The Effect of Lifestyle Intervention on Systemic Oxidative Stress in Women with Obesity and Infertility: A Post-Hoc Analysis of a Randomized Controlled Trial
Abstract
:1. Introduction
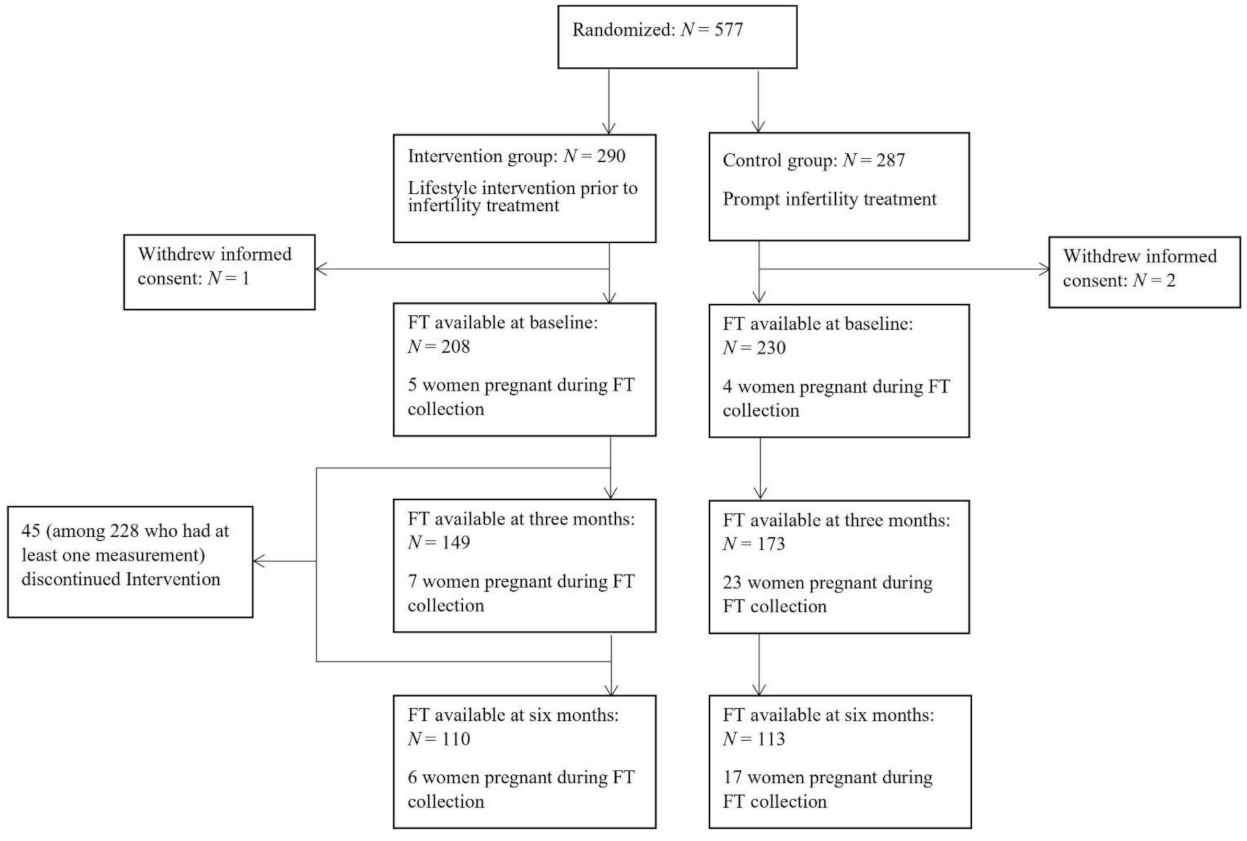
2. Materials and Methods
2.1. Subjects and Lifestyle Intervention
2.2. Clinical and Laboratory Measurements
2.3. Statistical Analysis
3. Results
3.1. Study Population Characteristics
3.2. Effect of the Lifestyle Intervention on OS
3.3. Associations between Serum FT Levels and Cardiometabolic Outcomes
3.4. Associations of Serum FT Levels with ∆weight/BMI, ∆triglycerides, ∆hs-CRP and ∆HOMA-IR (Three Months—Baseline)
3.5. Non-Linear Associations between Serum FT and Hs-CRP
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, G.J.; Jauniaux, E. Oxidative stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marseglia, L.; Manti, S.; D’Angelo, G.; Nicotera, A.G.; Parisi, E.; Di Rosa, G.; Gitto, E.; Arrigo, T. Oxidative Stress in Obesity: A Critical Component in Human Diseases. Int. J. Mol. Sci. 2014, 16, 378–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [Green Version]
- Pignatelli, P.; Menichelli, D.; Pastori, D.; Violi, F. Oxidative stress and cardiovascular disease: New insights. Kardiol. Pol. 2018, 76, 713–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Minno, A.; Stornaiuolo, M.; Novellino, E. Molecular Scavengers, Oxidative Stress and Cardiovascular Disease. J. Clin. Med. 2019, 8, 1895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracia, K.C.; Llanas-Cornejo, D.; Husi, H. CVD and Oxidative Stress. J. Clin. Med. 2017, 6, 22. [Google Scholar] [CrossRef] [Green Version]
- Essick, E.E.; Wilson, R.M.; Pimentel, D.R.; Shimano, M.; Baid, S.; Ouchi, N.; Sam, F. Adiponectin Modulates Oxidative Stress-Induced Autophagy in Cardiomyocytes. PLoS ONE 2013, 8, e68697. [Google Scholar] [CrossRef]
- Baba, S.P.; Bhatnagar, A. Role of thiols in oxidative stress. Curr. Opin. Toxicol. 2018, 7, 133–139. [Google Scholar] [CrossRef]
- Banne, A.F.; Amiri, A.; Pero, R.W. Reduced Level of Serum Thiols in Patients with a Diagnosis of Active Disease. J. Anti Aging Med. 2003, 6, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Cortese-Krott, M.M.; Koning, A.; Kuhnle, G.; Nagy, P.; Bianco, C.L.; Pasch, A.; Wink, D.A.; Fukuto, J.M.; Jackson, A.A.; Van Goor, H.; et al. The Reactive Species Interactome: Evolutionary Emergence, Biological Significance, and Opportunities for Redox Metabolomics and Personalized Medicine. Antioxid. Redox Signal. 2017, 27, 684–712. [Google Scholar] [CrossRef] [Green Version]
- Bourgonje, A.; Von Martels, J.Z.H.; Bulthuis, M.L.C.; Van Londen, M.; Faber, K.N.; Dijkstra, G.; Van Goor, H. Crohn’s Disease in Clinical Remission Is Marked by Systemic Oxidative Stress. Front. Physiol. 2019, 10, 499. [Google Scholar] [CrossRef]
- Koning, A.M.; Meijers, W.C.; Pasch, A.; Leuvenink, H.; Frenay, A.-R.S.; Dekker, M.M.; Feelisch, M.; de Boer, R.A.; van Goor, H. Serum free thiols in chronic heart failure. Pharmacol. Res. 2016, 111, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Schillern, E.E.; Pasch, A.; Feelisch, M.; Waanders, F.; Hendriks, S.H.; Mencke, R.; Harms, G.; Groenier, K.H.; Bilo, H.J.; Hillebrands, J.-L.; et al. Serum free thiols in type 2 diabetes mellitus: A prospective study. J. Clin. Transl. Endocrinol. 2019, 16, 100182. [Google Scholar] [CrossRef]
- Frenay, A.-R.S.; De Borst, M.; Bachtler, M.; Tschopp, N.; Keyzer, C.A.; Berg, E.V.D.; Bakker, S.J.; Feelisch, M.; Pasch, A.; van Goor, H. Serum free sulfhydryl status is associated with patient and graft survival in renal transplant recipients. Free Radic. Biol. Med. 2016, 99, 345–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kundi, H.; Ates, I.; Kiziltunc, E.; Cetin, M.; Cicekcioglu, H.; Neselioglu, S.; Erel, O.; Ornek, E. A novel oxidative stress marker in acute myocardial infarction; thiol/disulphide homeostasis. Am. J. Emerg. Med. 2015, 33, 1567–1571. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Ryan, D.H. New Obesity Guidelines. JAMA 2014, 311, 23–24. [Google Scholar] [CrossRef] [PubMed]
- Bray, G.A.; Frühbeck, G.; Ryan, D.; Wilding, J. Management of obesity. Lancet 2016, 387, 1947–1956. [Google Scholar] [CrossRef] [Green Version]
- Clifton, P.; Bastiaans, K.; Keogh, J. High protein diets decrease total and abdominal fat and improve CVD risk profile in overweight and obese men and women with elevated triacylglycerol. Nutr. Metab. Cardiovasc. Dis. 2009, 19, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Most, J.; Gilmore, L.A.; Smith, S.R.; Han, H.; Ravussin, E.; Redman, L.M. Significant improvement in cardiometabolic health in healthy nonobese individuals during caloric restriction-induced weight loss and weight loss maintenance. Am. J. Physiol. Metab. 2018, 314, E396–E405. [Google Scholar] [CrossRef] [Green Version]
- Zomer, E.; Gurusamy, K.; Leach, R.; Trimmer, C.; Lobstein, T.; Morris, S.; James, W.; Finer, N. Interventions that cause weight loss and the impact on cardiovascular risk factors: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 1001–1011. [Google Scholar] [CrossRef]
- Lastra, G.; Sowers, J.R. Obesity and cardiovascular disease: Role of adipose tissue, inflammation, and the renin-angiotensin-aldosterone system. Horm. Mol. Biol. Clin. Investig. 2013, 15, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Galarregui, C.; Zulet, M.; Cantero, I.; Marín-Alejandre, B.A.; Monreal, J.I.; Elorz, M.; Benito-Boillos, A.; Herrero, J.I.; Tur, J.A.; Abete, I.; et al. Interplay of Glycemic Index, Glycemic Load, and Dietary Antioxidant Capacity with Insulin Resistance in Subjects with a Cardiometabolic Risk Profile. Int. J. Mol. Sci. 2018, 19, 3662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Åsgård, R.; Rytter, E.; Basu, S.; Abramsson-Zetterberg, L.; Möller, L.; Vessby, B. High intake of fruit and vegetables is related to low oxidative stress and inflammation in a group of patients with type 2 diabetes. Scand. J. Food Nutr. 2007, 51, 149–158. [Google Scholar] [CrossRef]
- Pingitore, A.; Lima, G.; Mastorci, F.; Quinones, A.; Iervasi, G.; Vassalle, C. Exercise and oxidative stress: Potential effects of antioxidant dietary strategies in sports. Nutrition 2015, 31, 916–922. [Google Scholar] [CrossRef]
- Mutsaerts, M.A.Q.; Groen, H.; Ter Bogt, N.C.W.; Bolster, J.H.T.; Land, J.A.; Bemelmans, W.J.; Kuchenbecker, W.K.; Hompes, P.G.; Macklon, N.S.; Stolk, R.P.; et al. The LIFESTYLE study: Costs and effects of a structured lifestyle program in overweight and obese subfertile women to reduce the need for fertility treatment and improve reproductive outcome. A randomised controlled trial. BMC Women’s Health 2010, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Mutsaerts, M.A.Q.; Van Oers, A.M.; Groen, H.; Burggraaff, J.M.; Kuchenbecker, W.K.H.; Perquin, D.A.M.; Koks, C.A.M.; Van Golde, R.; Kaaijk, E.M.; Schierbeek, J.M.; et al. Randomized Trial of a Lifestyle Program in Obese Infertile Women. N. Engl. J. Med. 2016, 374, 1942–1953. [Google Scholar] [CrossRef]
- van der Steeg, J.W.; Steures, P.; Eijkemans, M.J.; Habbema, J.D.F.; Hompes, P.G.; Burggraaff, J.M.; Oosterhuis, G.J.E.; Bossuyt, P.M.; van der Veen, F.; Mol, B.W. Obesity affects spontaneous pregnancy chances in subfertile, ovulatory women. Hum. Reprod. 2007, 23, 324–328. [Google Scholar] [CrossRef] [Green Version]
- National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults—The Evidence Report. Obes. Res. 1998, 6 (Suppl. 2), 51s–209s. [Google Scholar]
- van Dammen, L.; Wekker, V.; van Oers, A.M.; Mutsaerts, M.A.Q.; Painter, R.C.; Zwinderman, A.H.; Groen, H.; van de Beek, C.; Kobold, A.C.M.; Kuchenbecker, W.K.H.; et al. Effect of a lifestyle intervention in obese infertile women on cardiometabolic health and quality of life: A randomized controlled trial. PLoS ONE 2018, 13, e0190662. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.; et al. Diagnosis and Management of the Metabolic Syndrome. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [Green Version]
- Hu, M.L.; Louie, S.; Cross, C.E.; Motchnik, P.; Halliwell, B. Antioxidant protection against hypochlorous acid in human plasma. J. Lab. Clin. Med. 1993, 121, 257–262. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Carmona-Maurici, J.; Amigó, N.; Cuello, E.; Bermúdez-López, M.; Baena-Fustegueras, J.A.; Peinado-Onsurbe, J.; Pardina, E. Bariatric surgery decreases oxidative stress and protein glycosylation in patients with morbid obesity. Eur. J. Clin. Investig. 2020, 50, e13320. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Gao, X.; Chen, M.; van Dam, R. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: A meta-analysis. Obes. Rev. 2009, 10, 313–323. [Google Scholar] [CrossRef]
- Messina, J.; Campbell, S.; Morris, R.; Eyles, E.; Sanders, C. A narrative systematic review of factors affecting diabetes prevention in primary care settings. PLoS ONE 2017, 12, e0177699. [Google Scholar] [CrossRef] [PubMed]
- Wekker, V.; Huvinen, E.; Van Dammen, L.; Rono, K.; Painter, R.C.; Zwinderman, A.H.; Van De Beek, C.; Sarkola, T.; Mol, B.W.J.; Groen, H.; et al. Long-term effects of a preconception lifestyle intervention on cardiometabolic health of overweight and obese women. Eur. J. Public Health 2018, 29, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, P.R.; Abdulle, A.E.; Bulthuis, M.L.; Perton, F.G.; Connelly, M.A.; van Goor, H.; Dullaart, R.P. The Systemic Redox Status Is Maintained in Non-Smoking Type 2 Diabetic Subjects Without Cardiovascular Disease: Association with Elevated Triglycerides and Large VLDL. J. Clin. Med. 2019, 9, 49. [Google Scholar] [CrossRef] [Green Version]
- Abdulle, A.E.; Bourgonje, A.; Kieneker, L.M.; Koning, A.M.; Gemert, S.L.B.-V.; Bulthuis, M.L.C.; Dijkstra, G.; Faber, K.N.; Dullaart, R.P.F.; Bakker, S.J.L.; et al. Serum free thiols predict cardiovascular events and all-cause mortality in the general population: A prospective cohort study. BMC Med. 2020, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Damba, T.; Bourgonje, A.R.; Abdulle, A.E.; Pasch, A.; Sydor, S.; Berg, E.H.V.D.; Gansevoort, R.T.; Bakker, S.J.L.; Blokzijl, H.; Dullaart, R.P.F.; et al. Oxidative stress is associated with suspected non-alcoholic fatty liver disease and all-cause mortality in the general population. Liver Int. 2020, 40, 2148–2159. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Al-Rawas, A.M.; Al-Maqbali, M.; Al-Saleh, M.; Enriquez, M.B.; Al-Siyabi, S.; Al-Hashmi, K.; Al-Lawati, I.; Bulthuis, M.L.C.; et al. Systemic Oxidative Stress Is Increased in Postmenopausal Women and Independently Associates with Homocysteine Levels. Int. J. Mol. Sci. 2020, 21, 314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayedi, A.; Rahimi, K.; Bautista, L.E.; Nazarzadeh, M.; Zargar, M.S.; Shab-Bidar, S. Inflammation markers and risk of developing hypertension: A meta-analysis of cohort studies. Heart 2019, 105, 686–692. [Google Scholar] [CrossRef]
- Neri, M.; Fineschi, V.; Paolo, M.; Pomara, C.; Riezzo, I.; Turillazzi, E.; Cerretani, D. Cardiac Oxidative Stress and Inflammatory Cytokines Response after Myocardial Infarction. Curr. Vasc. Pharmacol. 2015, 13, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruder, E.H.; Hartman, T.J.; Blumberg, J.; Goldman, M.B. Oxidative stress and antioxidants: Exposure and impact on female fertility. Hum. Reprod. Updat. 2008, 14, 345–357. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Gupta, S.; Sharma, R. Oxidative stress and its implications in female infertility—A clinician's perspective. Reprod. Biomed. Online 2005, 11, 641–650. [Google Scholar] [CrossRef]



| Total Group (N = 470) | Intervention Group (N = 228) | Control Group (N = 242) | p-Value | |
|---|---|---|---|---|
| Age (years) | 29.9 ± 4.5 | 30.0 ± 4.5 | 29.9 ± 4.6 | 0.84 |
| Weight (kg) | 103.4 ± 13.1 | 104.2 ± 14.0 | 102.7 ± 12.1 | 0.21 |
| BMI (kg/m2) | 35.9 ± 3.3 | 36.1 ± 3.4 | 35.8 ± 3.2 | 0.23 |
| Western European Ethnicity | 411 (87.4%) | 200 (87.7%) | 211 (87.2%) | 0.86 |
| Education | 0.54 | |||
| Primary school | 23 (4.9%) | 14 (6.1%) | 9 (3.7%) | |
| Secondary education | 107 (22.8%) | 56 (24.6%) | 51 (21.1%) | |
| Intermediate vocational education | 217 (46.2%) | 104 (45.6%) | 113 (46.7%) | |
| Advanced vocational education and university | 105 (22.3%) | 46 (20.1%) | 59 (24.4%) | |
| Unknown | 18 (3.8%) | 8 (3.5%) | 10 (4.1%) | |
| Current smoker | 110 (23.6%) | 60 (26.5%) | 50 (20.7%) | 0.14 |
| Baseline | Three Months | Six Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Intervention Group (N = 203) | Control Group (N = 226) | p-Value | Intervention Group (N = 142) | Control Group (N = 150) | p-Value | Intervention Group (N = 104) | Control Group (N = 96) | p-Value | |
| Serum FT (µM) | 222.1 ± 48.0 | 229.9 ± 47.8 | 0.09 | 222.4 ± 45.0 | 228.3 ± 43.2 | 0.26 | 214.0 ± 40.9 | 220.4 ± 39.8 | 0.26 |
| Weight (kg) | 104.2 ± 14.3 | 102.6 ± 12.0 | 0.20 | 99.8 ± 13.1 | 102.6 ± 12.5 | 0.08 | 98.9 ± 13.0 | 102.6 ± 13.4 | 0.06 |
| BMI (kg/m2) | 36.1 ± 3.4 | 35.6 ± 3.2 | 0.13 | 34.6 ± 3.8 | 35.5 ± 3.6 | 0.08 | 34.7 ± 3.8 | 35.5 ± 3.6 | 0.18 |
| Waist circumference (cm) | 108.3 ± 9.4 | 107.1 ± 9.1 | 0.16 | 104.3 ± 10.4 | 105.6 ± 9.4 | 0.30 | 104.1 ± 9.9 | 105.6 ± 10.5 | 0.30 |
| Hip circumference (cm) | 125.4 ± 9.2 | 124.8 ± 8.8 | 0.47 | 121.0 ± 9.5 | 124.4 ± 9.6 | 0.004 | 121.2 ± 9.0 | 124.8 ± 9.8 | 0.01 |
| Waist-hip circumference ratio | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.48 | 0.9 ± 0.1 | 0.9 ± 0.1 | 0.16 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.31 |
| Triglycerides (mmol/L) | 1.2 ± 0.9 | 1.4 ± 1.0 | 0.23 | 1.3 ± 0.8 | 1.4 ± 1.1 | 0.34 | 1.2 ± 1.0 | 1.5 ± 1.9 | 0.24 |
| Total cholesterol (mmol/L) | 4.8 ± 0.9 | 4.8 ± 0.8 | 0.85 | 4.8 ± 1.0 | 4.8 ± 0.9 | 0.80 | 4.7 ± 0.8 | 4.9 ± 0.9 | 0.19 |
| HDL-C (mmol/L) | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.75 | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.58 | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.47 |
| LDL-C (mmol/L) | 3.1 ± 0.8 | 3.1 ± 0.8 | 0.73 | 3.1 ± 0.9 | 3.1 ± 0.8 | 0.75 | 3.1 ± 0.8 | 3.1 ± 0.8 | 0.48 |
| hs-CRP (mg/L) | 4.2 (2.2; 7.0) | 4.0 (1.8; 7.8) | 0.61 | 3.6 (1.7; 7.1) | 4.2 (1.9; 7.8) | 0.32 | 3.4 (1.4; 6.5) | 3.9 (2.0; 7.4) | 0.36 |
| Glucose (mmol/L) | 5.3 ± 0.6 | 5.4 ± 0.7 | 0.42 | 5.3 ± 0.6 | 5.5 ± 0.8 | 0.09 | 5.2 ± 0.5 | 5.4 ± 0.9 | 0.09 |
| Insulin (pmol/L) | 96.1 ± 51.7 | 101.3 ± 60.6 | 0.34 | 90.1 ± 55.7 | 110.8 ± 70.6 | 0.006 | 85.9 ± 52.7 | 97.4 ± 49.0 | 0.12 |
| HOMA-IR | 3.3 ± 2.0 | 3.5 ± 2.3 | 0.34 | 3.1 ± 2.1 | 4.0 ± 2.8 | 0.005 | 2.9 ± 1.9 | 3.5 ± 2.1 | 0.05 |
| Metabolic syndrome | 100/193 (51.8%) | 124/216 (57.4%) | 0.26 | 54/116 (46.5%) | 81/125 (64.8%) | 0.004 | 34/82 (41.5%) | 48/83 (57.8%) | 0.04 |
| Crude B (95% CI) | p-Value | |
|---|---|---|
| Age (years) | −0.90 (−1.73 to −0.07) | 0.04 |
| BMI (kg/m2) | −0.71 (−1.61 to 0.20) | 0.13 |
| Waist circumference (cm) | −0.14 (−0.42 to 0.13) | 0.31 |
| Hip circumference (cm) | −0.50 (−0.82 to −0.18) | 0.002 |
| Waist-hip circumference ratio | 31.39 (−7.33 to 70.11) | 0.11 |
| Triglycerides (mmol/L) | 5.29 (1.08 to 9.50) | 0.01 |
| Total cholesterol (mmol/L) | 1.38 (−2.40 to 5.17) | 0.47 |
| HDL-C (mmol/L) | −10.98 (−24.59 to 2.63) | 0.11 |
| LDL-C (mmol/L) | −0.73 (−5.35 to 3.89) | 0.76 |
| hs-CRP (mg/L) | −0.60 (−1.11 to −0.10) | 0.02 |
| Glucose (mmol/L) | 3.80 (−0.12 to 7.72) | 0.06 |
| Insulin (pmol/L) | 0.62 (0.26 to 0.98) | 0.001 |
| HOMA-IR | 2.50 (1.16 to 3.38) | <0.001 |
| Metabolic syndrome | 3.12 (−1.77 to 8.01) | 0.21 |
| Crude B (95% CI) | p-Value | |
|---|---|---|
| Change in BMI | ||
| ∆BMI as continuous measure | 0.39 (−0.82 to 1.59) | 0.53 |
| ∆BMI categorized | ||
| Method 1: ∆BMI quartiles | ||
| Q1 (∆BMI ≤ −1.50) | −3.15 (−8.90 to 2.61) | 0.28 |
| Q2 (−1.40 ≤ ∆BMI ≤ −0.60) | −1.61 (−7.43 to 4.21) | 0.59 |
| Q3 (−0.50 ≤ ∆BMI ≤ 0.20) | 1.64 (−3.64 to 6.91) | 0.54 |
| Q4 (∆BMI ≥ 0.30) | reference | |
| Method 2: successful weight loss * | ||
| successful | 0.22 (−4.40 to 4.84) | 0.93 |
| unsuccessful | reference | |
| Change in triglycerides | ||
| ∆triglycerides as continuous measure | 0.67 (−1.55 to 2.90) | 0.55 |
| ∆triglycerides categorized (based on quartiles) | ||
| Q1 (∆triglycerides ≤ −0.26) | 2.10 (−3.20 to 7.41) | 0.44 |
| Q2 (−0.25 ≤ ∆triglycerides ≤ −0.01) | −0.93 (−6.67 to 4.80) | 0.75 |
| Q3 (0 ≤ ∆triglycerides ≤ 0.25) | 0.13 (−5.47 to 5.74) | 0.96 |
| Q4 (∆triglycerides ≥ 0.27) | reference | |
| Change in hs-CRP | ||
| ∆hs-CRP as continuous measure | −0.41 (−0.70 to −0.13) | 0.005 |
| Change in HOMA | ||
| ∆HOMA-IR as continuous measure | 0.67 (−0.08 to 1.42) | 0.08 |
| ∆HOMA-IR categorized (based on quartiles) | ||
| Q1 (∆HOMA-IR ≤ −0.80) | −2.63 (−7.26 to 2.01) | 0.27 |
| Q2 (−0.79 ≤ ∆HOMA-IR ≤ −0.08) | −4.96 (−10.03 to 0.11) | 0.06 |
| Q3 (−0.07 ≤ ∆HOMA-IR ≤ 0.72) | −0.91 (−6.55 to 4.72) | 0.75 |
| Q4 (∆HOMA-IR ≥ 0.73) | reference |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Bourgonje, A.R.; Groen, H.; Abdulle, A.E.; Cantineau, A.E.P.; van Oers, A.M.; van Dammen, L.; Bulthuis, M.L.C.; Wekker, V.; Mol, B.W.J.; et al. The Effect of Lifestyle Intervention on Systemic Oxidative Stress in Women with Obesity and Infertility: A Post-Hoc Analysis of a Randomized Controlled Trial. J. Clin. Med. 2021, 10, 4243. https://doi.org/10.3390/jcm10184243
Wang Z, Bourgonje AR, Groen H, Abdulle AE, Cantineau AEP, van Oers AM, van Dammen L, Bulthuis MLC, Wekker V, Mol BWJ, et al. The Effect of Lifestyle Intervention on Systemic Oxidative Stress in Women with Obesity and Infertility: A Post-Hoc Analysis of a Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(18):4243. https://doi.org/10.3390/jcm10184243
Chicago/Turabian StyleWang, Zheng, Arno R. Bourgonje, Henk Groen, Amaal E. Abdulle, Astrid E. P. Cantineau, Anne M. van Oers, Lotte van Dammen, Marian L. C. Bulthuis, Vincent Wekker, Ben W. J. Mol, and et al. 2021. "The Effect of Lifestyle Intervention on Systemic Oxidative Stress in Women with Obesity and Infertility: A Post-Hoc Analysis of a Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 18: 4243. https://doi.org/10.3390/jcm10184243
APA StyleWang, Z., Bourgonje, A. R., Groen, H., Abdulle, A. E., Cantineau, A. E. P., van Oers, A. M., van Dammen, L., Bulthuis, M. L. C., Wekker, V., Mol, B. W. J., Roseboom, T. J., van Goor, H., & Hoek, A., on behalf of the LIFEstyle Study Group. (2021). The Effect of Lifestyle Intervention on Systemic Oxidative Stress in Women with Obesity and Infertility: A Post-Hoc Analysis of a Randomized Controlled Trial. Journal of Clinical Medicine, 10(18), 4243. https://doi.org/10.3390/jcm10184243







