Effect of Intra- and Post-Operative Fluid and Blood Volume on Postoperative Pulmonary Edema in Patients with Intraoperative Massive Bleeding
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Patients
- Age < 18 years;
- Patients undergoing cardiac surgery;
- Patients who underwent a previous surgery within 7 days of the current surgery;
- Patients with preoperative pulmonary edema or hypoxemia (PaO2/FiO2 ≤ 300).
2.3. Primary and Secondary Outcomes
2.4. Major Variables
2.5. Other Variables
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Follow-Up Period
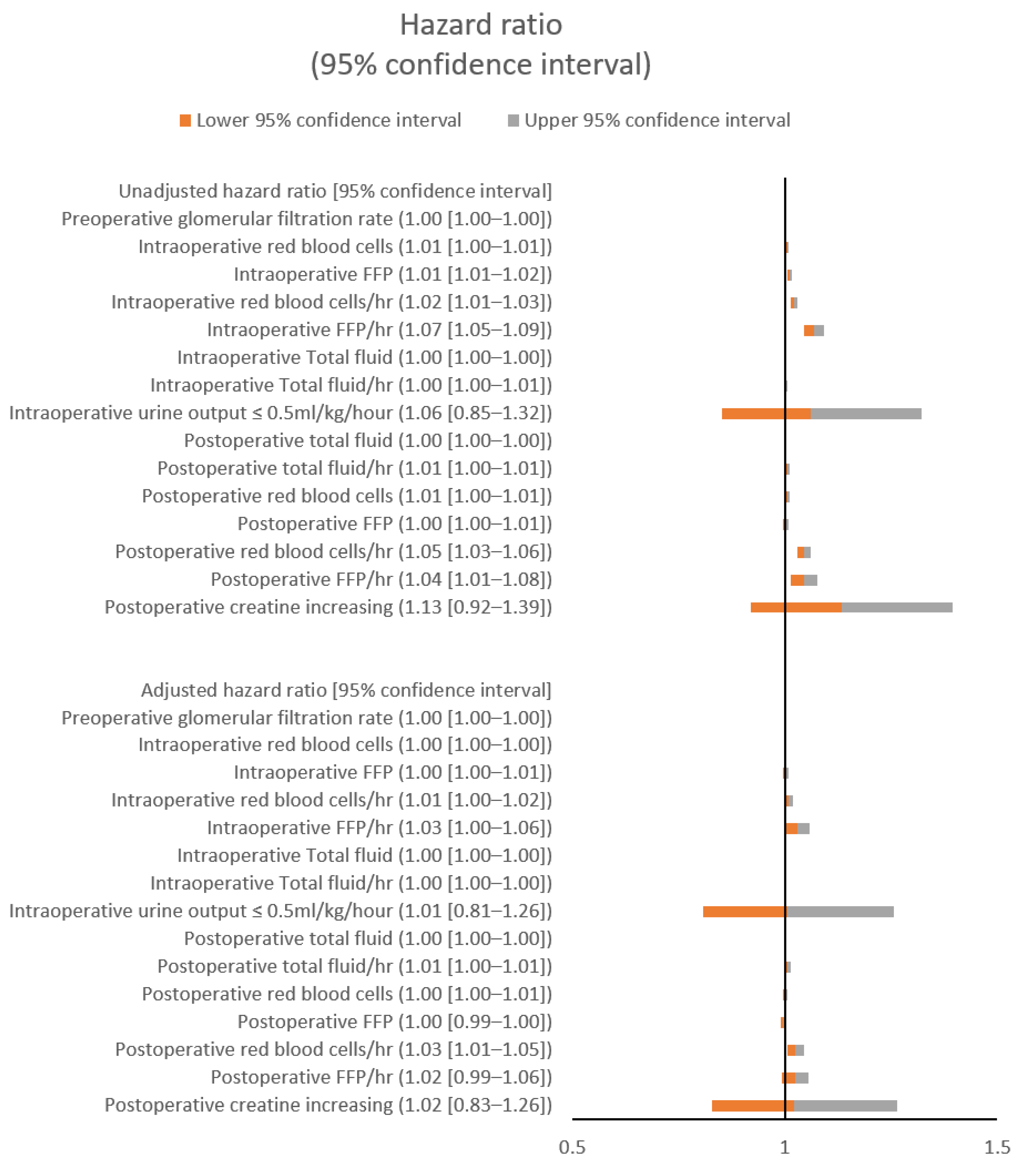
3.3. Hazard Ratios for Postoperative Pulmonary Edema with and without Hypoxemia
3.4. Blood and Fluid Cut-Off Values for Pulmonary Edema with and without Hypoxemia
3.5. Emergency Surgery
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Hazard Ratio | Lower 95% Confidence Interval | Upper 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Old age | 1.13 | 0.96 | 1.32 | 0.13 |
| Male sex | 1.04 | 0.90 | 1.21 | 0.56 |
| Obesity | 0.95 | 0.65 | 1.37 | 0.78 |
| Emergency | 1.09 | 0.93 | 1.27 | 0.30 |
| American Society of Anesthesiologists physical status > 2 | 1.09 | 0.94 | 1.27 | 0.24 |
| Anesthesia time | 1.00 | 0.98 | 1.03 | 0.77 |
| Anesthesia | 1.43 | 0.91 | 2.24 | 0.12 |
| Patient controlled analgesia | 1.01 | 0.87 | 1.19 | 0.85 |
| Tobacco use | 0.96 | 0.81 | 1.14 | 0.65 |
| Brain trauma | 0.96 | 0.77 | 1.19 | 0.73 |
| Multiple fractures | 1.09 | 0.84 | 1.41 | 0.52 |
| Acute abdomen surgery | 1.01 | 0.77 | 1.31 | 0.96 |
| Aorta surgery | 0.85 | 0.52 | 1.38 | 0.50 |
| Brain surgery | 0.93 | 0.77 | 1.12 | 0.42 |
| Spine surgery | 1.23 | 1.00 | 1.52 | 0.05 |
| Thoracic surgery | 1.05 | 0.72 | 1.52 | 0.80 |
| Massive transfusion | 1.24 | 0.87 | 1.77 | 0.24 |
| Estimated blood loss | 1.00 | 1.00 | 1.00 | 0.99 |
| Intraoperative inotropes | 1.04 | 0.88 | 1.24 | 0.62 |
| Preoperative hyponatremia | 0.94 | 0.76 | 1.16 | 0.57 |
| Preoperative hypoalbuminemia | 1.10 | 0.94 | 1.28 | 0.25 |
| Hazard Ratio | Lower 95% Confidence Interval | Upper 95% Confidence Interval | p-Value | |
|---|---|---|---|---|
| Old age | 1.27 | 1.03 | 1.58 | 0.03 |
| Male sex | 1.02 | 0.84 | 1.23 | 0.86 |
| Obesity | 1.10 | 0.62 | 1.93 | 0.74 |
| Emergency | 1.14 | 0.93 | 1.40 | 0.21 |
| American Society of Anesthesiologists physical status > 2 | 1.24 | 1.02 | 1.50 | 0.03 |
| Anesthesia time | 1.01 | 0.97 | 1.04 | 0.73 |
| Anesthesia | 1.37 | 0.78 | 2.43 | 0.27 |
| Patient controlled analgesia | 0.92 | 0.75 | 1.13 | 0.41 |
| Tobacco use | 1.01 | 0.80 | 1.26 | 0.96 |
| Brain Trauma | 0.96 | 0.73 | 1.26 | 0.75 |
| Multiple fractures | 1.08 | 0.76 | 1.53 | 0.67 |
| Acute abdomen surgery | 1.10 | 0.78 | 1.55 | 0.58 |
| Aorta surgery | 1.49 | 0.66 | 3.41 | 0.34 |
| Brain surgery | 0.95 | 0.75 | 1.20 | 0.67 |
| Spine surgery | 1.12 | 0.85 | 1.48 | 0.42 |
| Thoracic surgery | 1.01 | 0.58 | 1.74 | 0.98 |
| Massive transfusion | 1.59 | 0.97 | 2.62 | 0.07 |
| Estimated blood loss | 1.00 | 1.00 | 1.00 | 0.95 |
| Intraoperative inotropes | 1.12 | 0.89 | 1.41 | 0.35 |
| Preoperative hyponatremia | 1.04 | 0.79 | 1.38 | 0.77 |
| Preoperative hypoalbuminemia | 1.17 | 0.95 | 1.43 | 0.13 |
References
- Irita, K. Risk and crisis management in intraoperative hemorrhage: Human factors in hemorrhagic critical events. Korean J. Anesthesiol. 2011, 60, 151–160. [Google Scholar] [CrossRef]
- Lowell, J.A.; Schifferdecker, C.; Driscoll, D.F.; Benotti, P.N.; Bistrian, B.R. Postoperative fluid overload: Not a benign problem. Crit. Care Med. 1990, 18, 728–733. [Google Scholar] [CrossRef]
- Levy, J.H. Massive transfusion coagulopathy. Semin. Hematol. 2006, 43, S59–S63. [Google Scholar] [CrossRef]
- Sihler, K.C.; Napolitano, L.M. Complications of massive transfusion. Chest 2010, 137, 209–220. [Google Scholar] [CrossRef]
- Reynolds, B.R.; Forsythe, R.M.; Harbrecht, B.G.; Cuschieri, J.; Minei, J.P.; Maier, R.V.; Moore, E.E.; Billiar, T.R.; Peitzman, A.B.; Sperry, J.L.; et al. Hypothermia in massive transfusion: Have we been paying enough attention to it? J. Trauma Acute Care Surg. 2012, 73, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Toy, P.; Popovsky, M.A.; Abraham, E.; Ambruso, D.R.; Holness, L.G.; Kopko, P.M.; McFarland, J.G.; Nathens, A.B.; Silliman, C.C.; Stroncek, D.; et al. Transfusion-related acute lung injury: Definition and review. Crit. Care Med. 2005, 33, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Vercellini, P.; Rossi, R.; Pagnoni, B.; Fedele, L. Hypervolemic pulmonary edema and severe coagulopathy after intrauterine dextran instillation. Obstet. Gynecol. 1992, 79, 838–839. [Google Scholar] [CrossRef]
- Vaisrul, S. Acid-base imbalance in pulmonary edema. JAMA 1971, 216, 1337. [Google Scholar] [CrossRef]
- Su, Y.-J. Hypothermic lung edema after accidental hypothermia with out of hospital cardiac arrest. Heart Lung Vessel. 2015, 7, 328. [Google Scholar] [PubMed]
- Gajic, O.; Gropper, M.A.; Hubmayr, R.D. Pulmonary edema after transfusion: How to differentiate transfusion-associated circulatory overload from transfusion-related acute lung injury. Crit. Care Med. 2006, 34, S109–S113. [Google Scholar] [CrossRef]
- Butterworth, J.F.; Mackey, D.C.; Wasnick, J.D. Morgan and Mikhail’s Clinical Anesthesiology; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- KOO, B.N.; Kwon, M.A.; Kim, S.H.; Kim, J.Y.; Moon, Y.J.; Park, S.Y.; Lee, E.-H.; Chae, M.S.; Choi, S.U.; Choi, J.-H.; et al. Korean clinical practice guideline for perioperative red blood cell transfusion from Korean Society of Anesthesiologists. Korean J. Anesthesiol. 2019, 72, 91–118. [Google Scholar] [CrossRef]
- Chapleau, W.; Haskin, D.; LeBlanc, P.; Cardenas, G.; Borum, S.; Torres, N.; abi Saad, G.; al Ghanimi, O.; Al-Harthy, A.; al turki, S.; et al. Advanced Trauma Life Support (ATLS®): The ninth edition. J. Trauma Acute Care Surg. 2013, 74, 1363–1366. [Google Scholar] [CrossRef]
- Patil, V.; Shetmahajan, M. Massive transfusion and massive transfusion protocol. Indian J. Anaesth. 2014, 58, 590–595. [Google Scholar] [CrossRef]
- Mehta, R.L.; Kellum, J.A.; Shah, S.V.; Molitoris, B.A.; Ronco, C.; Warnock, D.G.; Levin, A. Acute Kidney Injury Network: Report of an initiative to improve outcomes in acute kidney injury. Crit. Care 2007, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Reinikainen, J.; Adeleke, K.A.; Pieterse, M.E.; Groothuis-Oudshoorn, C.G.M. Time-varying covariates and coefficients in Cox regression models. Ann. Transl. Med. 2018, 6, 121. [Google Scholar] [CrossRef]
- Warner, M.A.; Shore-Lesserson, L.; Shander, A.; Patel, S.Y.; Perelman, S.I.; Guinn, N.R. Perioperative anemia: Prevention, diagnosis, and management throughout the spectrum of perioperative care. Anesth. Analg. 2020, 130, 1364–1380. [Google Scholar] [CrossRef]
- Muñoz, M.; Acheson, A.G.; Bisbe, E.; Butcher, A.; Gómez-Ramírez, S.; Khalafallah, A.A.; Kehlet, H.; Kietaibl, S.; Liumbruno, G.M.; Meybohm, P.; et al. An international consensus statement on the management of postoperative anaemia after major surgical procedures. Anaesthesia 2018, 73, 1418–1431. [Google Scholar] [CrossRef]
- Koch, C.G.; Li, L.; Sun, Z.; Hixson, E.D.; Tang, A.S.; Phillips, S.C.; Blackstone, E.H.; Henderson, J.M. From bad to worse: Anemia on admission and hospital-acquired anemia. J. Patient Saf. 2017, 13, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Anand, I.S.; Chandrashekhar, Y.; Ferrari, R.; Poole-Wilson, P.A.; Harris, P.C. Pathogenesis of oedema in chronic severe anaemia: Studies of body water and sodium, renal function, haemodynamic variables, and plasma hormones. Br. Heart J. 1993, 70, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Rovellini, A.; Graziadei, G.; Folli, C.; Brambilla, A.M.; Cosentini, R.; Canetta, C.; Monzani, V. Causes and correlates of anemia in 200 patients with acute cardiogenic pulmonary edema. Eur. J. Intern. Med. 2012, 23, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Somers, K. Acute reversible heart failure in severe iron-deficiency anemia associated with hookworm infestation in Uganda Africans. Circulation 1959, 19, 672–675. [Google Scholar] [CrossRef]
- Varat, M.A.; Adolph, R.J.; Fowler, N.O. Cardiovascular effects of anemia. Am. Heart J. 1972, 83, 415–426. [Google Scholar] [CrossRef]
- Graettinger, J.S.; Parsons, R.L.; Campbell, J.A. A correlation of clinical and hemodynamic studies in patients with mild and severe anemia with and without congestive failure. Ann. Intern. Med. 1963, 58, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Sanghvi, L.; Sharma, V.; Mansharamani, G. Mechanism of oedema in chronic severe anaemia. Br. Heart J. 1963, 25, 89. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W.; Abraham, W.T. Hormones and hemodynamics in heart failure. N. Engl. J. Med. 1999, 341, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Sîrbu, O.; Floria, M.; Dascalita, P.; Stoica, A.; Adascalitei, P.; Sorodoc, V.; Sorodoc, L. Anemia in heart failure-from guidelines to controversies and challenges. Anatol. J. Cardiol. 2018, 20, 52. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C.; Hall, J.E. Textbook of Medical Physiology, 11th ed.; Elsevier: Philadelphia, PA, USA; Amsterdam, The Netherlands, 2006. [Google Scholar]
- Holte, K.; Sharrock, N.E.; Kehlet, H. Pathophysiology and clinical implications of perioperative fluid excess. Br. J. Anaesth. 2002, 89, 622–632. [Google Scholar] [CrossRef]
- Brandstrup, B.; Tønnesen, H.; Beier-Holgersen, R.; Hjortsø, E.; Ørding, H.; Lindorff-Larsen, K.; Rasmussen, M.S.; Lanng, C.; Wallin, L.; Iversen, L.H.; et al. Effects of intravenous fluid restriction on postoperative complications: Comparison of two perioperative fluid regimens: A randomized assessor-blinded multicenter trial. Ann. Surg. 2003, 238, 641–648. [Google Scholar] [CrossRef]
- Cooperman, L.H.; Price, H.L. Pulmonary edema in the operative and postoperative period: A review of 40 cases. Ann. Surg. 1970, 172, 883. [Google Scholar] [CrossRef] [PubMed]
- Arieff, A.I. Fatal postoperative pulmonary edema: Pathogenesis and literature review. Chest 1999, 115, 1371–1377. [Google Scholar] [CrossRef]
- Holte, K.; Jensen, P.; Kehlet, H. Physiologic effects of intravenous fluid administration in healthy volunteers. Anesth. Analg. 2003, 96, 1504–1509. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Kulshrestha, A. Diagnosis, prevention and management of postoperative pulmonary edema. Ann. Med. Health Sci. Res. 2012, 2, 180–185. [Google Scholar]
- Díaz, M.; Becker, D.E. Thermoregulation: Physiological and clinical considerations during sedation and general anesthesia. Anesth. Prog. 2010, 57, 25–34. [Google Scholar] [CrossRef]
- Torossian, A.; Bräuer, A.; Höcker, J.; Bein, B.; Wulf, H.; Horn, E.P. Preventing inadvertent perioperative hypothermia. Dtsch. Arztebl. Int. 2015, 112, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Oshvandi, K.; Shiri, F.H.; Fazel, M.R.; Safari, M.; Ravari, A. The effect of pre-warmed intravenous fluids on prevention of intraoperative hypothermia in cesarean section. Iran. J. Nurs. Midwifery Res. 2014, 19, 64–69. [Google Scholar] [PubMed]
- Martini, W.Z. Coagulopathy by hypothermia and acidosis: Mechanisms of thrombin generation and fibrinogen availability. J. Trauma 2009, 67, 202–208; discussion 208–209. [Google Scholar] [CrossRef] [PubMed]
- Wolberg, A.S.; Meng, Z.H.; Monroe, D.M.; Hoffman, M. A systematic evaluation of the effect of temperature on coagulation enzyme activity and platelet function. J. Trauma 2004, 56, 1221–1228. [Google Scholar] [CrossRef]
- Ghadimi, K.; Levy, J.H.; Welsby, I.J. Perioperative management of the bleeding patient. BJA Br. J. Anaesth. 2016, 117, iii18–iii30. [Google Scholar] [CrossRef]
- Meißner, A.; Schlenke, P. Massive bleeding and massive transfusion. Transfus. Med. Hemother. 2012, 39, 73–84. [Google Scholar] [CrossRef] [PubMed]
- van de Wiel, A. Anemia in critically ill patients. Eur. J. Intern. Med. 2004, 15, 481–486. [Google Scholar] [CrossRef]
- Levi, M.; Opal, S.M. Coagulation abnormalities in critically ill patients. Crit. Care 2006, 10, 222. [Google Scholar] [CrossRef]
- Liumbruno, G.M.; Bennardello, F.; Lattanzio, A.; Piccoli, P.; Rossetti, G. Recommendations for the transfusion management of patients in the peri-operative period. III. The post-operative period. Blood Transfus. 2011, 9, 320–335. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B. Pulmonary complications of transfused blood components. Crit. Care Nurs. Clin. N. Am. 2012, 24, 403–418. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roubinian, N. TACO and TRALI: Biology, risk factors, and prevention strategies. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 585–594. [Google Scholar] [CrossRef]
- Handy, J.; Soni, N. Physiological effects of hyperchloraemia and acidosis. Br. J. Anaesth. 2008, 101, 141–150. [Google Scholar] [CrossRef] [PubMed]
- DiFrancesco, N.R.; Gaffney, T.P.; Lashley, J.L.; Hickerson, K.A. Hypocalcemia and massive blood transfusions: A pilot study in a level I trauma center. J. Trauma Nurs. Off. J. Soc. Trauma Nurses 2019, 26, 186–192. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Gibbs, J.S.R.; Wachter, R.; De Marco, T.; Vonk-Noordegraaf, A.; Vachiery, J.L. Left ventricular heart failure and pulmonary hypertension. Eur. Heart J. 2016, 37, 942–954. [Google Scholar] [CrossRef]
- Barker, S.J.; Shander, A.; Ramsay, M.A. Continuous noninvasive hemoglobin monitoring: A measured response to a critical review. Anesth. Analg. 2016, 122, 565–572. [Google Scholar] [CrossRef]
- Endo, Y.; Tamura, J.; Ishizuka, T.; Itami, T.; Hanazono, K.; Miyoshi, K.; Sano, T.; Yamashita, K.; Muir, W.W. Stroke volume variation (SVV) and pulse pressure variation (PPV) as indicators of fluid responsiveness in sevoflurane anesthetized mechanically ventilated euvolemic dogs. J. Vet. Med. Sci. 2017, 79, 1437–1445. [Google Scholar] [CrossRef]
- Checketts, M.R.; Alladi, R.; Ferguson, K.; Gemmell, L.; Handy, J.M.; Klein, A.A.; Love, N.J.; Misra, U.; Morris, C.; Nathanson, M.H.; et al. Recommendations for standards of monitoring during anaesthesia and recovery 2015: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2016, 71, 85–93. [Google Scholar] [CrossRef]
- Pistolesi, M.; Giuntini, C. Assessment of extravascular lung water. Radiol. Clin. N. Am. 1978, 16, 551–574. [Google Scholar] [PubMed]
- Waxman, K. Postoperative multiple organ failure. Crit. Care Clin. 1987, 3, 429–440. [Google Scholar] [CrossRef]
- Ware, L.B.; Matthay, M.A. Acute pulmonary edema. N. Engl. J. Med. 2005, 353, 2788–2796. [Google Scholar] [CrossRef]
- Clark, S.B.; Soos, M.P. Noncardiogenic pulmonary edema. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Basu, R.K.; Wheeler, D. Effects of ischemic acute kidney injury on lung water balance: Nephrogenic pulmonary edema? Pulm. Med. 2011, 2011, 414253. [Google Scholar] [CrossRef] [PubMed]
- Shires, G.T.; Canizaro, P.C.; Lowry, S. Fluid, electrolyte, and nutritional management of the surgical patient. In Principles of Surgery; McGraw-Hill: New York, NY, USA, 1994; Volume 6, pp. 61–80. [Google Scholar]
- Darby, J.; Nelson, P. Fluid, electrolyte, and acid-base balance in neurosurgical intensive care. In Neurosurgical Intensive Care; McGraw-Hill: New York, NY, USA, 1993; pp. 133–162. [Google Scholar]
- Liumbruno, G.; Bennardello, F.; Lattanzio, A.; Piccoli, P.; Rossetti, G. Recommendations for the transfusion of plasma and platelets. Blood Transfus. 2009, 7, 132–150. [Google Scholar] [CrossRef] [PubMed]
- Faubel, S. Pulmonary complications after acute kidney injury. Adv. Chronic Kidney Dis. 2008, 15, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Farha, N.; Munguti, C. A dramatic presentation of pulmonary edema due to renal failure. Kans. J. Med. 2020, 13, 56–57. [Google Scholar] [CrossRef] [PubMed]
- Park, J.T. Postoperative acute kidney injury. Korean J. Anesthesiol. 2017, 70, 258–266. [Google Scholar] [CrossRef]
- Han, S.S.; Baek, S.H.; Ahn, S.Y.; Chin, H.J.; Na, K.Y.; Chae, D.W.; Kim, S. Anemia is a risk factor for acute kidney injury and long-term mortality in critically ill patients. Tohoku J. Exp. Med. 2015, 237, 287–295. [Google Scholar] [CrossRef]
- Terceros-Almanza, L.J.; García-Fuentes, C.; Bermejo-Aznárez, S.; Prieto-Del Portillo, I.J.; Mudarra-Reche, C.; Saez-de la Fuente, I.; Chico-Fernández, M. Prediction of massive bleeding. Shock index and modified shock index. Med. Intensiva 2017, 41, 532–538. [Google Scholar] [CrossRef]
- Alder, L.; Tambe, A. Acute anemia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Bolliger, D.; Görlinger, K.; Tanaka, K.A.; Warner, D.S. Pathophysiology and treatment of coagulopathy in massive hemorrhage and hemodilution. Anesthesiology 2010, 113, 1205–1219. [Google Scholar] [CrossRef]
- Hahn, R.G.; Lyons, G. The half-life of infusion fluids: An educational review. Eur. J. Anaesthesiol. 2016, 33, 475–482. [Google Scholar] [CrossRef]
- Hobson, C.; Ruchi, R.; Bihorac, A. Perioperative acute kidney injury: Risk factors and predictive strategies. Crit. Care Clin. 2017, 33, 379–396. [Google Scholar] [CrossRef]
- Liu, R.; Wang, J.; Zhao, G.; Su, Z. Negative pressure pulmonary edema after general anesthesia: A case report and literature review. Medicine 2019, 98, e15389. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.C. Generating survival times to simulate Cox proportional hazards models with time-varying covariates. Stat. Med. 2012, 31, 3946–3958. [Google Scholar] [CrossRef]
- de la Ventilacio Mecanica, S.B.; Miranda, M.; Kelly, C.R.; Rabbani, L.E. Pulmonary-artery catheterization. N. Engl. J. Med. 2013, 369, e35. [Google Scholar]
- Milne, E.N.; Pistolesi, M.; Miniati, M.; Giuntini, C. The radiologic distinction of cardiogenic and noncardiogenic edema. AJR Am. J. Roentgenol. 1985, 144, 879–894. [Google Scholar] [CrossRef] [PubMed]

| Variable | No Postoperative Pulmonary Edema (n = 1790) | Postoperative Pulmonary Edema (n = 300) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Old age, n (%) | 496 (27.7) | 108 (36.0) | 0.003 | |
| Males, n (%) | 904 (50.5) | 148 (49.3) | 0.71 | |
| Obesity, n (%) | 68 (3.8) | 11 (3.7) | 0.91 | |
| Preoperative clinical features | ||||
| Emergency, n (%) | 647 (36.1) | 148 (49.3) | <0.001 | |
| American Society of Anesthesiologists physical status > 2, n (%) | 862 (48.2) | 186 (62.0) | <0.001 | |
| Tobacco use, n (%) | 415 (23.2) | 62 (20.7) | 0.346 | |
| Brain trauma, n (%) | 230 (12.8) | 42 (14.0) | 0.58 | |
| Multiple fractures, n (%) | 127 (7.1) | 33 (11.0) | 0.02 | |
| Hyponatremia, n (%) | 240 (13.4) | 34 (11.3) | 0.33 | |
| Hypoalbuminemia, n (%) | 542 (30.3) | 117 (39.0) | 0.003 | |
| Glomerular filtration rate, median (interquartile range), mL/min/1.73 m2 | 93.4 (72.7–93.4) | 84.4 (68.3–84.4) | <0.001 | |
| Intraoperative clinical features | ||||
| General anesthesia, n (%) | 1730 (96.6) | 299 (99.7) | 0.004 | |
| Anesthesia time, median (interquartile range), h | 5.0 (3.5–5.0) | 5.1 (2.9–5.1) | 0.54 | |
| Acute abdomen surgery, n (%) | 119 (6.6) | 34 (11.3) | 0.004 | |
| Aorta surgery, n (%) | 30 (1.7) | 7 (2.3) | 0.42 | |
| Brain surgery, n (%) | 391 (21.8) | 59 (19.7) | 0.40 | |
| Spine surgery, n (%) | 270 (15.1) | 58 (19.3) | 0.06 | |
| Thoracic surgery, n (%) | 53 (3.0) | 13 (4.3) | 0.21 | |
| Massive transfusion, n (%) | 57 (3.2) | 33 (11.0) | <0.001 | |
| Urine output ≤ 0.5 mL/kg/h, n (%) | 202 (11.3) | 39 (13.0) | 0.39 | |
| Continuous inotrope use, n (%) | 375 (20.9) | 96 (32.0) | <0.001 | |
| Red blood cells, median (interquartile range), % | 24.3 (12.1–24.3) | 32.9 (20.9–32.9) | <0.001 | |
| Red blood cells per hour, median (interquartile range), %/h | 4.6 (2.0–4.6) | 6.3 (3.7–6.3) | <0.001 | |
| FFP, median (interquartile range), % | 1.5 (0.0–1.5) | 9.5 (0.0–9.5) | <0.001 | |
| FFP per hour, median (interquartile range), %/h | 0.1 (0.0–0.1) | 1.7 (0.0–1.7) | <0.001 | |
| Total fluid, median (interquartile range), % | 113.7 (87.9–113.7) | 119.7 (89.7–119.7) | 0.04 | |
| Total fluid per hour, median (interquartile range), %/h | 22.6 (17.2–22.6) | 24.3 (17.8–24.3) | 0.01 | |
| Estimated blood loss, median (interquartile range), % | 54.4 (45.5–54.4) | 63.0 (48.6–63.0) | <0.001 | |
| Postoperative clinical features | ||||
| Patient controlled analgesia, n (%) | 1139 (63.6) | 181 (60.3) | 0.27 | |
| Time-varying postoperative clinical features | ||||
| Period 1 (No postoperative pulmonary edema, n = 1866; postoperative pulmonary edema, n = 192) | Start time, median (interquartile range), h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.000 |
| End time, median (interquartile range), h | 10.4 (5.6–16.1) | 0.8 (0.3–6.2) | <0.001 | |
| Total fluid, median (interquartile range), % | 29.0 (10.2–58.2) | 2.4 (0.0–24.5) | <0.001 | |
| Total fluid per hour, median (interquartile range), %/h | 2.8 (1.3–4.7) | 1.5 (0.0–4.4) | <0.001 | |
| Red blood cells, median (interquartile range), % | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.79 | |
| FFP, median (interquartile range), % | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.76 | |
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.34 | |
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 0.88 | |
| Creatinine increase, n (%) | 190 (10.2) | 20 (10.4) | 0.92 | |
| Period 2 (No postoperative pulmonary edema, n = 20; postoperative pulmonary edema, n = 1) | Start time, median (interquartile range), h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | 1.000 |
| End time, median (interquartile range), h | 33.8 (29.2–37.2) | 43.4 (43.4–43.4) | 0.48 | |
| Total fluid, median (interquartile range), % | 69.9 (42.7–119.4) | 25.6 (25.6–25.6) | 0.38 | |
| Total fluid per hour, median (interquartile range), %/h | 2.5 (1.5–3.4) | 0.6 (0.6–0.6) | 0.29 | |
| Red blood cells, median (interquartile range), % | 0.0 (0.0–12.8) | 5.0 (5.0–5.0) | 0.86 | |
| FFP, median (interquartile range), % | 0.0 (0.0–4.5) | 0.0 (0.0–0.0) | 0.67 | |
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–0.4) | 0.1 (0.1–0.1) | 0.86 | |
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–0.1) | 0.0 (0.0–0.0) | 0.67 | |
| Creatinine increase, n (%) | 2 (10.0) | 1 (100.0) | 0.14 | |
| Period 3 (No postoperative pulmonary edema, n = 11; postoperative pulmonary edema, n = 0) | Start time, median (interquartile range), h | 0.0 (0.0–0.0) | ||
| End time, median (interquartile range), h | 59.2 (58.3–64.1) | |||
| Total fluid, median (interquartile range), % | 74.8 (22.0–140.3) | |||
| Total fluid per hour, median (interquartile range), %/h | 1.2 (0.4–2.3) | |||
| Red blood cells, median (interquartile range), % | 0.0 (0.0–0.0) | |||
| FFP, median (interquartile range), % | 0.0 (0.0–0.0) | |||
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–0.0) | |||
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–0.0) | |||
| Creatinine increase, n (%) | 2 (18.2) | |||
| Period 4 (No postoperative pulmonary edema, n = 1301; postoperative pulmonary edema, n = 63) | Start time, median (interquartile range), h | 11.4 (7.0–17.1) | 11.0 (8.8–16.0) | 0.73 |
| Start time, median (interquartile range), h | 35.6 (31.1–41.2) | 34.2 (30.9–37.5) | 0.08 | |
| End time, median (interquartile range), h | 106.1 (68.3–163.5) | 111.1 (77.9–158.9) | 0.29 | |
| Total fluid, median (interquartile range), % | 4.6 (2.8–7.2) | 5.0 (3.7–7.7) | 0.09 | |
| Total fluid per hour, median (interquartile range), %/h | 0.0 (0.0–10.3) | 9.5 (0.0–25.9) | <0.001 | |
| Red blood cells, median (interquartile range), % | 0.0 (0.0–7.0) | 4.0 (0.0–17.9) | <0.001 | |
| FFP, median (interquartile range), % | 0.0 (0.0–0.4) | 0.4 (0.0–1.5) | <0.001 | |
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–0.3) | 0.2 (0.0–0.8) | <0.001 | |
| FFP per hour, median (interquartile range), %/h | 198 (15.2) | 16 (25.4) | 0.03 | |
| Period 5 (No postoperative pulmonary edema, n = 81; postoperative pulmonary edema, n = 2) | Start time, median (interquartile range), h | 10.3 (5.1–15.7) | 9.6 (9.2–10.0) | 0.78 |
| End time, median (interquartile range), h | 58.5 (53.8–64.6) | 55.9 (54.8–57.0) | 0.49 | |
| Total fluid, median (interquartile range), % | 89.2 (60.9–160.2) | 124.3 (111.2–137.5) | 0.53 | |
| Total fluid per hour, median (interquartile range), %/h | 2.0 (1.4–3.2) | 2.7 (2.4–2.9) | 0.43 | |
| Red blood cells, median (interquartile range), % | 0.0 (0.0–10.7) | 2.6 (1.3–4.0) | 0.81 | |
| FFP, median (interquartile range), % | 0.0 (0.0–0.0) | 1.8 (0.9–2.7) | 0.53 | |
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–0.2) | 0.1 (0.0–0.1) | 0.81 | |
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–0.0) | 0.0 (0.0–0.1) | 0.53 | |
| Creatinine increase, n (%) | 1 (1.2) | 0 (0.0) | >0.999 | |
| Period 6 (No postoperative pulmonary edema, n = 1008; postoperative pulmonary edema, n = 42) | Start time, median (interquartile range), h | 35.3 (30.9–40.9) | 35.4 (30.9–41.5) | 0.96 |
| End time, median (interquartile range), h | 59.0 (54.9–64.9) | 58.2 (52.4–63.1) | 0.21 | |
| Total fluid, median (interquartile range), % | 178.7 (109.1–268.1) | 166.5 (108.0–237.0) | 0.46 | |
| Total fluid per hour, median (interquartile range), %/h | 7.7 (4.6–11.5) | 6.9 (4.9–11.1) | 0.79 | |
| Red blood cells, median (interquartile range), % | 0.0 (0.0–13.9) | 11.6 (0.0–25.2) | 0.001 | |
| FFP, median (interquartile range), % | 0.0 (0.0–8.3) | 0.0 (0.0–14.4) | 0.19 | |
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–0.6) | 0.4 (0.0–1.2) | 0.001 | |
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–0.4) | 0.0 (0.0–0.6) | 0.17 | |
| Creatinine increase, n (%) | 153 (15.2) | 13 (31.0) | 0.01 | |
| Variable | No Postoperative Pulmonary Edema with Hypoxemia (n = 1499) | Postoperative Pulmonary Edema with Hypoxemia (n = 161) | p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Old age, n (%) | 384 (25.6) | 63 (39.1) | <0.001 | |
| Males, n (%) | 730 (48.7) | 88 (54.7) | 0.15 | |
| Obesity, n (%) | 46 (3.1) | 6 (3.7) | 0.65 | |
| Preoperative clinical features | ||||
| Emergency, n (%) | 516 (34.4) | 89 (55.3) | <0.001 | |
| American Society of Anesthesiologists physical status > 2, n (%) | 698 (46.6) | 117 (72.7) | <0.001 | |
| Tobacco use, n (%) | 332 (22.1) | 36 (22.4) | 0.95 | |
| Brain trauma, n (%) | 183 (12.2) | 24 (14.9) | 0.33 | |
| Multiple fracture, n (%) | 106 (7.1) | 19 (11.8) | 0.03 | |
| Hyponatremia, n (%) | 195 (13.0) | 21 (13.0) | 0.99 | |
| Hypoalbuminemia, n (%) | 443 (29.6) | 69 (42.9) | 0.001 | |
| Glomerular filtration rate, median (interquartile range), mL/min/1.73 m2 | 94.1 (72.8–122.9) | 83.7 (65.4–108.6) | <0.001 | |
| Intraoperative clinical features | ||||
| General anesthesia, n (%) | 1445 (96.4) | 160 (99.4) | 0.05 | |
| Anesthesia time, median (interquartile range), h | 5.0 (3.5–6.9) | 4.7 (2.8–7.2) | 0.58 | |
| Acute abdomen surgery, n (%) | 97 (6.5) | 26 (16.1) | <0.001 | |
| Aorta surgery, n (%) | 12 (0.8) | 5 (3.1) | 0.01 | |
| Brain surgery, n (%) | 319 (21.3) | 35 (21.7) | 0.89 | |
| Spine surgery, n (%) | 235 (15.7) | 25 (15.5) | 0.96 | |
| Thoracic surgery, n (%) | 41 (2.7) | 6 (3.7) | 0.47 | |
| Massive transfusion, n (%) | 35 (2.3) | 25 (15.5) | <0.001 | |
| Urine output ≤ 0.5 mL/kg/h, n (%) | 154 (10.3) | 21 (13.0) | 0.28 | |
| Continuous inotropes use, n (%) | 279 (18.6) | 62 (38.5) | <0.001 | |
| Red blood cells, median (interquartile range), % | 23.7 (11.8–37.2) | 34.6 (21.1–61.0) | <0.001 | |
| Red blood cells per hour, median (interquartile range), %/h | 4.6 (1.9–8.0) | 6.9 (3.8–16.7) | <0.001 | |
| FFP, median (interquartile range), % | 0.0 (0.0–10.1) | 9.7 (0.0–18.6) | <0.001 | |
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–2.1) | 1.9 (0.0–5.4) | <0.001 | |
| Total fluid, median (interquartile range), % | 113.6 (87.8–146.9) | 126.9 (91.9–178.0) | 0.02 | |
| Total fluid per hour, median (interquartile range), % | 22.8 (17.3–31.6) | 26.5 (18.0–40.1) | 0.02 | |
| Estimated blood loss, median (interquartile range), % | 54.3 (45.5–72.6) | 66.7 (51.0–104.2) | <0.001 | |
| Postoperative clinical features | ||||
| Patient controlled analgesia, n (%) | 979 (65.3) | 83 (51.6) | 0.001 | |
| Time-varying postoperative clinical features | ||||
| Period 1 (No postoperative pulmonary edema with hypoxemia, n = 1275; postoperative pulmonary edema with hypoxemia, n = 72) | Start time, median (interquartile range), h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | >0.999 |
| End time, median (interquartile range), h | 9.9 (3.3–15.3) | 1.3 (0.8–7.1) | <0.001 | |
| Total fluid, median (interquartile range), % | 27.1 (5.4–54.5) | 0.9 (0.0–22.4) | <0.001 | |
| Total fluid per hour, median (interquartile range), %/h | 2.7 (1.1–4.5) | 0.3 (0.0–2.8) | <0.001 | |
| Red blood cells, median (interquartile range), % | 0.0 (0.0–0.0) | 0.0 (0.0–7.1) | 0.001 | |
| FFP, median (interquartile range), % | 0.0 (0.0–0.0) | 0.0 (0.0–5.3) | 0.004 | |
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–0.0) | 0.0 (0.0–1.7) | <0.001 | |
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–0.0) | 0.0 (0.0–0.6) | 0.002 | |
| Creatinine increase, n (%) | 99 (7.8) | 11 (15.3) | 0.02 | |
| Period 2 (No postoperative pulmonary edema with hypoxemia, n = 148; postoperative pulmonary edema with hypoxemia, n = 42) | Start time, median (interquartile range), h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | >0.999 |
| End time, median (interquartile range), h | 37.2 (31.3–41.6) | 35.3 (30.0–39.6) | 0.12 | |
| Total fluid, median (interquartile range), % | 102.3 (70.7–150.7) | 102.9 (54.0–145.5) | 0.76 | |
| Total fluid per hour, median (interquartile range), %/h | 2.9 (1.9–4.1) | 2.8 (1.6–4.4) | 0.93 | |
| Red blood cells, median (interquartile range), % | 0.0 (0.0–7.4) | 0.0 (0.0–21.2) | 0.05 | |
| FFP, median (interquartile range), % | 0.0 (0.0–0.0) | 7.9 (0.0–21.6) | <0.001 | |
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–0.2) | 0.0 (0.0–0.6) | 0.04 | |
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–0.0) | 0.3 (0.0–0.6) | <0.001 | |
| Creatinine increase, n (%) | 17 (11.5) | 19 (45.2) | <0.001 | |
| Period 3 (No postoperative pulmonary edema with hypoxemia, n = 86; postoperative pulmonary edema with hypoxemia n = 21) | Start time, median (interquartile range), h | 0.0 (0.0–0.0) | 0.0 (0.0–0.0) | >0.999 |
| End time, median (interquartile range), h | 59.1 (55.3–64.8) | 57.2 (54.5–61.2) | 0.20 | |
| Total fluid, median (interquartile range), % | 132.7 (75.9–222.2) | 198.8 (118.1–279.2) | 0.05 | |
| Total fluid per hour, median (interquartile range), %/h | 4.3 (0.0–21.7) | 38.5 (10.0–82.3) | 0.04 | |
| Red blood cells, median (interquartile range), % | 2.2 (1.3–3.8) | 3.4 (2.1–5.0) | 0.01 | |
| FFP, median (interquartile range), % | 0.1 (0.0–0.3) | 0.6 (0.2–1.4) | 0.01 | |
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–14.0) | 11.0 (0.0–44.0) | 0.01 | |
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–8.5) | 8.3 (0.0–40.0) | 0.01 | |
| Creatinine increase, n (%) | 9 (10.5) | 7 (33.3) | 0.01 | |
| Period 4 (No postoperative pulmonary edema with hypoxemia, n = 583; postoperative pulmonary edema with hypoxemia, n = 0) | Start time, median (interquartile range), h | 11.4 (5.6–16.0) | ||
| End time, median (interquartile range), h | 36.9 (31.9–40.8) | |||
| Total fluid, median (interquartile range), % | 116.0 (78.3–178.2) | |||
| Total fluid per hour, median (interquartile range), %/h | 0.0 (0.0–20.7) | |||
| Red blood cells, median (interquartile range), % | 4.6 (3.0–7.4) | |||
| FFP, median (interquartile range), % | 0.0 (0.0–0.8) | |||
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–10.2) | |||
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–7.9) | |||
| Creatinine increase, n (%) | 71 (12.2) | |||
| Period 5 (No postoperative pulmonary edema with hypoxemia, n = 148; postoperative pulmonary edema with hypoxemia, n = 12) | Start time, median (interquartile range), h | 10.5 (5.5–15.5) | 9.4 (7.1–16.9) | 0.66 |
| End time, median (interquartile range), h | 60.0 (55.6–64.3) | 59.5 (55.1–63.2) | 0.77 | |
| Total fluid, median (interquartile range), % | 137.2 (79.0–211.5) | 227.4 (186.0–284.8) | 0.003 | |
| Total fluid per hour, median (interquartile range), %/h | 0.0 (0.0–16.9) | 22.3 (0.0–41.4) | 0.003 | |
| Red blood cells, median (interquartile range), % | 2.8 (1.7–4.4) | 5.0 (3.5–5.9) | 0.39 | |
| FFP, median (interquartile range), % | 0.0 (0.0–0.4) | 0.5 (0.0–0.9) | 0.03 | |
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–13.9) | 5.9 (0.0–14.0) | 0.32 | |
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–5.2) | 6.8 (0.0–24.2) | 0.03 | |
| Creatinine increase, n (%) | 14 (9.5) | 5 (41.7) | 0.001 | |
| Period 6 (No postoperative pulmonary edema with hypoxemia, n = 384; postoperative pulmonary edema with hypoxemia, n = 14) | Start time, median (interquartile range), h | 37.3 (32.2–41.3) | 36.6 (32.9–38.6) | 0.63 |
| End time, median (interquartile range), h | 60.9 (55.6–65.6) | 60.1 (57.3–63.2) | 0.61 | |
| Total fluid, median (interquartile range), % | 204.7 (125.8–297.4) | 172.5 (135.9–218.7) | 0.58 | |
| Total fluid per hour, median (interquartile range), %/h | 7.5 (0.0–29.0) | 30.1 (15.6–68.9) | 0.56 | |
| Red blood cells, median (interquartile range), % | 8.6 (5.3–12.8) | 7.1 (5.5–9.0) | >0.999 | |
| FFP, median (interquartile range), % | 0.3 (0.0–1.3) | 1.4 (0.5–2.8) | 0.27 | |
| Red blood cells per hour, median (interquartile range), %/h | 0.0 (0.0–14.0) | 18.4 (14.7–37.0) | >0.999 | |
| FFP per hour, median (interquartile range), %/h | 0.0 (0.0–13.3) | 2.6 (0.0–22.9) | 0.30 | |
| Creatinine increase, n (%) | 47 (12.2) | 4 (28.6) | 0.07 | |
| Pulmonary Edema | Cut-Off Value | ROC-AUC | 95% CI | p-Value |
|---|---|---|---|---|
| Intraop. RBCs | 27.7 | 0.61 | 0.58–0.64 | <0.001 |
| Intraop. FFP | 9.0 | 0.62 | 0.59–0.65 | <0.001 |
| Intraop. RBCs/h | 3.7 | 0.61 | 0.58–0.64 | <0.001 |
| Intraop. FFP/h | 2.4 | 0.62 | 0.59–0.65 | <0.001 |
| Intraop. total fluid | 120.7 | 0.53 | 0.50–0.56 | 0.07 |
| Intraop. total fluid/h | 21.5 | 0.55 | 0.52–0.58 | <0.001 |
| Postop. total fluid | 579.0 | 0.35 | 0.32–0.38 | <0.001 |
| Postop. total fluid/h | 11.8 | 0.44 | 0.40–0.47 | <0.001 |
| Postop. RBCs | 15.2 | 0.53 | 0.50–0.56 | 0.02 |
| Postop. FFP | 9.1 | 0.52 | 0.49–0.55 | 0.17 |
| Postop. RBCs/h | 1.2 | 0.54 | 0.51–0.58 | <0.001 |
| Postop. FFP/h | 0.5 | 0.53 | 0.50–0.56 | 0.06 |
| Pulmonary edema with hypoxemia | ||||
| Intraop. RBCs | 239.6 | 0.65 | 0.61–0.70 | <0.001 |
| Intraop. FFP | 87.4 | 0.64 | 0.59–0.68 | <0.001 |
| Intraop. RBCs/h | 54.5 | 0.65 | 0.61–0.70 | <0.001 |
| Intraop. FFP/h | 15.1 | 0.64 | 0.59–0.69 | <0.001 |
| Intraop. total fluid | 510.8 | 0.56 | 0.51–0.61 | 0.01 |
| Intraop. total fluid/h | 124.3 | 0.57 | 0.52–0.62 | <0.001 |
| Postop. total fluid | 718.9 | 0.48 | 0.43–0.53 | 0.36 |
| Postop. total fluid/h | 10.0 | 0.41 | 0.36–0.45 | <0.001 |
| Postop. RBCs | 100.5 | 0.61 | 0.56–0.66 | <0.001 |
| Postop. FFP | 123.3 | 0.61 | 0.56–0.66 | <0.001 |
| Postop. RBCs/h | 13.3 | 0.61 | 0.56–0.66 | <0.001 |
| Postop. FFP/h | 8.4 | 0.60 | 0.55–0.65 | <0.001 |
| Pulmonary Edema | HR | 95% CI | p-Value |
|---|---|---|---|
| Intraop. RBCs | 1.00 | 1.00–1.00 | 0.96 |
| Intraop. FFP | 1.00 | 0.99–1.01 | 0.91 |
| Intraop. RBCs/h | 1.01 | 1.00–1.02 | 0.24 |
| Intraop. FFP/h | 1.03 | 1.00–1.07 | 0.05 |
| Intraop. total fluid | 1.00 | 1.00–1.00 | 0.65 |
| Intraop. total fluid/h | 1.00 | 1.00–1.00 | 0.63 |
| Oliguria | 1.05 | 0.83–1.33 | 0.66 |
| Preop. GFR | 1.00 | 1.00–1.00 | 0.38 |
| Postop. total fluid | 1.00 | 1.00–1.00 | 0.00 |
| Postop. total fluid/h | 1.00 | 0.99–1.01 | 0.65 |
| Postop. RBCs | 1.00 | 0.99–1.01 | 0.97 |
| Postop. FFP | 1.00 | 0.99–1.00 | 0.32 |
| Postop. RBCs/h | 1.02 | 1.00–1.05 | 0.05 |
| Postop. FFP/h | 1.02 | 0.99–1.06 | 0.16 |
| Postop. Cr increase | 1.03 | 0.77–1.38 | 0.83 |
| Pulmonary edema with hypoxemia | |||
| Intraop. RBCs | 1.00 | 0.99–1.00 | 0.85 |
| Intraop. FFP | 1.00 | 0.99–1.01 | 0.87 |
| Intraop. RBCs/h | 1.00 | 0.99–1.02 | 0.70 |
| Intraop. FFP/h | 1.03 | 0.99–1.08 | 0.14 |
| Intraop. total fluid | 1.00 | 1.00–1.00 | 0.78 |
| Intraop. total fluid/h | 1.00 | 1.00–1.01 | 0.76 |
| Oliguria | 1.35 | 0.80–2.27 | 0.26 |
| Preop. GFR | 1.00 | 0.99–1.00 | 0.11 |
| Postop. total fluid | 1.00 | 1.00–1.00 | 0.00 |
| Postop. total fluid/h | 1.00 | 0.97–1.02 | 0.80 |
| Postop. RBCs | 1.00 | 1.00–1.01 | 0.36 |
| Postop. FFP | 1.00 | 0.99–1.01 | 0.81 |
| Postop. RBCs/h | 1.03 | 0.99–1.07 | 0.11 |
| Postop. FFP/h | 1.06 | 0.99–1.14 | 0.10 |
| Postop. Cr increase | 1.39 | 0.94–2.05 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, Y.-S.; Kim, H.; Lee, H.; Kim, J.-H.; Jang, J.-S.; Hwang, S.-M.; Hong, J.-Y.; Yang, G.-E.; Kim, Y.; Lee, J.-J. Effect of Intra- and Post-Operative Fluid and Blood Volume on Postoperative Pulmonary Edema in Patients with Intraoperative Massive Bleeding. J. Clin. Med. 2021, 10, 4224. https://doi.org/10.3390/jcm10184224
Kwon Y-S, Kim H, Lee H, Kim J-H, Jang J-S, Hwang S-M, Hong J-Y, Yang G-E, Kim Y, Lee J-J. Effect of Intra- and Post-Operative Fluid and Blood Volume on Postoperative Pulmonary Edema in Patients with Intraoperative Massive Bleeding. Journal of Clinical Medicine. 2021; 10(18):4224. https://doi.org/10.3390/jcm10184224
Chicago/Turabian StyleKwon, Young-Suk, Haewon Kim, Hanna Lee, Jong-Ho Kim, Ji-Su Jang, Sung-Mi Hwang, Ji-Young Hong, Go-Eun Yang, Youngmi Kim, and Jae-Jun Lee. 2021. "Effect of Intra- and Post-Operative Fluid and Blood Volume on Postoperative Pulmonary Edema in Patients with Intraoperative Massive Bleeding" Journal of Clinical Medicine 10, no. 18: 4224. https://doi.org/10.3390/jcm10184224
APA StyleKwon, Y.-S., Kim, H., Lee, H., Kim, J.-H., Jang, J.-S., Hwang, S.-M., Hong, J.-Y., Yang, G.-E., Kim, Y., & Lee, J.-J. (2021). Effect of Intra- and Post-Operative Fluid and Blood Volume on Postoperative Pulmonary Edema in Patients with Intraoperative Massive Bleeding. Journal of Clinical Medicine, 10(18), 4224. https://doi.org/10.3390/jcm10184224






