Technological Advancements in Male Infertility Microsurgery
Abstract
:1. Introduction
2. History of Male Infertility Microsurgery
2.1. Vasal Obstruction
2.2. Varicoceles
2.3. Microsurgical Sperm Retrieval
3. Training and Male Infertility Microsurgery
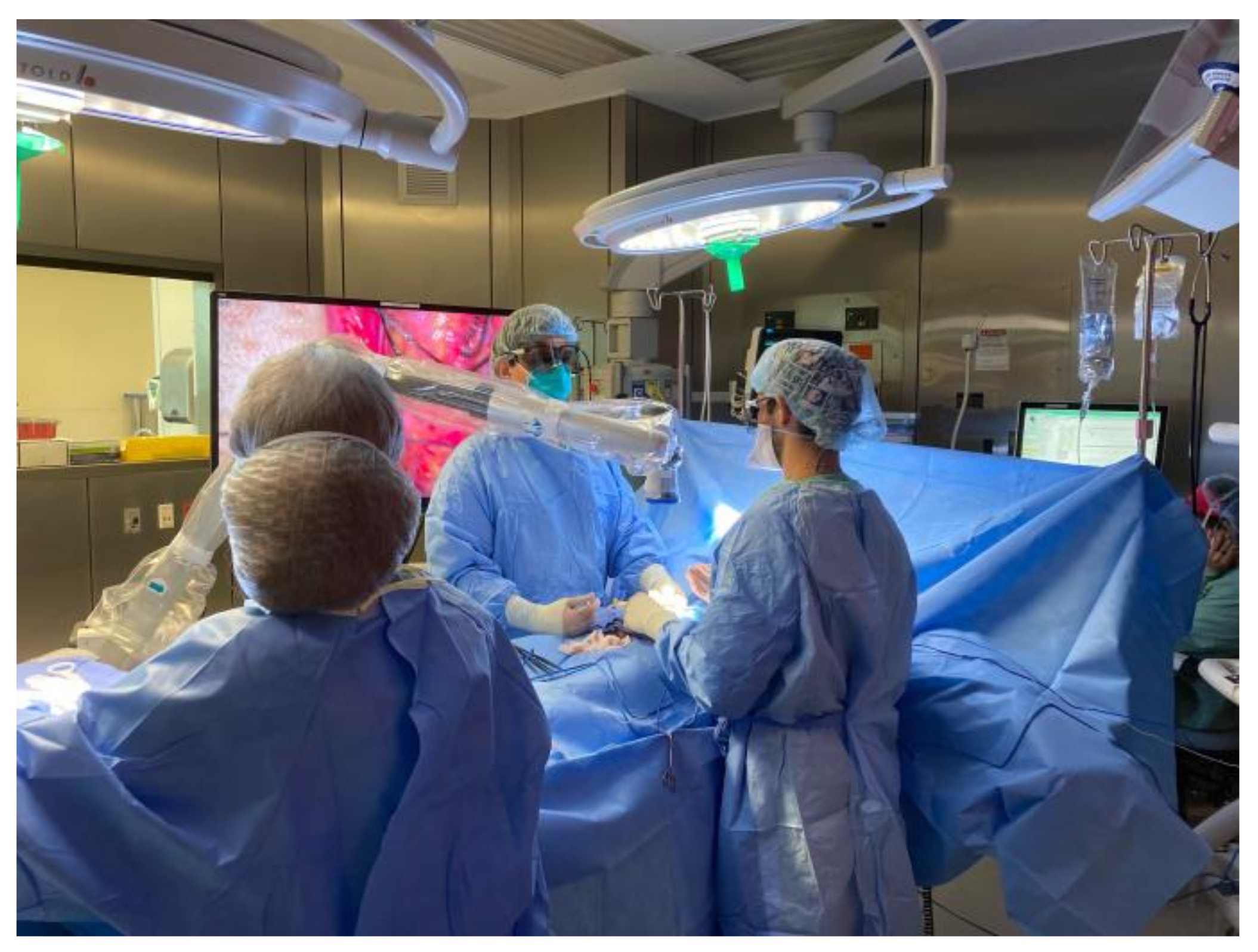
4. Video Microsurgery and 4K3D Operating Microscopes
5. Robotics and Male Infertility Microsurgery
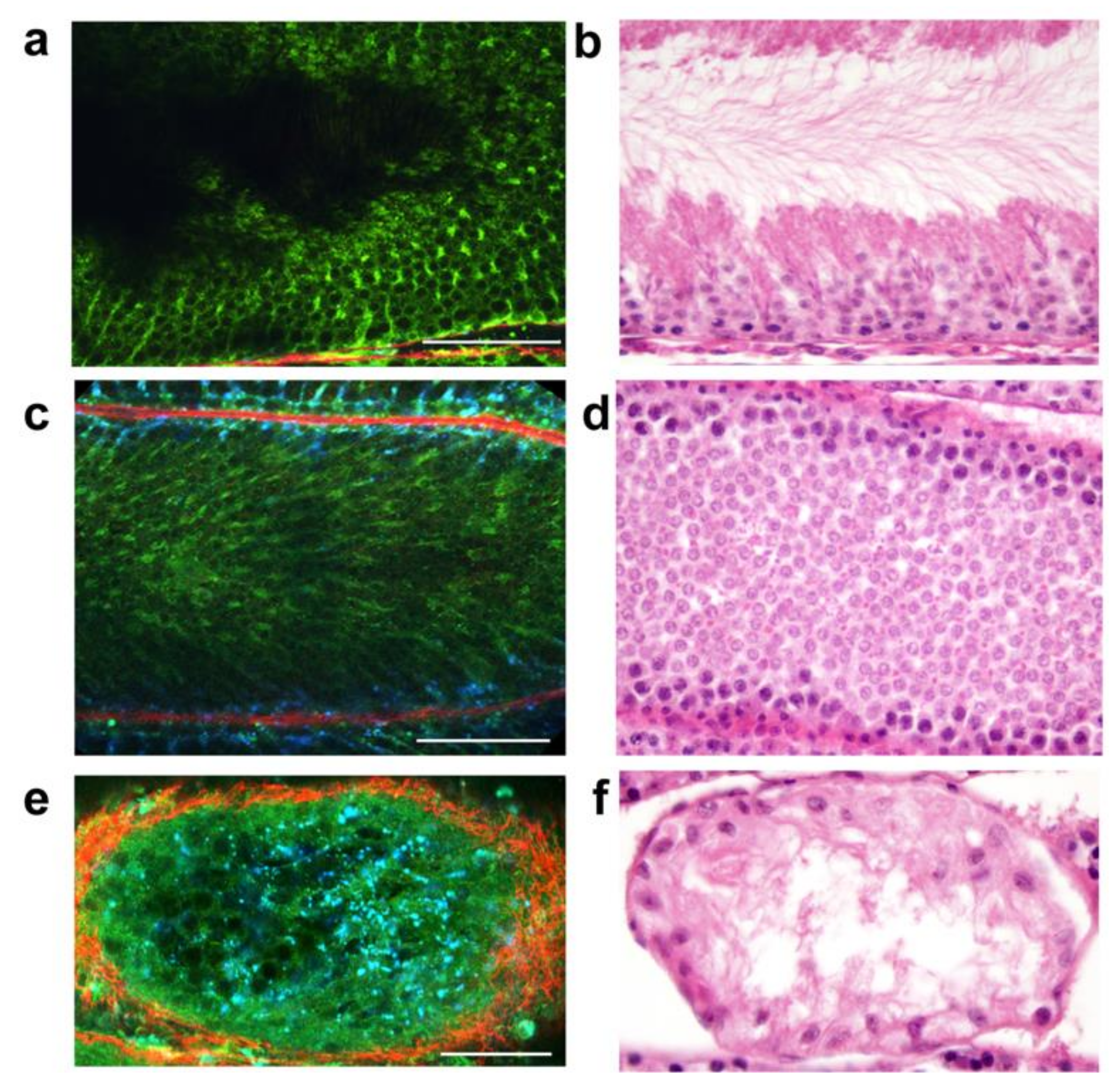
6. Multiphoton Microscopy
7. Artificial Intelligence, Deep Learning and Machine Learning
7.1. AI and Microsurgery
7.2. AI and Infertile Men
7.3. AI and Semen Analysis/Sperm Selection
7.4. Limitations of AI/ML in Male Infertility
8. Limitations and Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, N.; Singh, A.K. Trends of male factor infertility, an important cause of infertility: A review of literature. J. Hum. Reprod. Sci. 2015, 8, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Punjani, N.; Kang, C.; Schlegel, P. Two Decades from the Introduction of Microdissection Testicular Sperm Extraction: How This Surgical Technique Has Improved the Management of NOA. J. Clin. Med. 2021, 10, 1374. [Google Scholar] [CrossRef]
- Chen, M.L.; Buncke, G.M.; Turek, P.J. Narrative review of the history of microsurgery in urological practice. Transl. Androl. Urol. 2021, 10, 1780–1791. [Google Scholar] [CrossRef] [PubMed]
- Dohlman, G.F. Carl Olof Nylen and the Birth of the Otomicroscope and Microsurgery. Arch. Otolaryngol. Head Neck Surg. 1969, 90, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, D.; Denil, J. History of the microscope and development of microsurgery: A revolution for reproductive tract surgery. Andrologia 2002, 34, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Kriss, T.C.; Kriss, V.M. History of the Operating Microscope: From Magnifying Glass to Microneurosurgery. Neurosurgery 1998, 42, 899–907. [Google Scholar] [CrossRef]
- Owen, E.R. Microsurgical Vasovasostomy: A Reliable Vasectomy Reversal. ANZ J. Surg. 1977, 47, 305–309. [Google Scholar] [CrossRef]
- Silber, S.J. Microscopic vasectomy reversal. Fertil Steril 1977, 28, 1191–1202. [Google Scholar] [CrossRef]
- Silber, S.J. Microscopic vasoepididymostomy: Specific microanastomosis to the epididymal tubule. Fertil Steril 1978, 30, 565–571. [Google Scholar] [CrossRef]
- Thomas, A.J., Jr. Vasoepididymostomy. Urol. Clin. N. Am. 1987, 14, 527–538. [Google Scholar] [CrossRef]
- Fogdestam, I.; Fall, M.; Nilsson, S. Microsurgical epididymovasostomy in the treatment of occlusive azoospermia. Fertil Steril 1986, 46, 925–929. [Google Scholar] [CrossRef]
- Chan, P.T.; Li, P.S.; Goldstein, M. Microsurgical Vasoepididymostomy: A Prospective Randomized Study of 3 Intussusception Techniques in Rats. J. Urol. 2003, 169, 1924–1929. [Google Scholar] [CrossRef]
- Berger, R.E. Triangulation end-to-side vasoepididymostomy. J. Urol. 1998, 159, 1951–1953. [Google Scholar] [CrossRef]
- Shekarriz, M.; Pomer, S. Microsurgical vasoepididymostomy: A comparison between the end-to-side anastomosis and the in-vagination technique. Urol. Res. 1991, 19, 285–287. [Google Scholar] [CrossRef]
- Stefanović, K.B.; Clark, S.A.; Buncke, H.J. Microsurgical Epididymovasostomy by Loop Intussusception. BJU Int. 1991, 68, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Monoski, M.A.; Schiff, J.; Li, P.S.; Chan, P.T.; Goldstein, M. Innovative single-armed suture technique for microsurgical vaso-epididymostomy. Urology 2007, 69, 800–804. [Google Scholar] [CrossRef]
- Kang, C.; Punjani, N.; Lee, R.K.; Li, P.S.; Goldstein, M. Effect of varicoceles on spermatogenesis. Semin. Cell Dev. Biol. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Marmar, J.; Kim, Y. Subinguinal Microsurgical Varicocelectomy: A Technical Critique and Statistical Analysis of Semen and Pregnancy Data. J. Urol. 1994, 152, 1127–1132. [Google Scholar] [CrossRef]
- Gorelick, J.I.; Goldstein, M. Loss of fertility in men with varicocele. Fertil Steril 1993, 59, 613–616. [Google Scholar] [CrossRef]
- Steckel, J.; Dicker, A.; Goldstein, M. Relationship Between Varicocele Size and Response to Varicocelectomy. J. Urol. 1993, 149, 769–771. [Google Scholar] [CrossRef]
- Ding, H.; Tian, J.; Du, W.; Zhang, L.; Wang, H.; Wang, Z. Open non-microsurgical, laparoscopic or open microsurgical vari-cocelectomy for male infertility: A meta-analysis of randomized controlled trials. BJU Int. 2012, 110, 1536–1542. [Google Scholar] [CrossRef]
- Wang, J.; Sauer, M.V. In vitro fertilization (IVF): A review of 3 decades of clinical innovation and technological advancement. Ther. Clin. Risk Manag. 2006, 2, 355–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temple-Smith, P.D.; Southwick, G.J.; Yates, C.A.; Trounson, A.; De Kretser, D.M. Human pregnancy by in vitro fertilization (IVF) using sperm aspirated from the epididymis. J. Assist. Reprod. Genet. 1985, 2, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Devroey, P.; Liu, J.; Nagy, Z.; Goossens, A.; Tournaye, H.; Camus, M.; van Steirteghem, A.; Silber, S. Pregnancies after testicular sperm extraction and intra-cytoplasmic sperm injection in non-obstructive azoospermia. Hum. Reprod. 1995, 10, 1457–1460. [Google Scholar] [CrossRef]
- Schlegel, P.N. Testicular sperm extraction: Microdissection improves sperm yield with minimal tissue excision. Hum. Reprod. 1999, 14, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, P.N.; Li, P.S. Microdissection TESE: Sperm retrieval in non-obstructive azoospermia. Hum. Reprod. Update 1998, 4, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corona, G.; Minhas, S.; Giwercman, A.; Bettocchi, C.; Dinkelman-Smit, M.; Dohle, G.; Fusco, F.; Kadioglou, A.; Kliesch, S.; Kopa, Z.; et al. Sperm recovery and ICSI outcomes in men with non-obstructive azoospermia: A systematic review and meta-analysis. Hum. Reprod. Updat. 2019, 25, 733–757. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Yagan, N.; Schlegel, P.N. Structural and functional changes to the testis after conventional versus microdissection testicular sperm extraction. Urology 2005, 65, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Li, P.S. Male infertility microsurgical training. Asian J. Androl. 2013, 15, 61–66. [Google Scholar] [CrossRef] [Green Version]
- Mehta, A.; Li, P.S.; Goldstein, M. Male infertility microsurgical training. Transl. Androl. Urol. 2014, 3, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Masterson, T.A.; Nackeeran, S.; Rainer, Q.; Hauser, N.; Marcovich, R.; Ramasamy, R. Survey of Microsurgery Training Availability in US Urology Residency Programs. World J. Men’s Health 2021, 39, 376–380. [Google Scholar] [CrossRef]
- Uluç, K.; Kujoth, G.; Başkaya, M.K. Operating microscopes: Past, present, and future. Neurosurg. Focus 2009, 27, E4. [Google Scholar] [CrossRef] [Green Version]
- Best, J.C.; Gonzalez, D.; Alawamlh, O.A.H.; Li, P.S.; Ramasamy, R. Use of 4K3D video microscope in male infertility microsurgery. Urol. Video J. 2020, 7, 100046. [Google Scholar] [CrossRef]
- Hayden, R.P.; Chen, H.; Li, P.S.; Goldstein, M. Promising 4K3D Reconstructive Macrosurgery and Microsurgery. AUA News 2019, 24, 6–7. [Google Scholar]
- Chen, H.; Hayden, R.P.; Al Hussein Alawamlh, O.; Schlegel, P.N.; Goldstein, M.; Li, P.S. New era of male infertility microsurgery: 4K3D ORBEYE video operating microscopy. Fertil Steril 2020, 114. [Google Scholar]
- Frykman, P.K.; Duel, B.P.; Gangi, A.; Williams, J.A.; Berci, G.; Freedman, A.L. Evaluation of a Video Telescopic Operating Microscope (VITOM) for Pediatric Surgery and Urology: A Preliminary Report. J. Laparoendosc. Adv. Surg. Tech. 2013, 23, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Wahba, R.; Datta, R.; Bußhoff, J.; Bruns, T.; Hedergott, A.; Gietzelt, C.; Dieplinger, G.; Fuchs, H.; Morgenstern, B.; Möller, D.; et al. 3D Versus 4K Display System–Influence of “State-of-the-art”—Display Technique on Surgical Performance (IDOSP-study) in Minimally Invasive Surgery: A Randomized Cross-over Trial. Ann. Surg. 2020, 272, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Hayden, R.P.; Chen, H.; Goldstein, M.; Li, P.S. A randomized controlled animal trial: Efficacy of a 4K3D video microscope versus an optical operating microscope for urologic microsurgery. Fertil Steril 2019, 112, e93. [Google Scholar] [CrossRef]
- Ahmad, F.I.; Mericli, A.F.; DeFazio, M.V.; Chang, E.I.; Hanasono, M.M.; Pederson, W.C.; Kaufman, M.; Selber, J.C. Application of the ORBEYE three-dimensional exoscope for microsurgical procedures. Microsurgery 2019, 40, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S.; Toda, M.; Nishimoto, M.; Ishihara, E.; Miwa, T.; Akiyama, T.; Takashi, H.; Hikaru, S.; Kazunari, Y. Pros and cons of using ORBEYE for microneuro-surgery. Clin. Neurol. Neurosurg. 2018, 174, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Izumo, T.; Ujifuku, K.; Baba, S.; Morofuji, Y.; Horie, N.; Matsuo, T. Initial experience of ORBEYE™ surgical microscope for carotid endarterectomy. Asian J. Neurosurg. 2019, 14, 839–842. [Google Scholar] [CrossRef]
- Challacombe, B.; Khan, M.S.; Murphy, D.; Dasgupta, P. The history of robotics in urology. World J. Urol. 2006, 24, 120–127. [Google Scholar] [CrossRef]
- Darves-Bornoz, A.; Panken, E.; Brannigan, R.E.; Halpern, J.A. Robotic Surgery for Male Infertility. Urol. Clin. N. Am. 2020, 48, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Pastuszak, A.W.; Wenker, E.P.; Lipshultz, L.I. The History of Microsurgery in Urology. Urology 2015, 85, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Schiff, J.; Li, P.S.; Goldstein, M. Robotic microsurgical vasovasostomy and vasoepididymostomy: A prospective randomized study in a rat model. J. Urol. 2004, 171, 1720–1725. [Google Scholar] [CrossRef] [PubMed]
- Etafy, M.; Gudeloglu, A.; Brahmbhatt, J.V.; Parekattil, S.J. Review of the role of robotic surgery in male infertility. Arab. J. Urol. 2017, 16, 148–156. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.; Parekattil, S.J.; Goldstein, M.; Lipshultz, L.I.; Kavoussi, P.; McCullough, A.; Sigman, M. Pros and cons of robotic microsurgery as an appropriate approach to male reproductive surgery for vasectomy reversal and varicocele repair. Fertil Steril 2018, 110, 816–823. [Google Scholar] [CrossRef] [Green Version]
- Ramasamy, R.; Sterling, J.; Fisher, E.S.; Li, P.S.; Jain, M.; Robinson, B.D.; Maria, S.; David, H.; Chris, X.; Sushmita, M.; et al. Identification of spermatogenesis with multiphoton mi-croscopy: An evaluation in a rodent model. J. Urol. 2011, 186, 2487–2492. [Google Scholar] [CrossRef]
- Katz, M.J.; Huland, D.M.; Ramasamy, R. Multiphoton microscopy: Applications in Urology and Andrology. Transl. Androl. Urol. 2014, 3, 77–83. [Google Scholar] [CrossRef]
- Chiba, K.; Enatsu, N.; Fujisawa, M. Management of non-obstructive azoospermia. Reprod. Med. Biol. 2016, 15, 165–173. [Google Scholar] [CrossRef] [Green Version]
- Najari, B.B.; Ramasamy, R.; Sterling, J.; Aggarwal, A.; Sheth, S.; Li, P.S.; Dubin, J.M.; Goldenberg, S.; Jain, M.; Robinson, B.D.; et al. Pilot Study of the Correlation of Multiphoton Tomography of Ex Vivo Human Testis with Histology. J. Urol. 2012, 188, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Enatsu, N.; Miyake, H.; Chiba, K.; Fujisawa, M. Identification of Spermatogenically Active Regions in Rat Testes by Using Nar-row-band Imaging System. Urology 2015, 86, 929–935. [Google Scholar] [CrossRef]
- Bi, Q.; Goodman, K.E.; Kaminsky, J.; Lessler, J. What is Machine Learning? A Primer for the Epidemiologist. Am. J. Epidemiol. 2019, 188, 2222–2239. [Google Scholar] [CrossRef]
- French, R.M. The Turing Test: The first 50 years. Trends Cogn. Sci. 2000, 4, 115–122. [Google Scholar] [CrossRef]
- Chang, T.C.; Seufert, C.; Eminaga, O.; Shkolyar, E.; Hu, J.C.; Liao, J. Current Trends in Artificial Intelligence Application for En-dourology and Robotic Surgery. Urol. Clin. N. Am. 2021, 48, 151–160. [Google Scholar] [CrossRef]
- Sidey-Gibbons, J.A.M.; Sidey-Gibbons, C.J. Machine learning in medicine: A practical introduction. BMC Med Res. Methodol. 2019, 19, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murdoch, W.J.; Singh, C.; Kumbier, K.; Abbasi-Asl, R.; Yu, B. Definitions, methods, and applications in interpretable machine learning. Proc. Natl. Acad. Sci. USA 2019, 116, 22071–22080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, B.; Dhavileswarapu, A.S.; Raza, S.; Karimi, H.; Khanuja, H.; Shetty, D.; Ibrahim, S.; Shah, M.; Naik, N.; Paul, R.; et al. Artificial Intelligence and Its Impact on Urological Diseases and Management: A Comprehensive Review of the Literature. J. Clin. Med. 2021, 10, 1864. [Google Scholar] [CrossRef] [PubMed]
- Chu, K.Y.; Nassau, D.E.; Arora, H.; Lokeshwar, S.D.; Madhusoodanan, V.; Ramasamy, R. Artificial Intelligence in Reproductive Urology. Curr. Urol. Rep. 2019, 20, 52. [Google Scholar] [CrossRef]
- Akinsal, E.C.; Haznedar, B.; Baydilli, N.; Kalinli, A.; Ozturk, A.; Ekmekçioğlu, O. Artificial Neural Network for the Prediction of Chromosomal Abnormalities in Azoospermic Males. Urol. J. 2018, 15, 122–125. [Google Scholar] [PubMed]
- Punjani, N.; Lamb, D.J. Canary in the Coal Mine? Male Infertility as a Marker of Overall Health. Annu. Rev. Genet. 2020, 54, 465–486. [Google Scholar]
- Libbrecht, M.; Noble, W.S. Machine learning applications in genetics and genomics. Nat. Rev. Genet. 2015, 16, 321–332. [Google Scholar] [CrossRef] [Green Version]
- Gil, D.; Girela, J.L.; De Juan, J.; Gomez-Torres, M.J.; Johnsson, M. Predicting seminal quality with artificial intelligence methods. Expert Syst. Appl. 2012, 39, 12564–12573. [Google Scholar] [CrossRef]
- Girela, J.L.; Gil, D.; Johnsson, M.; Gómez-Torres, M.J.; De Juan, J. Semen Parameters Can Be Predicted from Environmental Factors and Lifestyle Using Artificial Intelligence Methods. Biol. Reprod. 2013, 88, 99. [Google Scholar] [CrossRef]
- Shi, X.; Chan, C.P.S.; Waters, T.; Chi, L.; Chan, D.Y.; Li, T.C. Lifestyle and demographic factors associated with human semen quality and sperm function. Syst. Biol. Reprod. Med. 2018, 64, 358–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurewicz, J.; Radwan, M.; Sobala, W.; Ligocka, D.; Radwan, P.; Bochenek, M.; Hanke, W. Lifestyle and semen quality: Role of modifiable risk factors. Syst. Biol. Reprod. Med. 2013, 60, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeadna, A.; Khateeb, N.; Rokach, L.; Lior, Y.; Har-Vardi, I.; Harlev, A.; Huleihel, M.; Lunenfeld, E.; Levitas, E. Prediction of sperm extraction in non-obstructive azo-ospermia patients: A machine-learning perspective. Hum. Reprod. 2020, 35, 1505–1514. [Google Scholar] [CrossRef]
- You, J.B.; McCallum, C.; Wang, Y.; Riordon, J.; Nosrati, R.; Sinton, D. Machine learning for sperm selection. Nat. Rev. Urol. 2021, 18, 387–403. [Google Scholar] [CrossRef]
- Hicks, S.A.; Andersen, J.M.; Witczak, O.; Thambawita, V.; Halvorsen, P.; Hammer, H.L.; Haugen, T.B.; Riegler, M.A. Machine Learning-Based Analysis of Sperm Videos and Participant Data for Male Fertility Prediction. Sci. Rep. 2019, 9, 16770. [Google Scholar] [CrossRef]
- Wang, R.; Pan, W.; Jin, L.; Li, Y.; Geng, Y.; Gao, C.; Chen, G.; Wang, H.; Ma, D.; Liao, S. Artificial intelligence in reproductive medicine. Reproduction 2019, 158, R139–R154. [Google Scholar] [CrossRef] [PubMed]
- Vaughan, A.D.; Sakkas, D. Sperm selection methods in the 21st century. Biol. Reprod. 2019, 101, 1076–1082. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.P.; Gross, K.X.; Hotaling, J.M. Can artificial intelligence drive optimal sperm selection for in vitro fertilization? Fertil Steril 2021, 115, 883. [Google Scholar] [CrossRef] [PubMed]
- Zaninovic, N.; Rosenwaks, Z. Artificial intelligence in human in vitro fertilization and embryology. Fertil Steril 2020, 114, 914–920. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ramasamy, R.; Manzoor, M.; Jain, M.; Li, P.S.; Sterling, J.; Salamoon, B.; Fisher, E.; Schlegel, P.N. Full field optical coherence tomography can identify spermatogenesis in a rodent sertoli-cell only model. J. Pathol. Inform. 2012, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Ramasamy, R.; Sterling, J.; Li, P.S.; Robinson, B.D.; Parekattil, S.; Chen, J.; Felsen, D.; Mukherjee, S.; Goldstein, M.; Schlegel, P.N. Multiphoton Imaging and Laser Ablation of Rodent Spermatic Cord Nerves: Potential Treatment for Patients with Chronic Orchialgia. J. Urol. 2012, 187, 733–738. [Google Scholar] [CrossRef] [PubMed]
| Technology | Pros | Cons |
|---|---|---|
| Video Microsurgery and 4K3D Operating Microscopes [34,35,36] | More ergonomic High definition/quality displays Easy transport Less space | Expensive upfront cost Learning curve Surgeon comfort |
| Robotics and Male Infertility Microsurgery [44,46,48] | Reduce tremor Additional arm can replace an assistant Improved visualization | Large upfront cost Requires extra microsurgical robotic and male infertility microsurgery training No concrete clinical evidence suggesting better outcomes Extra microsurgical training required Large space and operating room staff |
| Multiphoton Microscopy [49,50,52,75,76] | Identification of real-time spermatogenesis Potentially reduce unnecessary dissection | Safety concerns Technological limitations Cost and learning curve Limited human studies |
| Artificial Intelligence, Deep Learning and Machine Learning [60,64,65,74] | Powerful Efficient Novel | Interpretability can be challenging May require significant computational power Requires further research |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Punjani, N.; Kang, C.; Lee, R.K.; Goldstein, M.; Li, P.S. Technological Advancements in Male Infertility Microsurgery. J. Clin. Med. 2021, 10, 4259. https://doi.org/10.3390/jcm10184259
Punjani N, Kang C, Lee RK, Goldstein M, Li PS. Technological Advancements in Male Infertility Microsurgery. Journal of Clinical Medicine. 2021; 10(18):4259. https://doi.org/10.3390/jcm10184259
Chicago/Turabian StylePunjani, Nahid, Caroline Kang, Richard K. Lee, Marc Goldstein, and Philip S. Li. 2021. "Technological Advancements in Male Infertility Microsurgery" Journal of Clinical Medicine 10, no. 18: 4259. https://doi.org/10.3390/jcm10184259
APA StylePunjani, N., Kang, C., Lee, R. K., Goldstein, M., & Li, P. S. (2021). Technological Advancements in Male Infertility Microsurgery. Journal of Clinical Medicine, 10(18), 4259. https://doi.org/10.3390/jcm10184259






