthritis in general practice: A registry-based study. BMJ Open 2020, 10, e031734. [Google Scholar] [CrossRef] [Green Version]
- Kiadaliri, A.A.; Rinaldi, G.; Lohmander, L.S.; Petersson, I.F.; Englund, M. Temporal trend and regional disparity in osteoarthritis hospitalisations in Sweden 1998–2015. Scand. J. Public Health 2019, 47, 53–60. [Google Scholar] [CrossRef]
- Zhang, Y.; Jordan, J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010, 26, 355–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto-Alhambra, D.; Judge, A.; Javaid, M.K.; Cooper, C.; Diez-Perez, A.; Arden, N.K. Incidence and risk factors for clinically diagnosed knee, hip and hand osteoarthritis: Influences of age, gender and osteoarthritis affecting other joints. Ann. Rheum. Dis. 2014, 73, 1659–1664. [Google Scholar] [CrossRef] [Green Version]
- Shane Anderson, A.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract. Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, V.L.; Hunter, D.J. The epidemiology of osteoarthritis. Best Pract. Res. Clin. Rheumatol. 2014, 28, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; You, X.; Cohen, J.D.; Zhou, H.; He, W.; Li, Z.; Xiong, Y.; Yu, T. Sex Differences in Osteoarthritis Pathogenesis: A Comprehensive Study Based on Bioinformatics. Med. Sci. Monit. 2020, 26, e923331. [Google Scholar] [CrossRef]
- Haq, S.A.; Davatchi, F. Osteoarthritis of the knees in the COPCORD world. Int. J. Rheum. Dis. 2011, 14, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Silverwood, V.; Blagojevic-Bucknall, M.; Jinks, C.; Jordan, J.L.; Protheroe, J.; Jordan, K.P. Current evidence on risk factors for knee osteoarthritis in older adults: A systematic review and meta-analysis. Osteoarthr. Cartil. 2015, 23, 507–515. [Google Scholar] [CrossRef] [Green Version]
- Felson, D.T.; Zhang, Y.; Anthony, J.M.; Naimark, A.; Anderson, J.J. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann. Intern. Med. 1992, 116, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; Guermazi, A.; Roemer, F.; Zhang, Y.; Neogi, T. Structural correlates of pain in joints with osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1170–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristic | OA Patients n = 180,126 (%) |
|---|---|
| Mean age at OA diagnosis, years (SD) | 58.5 ± 11.9 |
| Age distribution, years | |
| 18–29 | 637 (0.35) |
| 30–39 | 2255 (1.25) |
| 40–49 | 10,183 (5.65) |
| 50–59 | 29,467 (16.36) |
| 60–69 | 53,497 (29.70) |
| 70–79 | 49,795 (27.64) |
| ≥80 | 34,292 (19.04) |
| Sex | |
| Females | 115,856 (64.32) |
| Males | 64,270 (35.68) |
| District | |
| Center | 34,534 (19.17) |
| Jerusalem and Shfela | 43,025 (23.89) |
| North | 34,848 (19.35) |
| Sharon | 33,835 (18.78) |
| South | 33,884 (18.81) |
| Mean time elapsed since OA diagnosis, years | 9.0 ± 5.9 |
| Time elapsed since OA diagnosis distribution, years | |
| <4 | 41,455 (23.01) |
| 4–7 | 40,161 (22.30) |
| 8–13 | 49,862 (27.68) |
| ≥14 | 48,648 (27.01) |
| Mean BMI, kg/m2 (SD) | 29.5 ± 6.2 |
| Mean weight, kg (SD) | 78.8 ± 17.2 |
| Smoking | |
| Ever | 43,894 (24.37) |
| Never | 126,552 (70.26) |
| Unknown | 9680 (5.37) |
| Comorbid conditions between 2013–2018 | |
| Ischemic heart disease | 21,359 (11.86) |
| Cancer | 36,212 (20.10) |
| Diabetes | 47,229 (26.22) |
| Hypertension | 105,252 (58.43) |
| Osteoporosis | 41,260 (22.91) |
| Chronic kidney disease | 61,490 (34.14) |
| Rheumatoid arthritis | 4054 (2.25) |
| Inflammatory bowel disease | 1959 (1.09) |
| Psoriasis | 9562 (5.31) |
| Psoriatic arthritis | 1628 (0.90) |
| Ankylosing spondylitis | 700 (0.39) |
| Fibromyalgia | 31,498 (17.49) |
| Depression/Anxiety | 21,455 (11.91) |
| Characteristic | Mean BMI (kg/m2) (SD) | p |
|---|---|---|
| Age, years | ||
| 18–29 | 24.7 (4.9) | <0.0001 |
| 30–39 | 27.3 (5.8) | |
| 40–49 | 29.2 (5.7) | |
| 50–59 | 29.7 (5.5) | |
| 60–69 | 29.6 (5.2) | |
| 70–79 | 29.3 (5.0) | |
| ≥80 | 28.6 (4.9) | |
| Sex | ||
| Females | 29.5 (5.5) | <0.0001 |
| Males | 28.9 (4.6) | |
| District | ||
| Center | 28.4 (5.0) | <0.0001 |
| Sharon | 28.8 (5.0) | |
| Jerusalem and Shfela | 29.4 (5.1) | |
| North | 29.7 (5.2) | |
| South | 30.0 (5.2) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamood, R.; Tirosh, M.; Fallach, N.; Chodick, G.; Eisenberg, E.; Lubovsky, O. Prevalence and Incidence of Osteoarthritis: A Population-Based Retrospective Cohort Study. J. Clin. Med. 2021, 10, 4282. https://doi.org/10.3390/jcm10184282
Hamood R, Tirosh M, Fallach N, Chodick G, Eisenberg E, Lubovsky O. Prevalence and Incidence of Osteoarthritis: A Population-Based Retrospective Cohort Study. Journal of Clinical Medicine. 2021; 10(18):4282. https://doi.org/10.3390/jcm10184282
Chicago/Turabian StyleHamood, Rola, Matanya Tirosh, Noga Fallach, Gabriel Chodick, Elon Eisenberg, and Omri Lubovsky. 2021. "Prevalence and Incidence of Osteoarthritis: A Population-Based Retrospective Cohort Study" Journal of Clinical Medicine 10, no. 18: 4282. https://doi.org/10.3390/jcm10184282
APA StyleHamood, R., Tirosh, M., Fallach, N., Chodick, G., Eisenberg, E., & Lubovsky, O. (2021). Prevalence and Incidence of Osteoarthritis: A Population-Based Retrospective Cohort Study. Journal of Clinical Medicine, 10(18), 4282. https://doi.org/10.3390/jcm10184282







