Recurrence Kinetics after Laparoscopic Versus Open Surgery in Colon Cancer. A Meta-Analysis
Abstract
:1. Introduction
Aims and Objectives
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
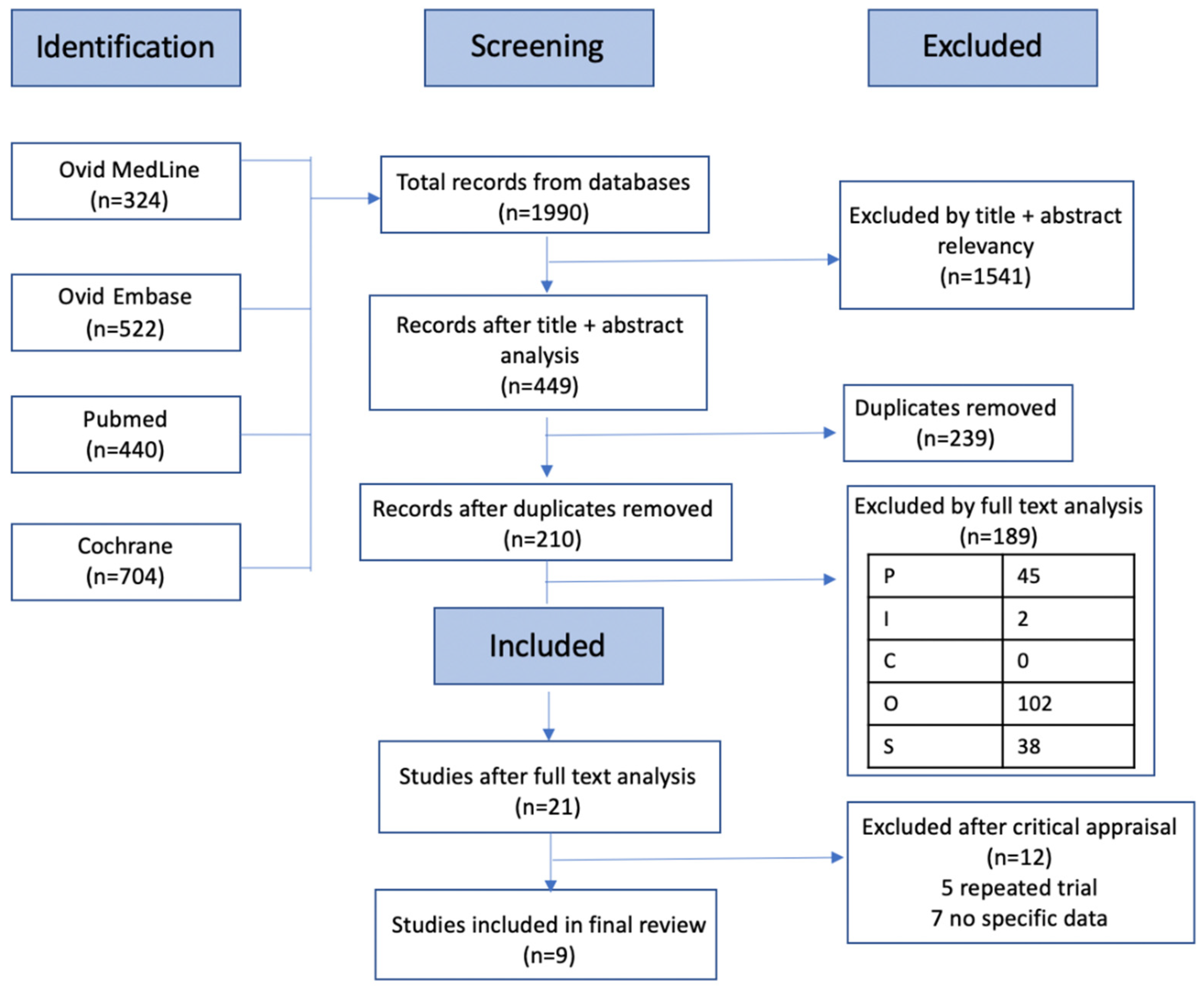
2.2. Search Strategy
2.3. Data Collection and Analysis
2.4. Critical Appraisal
3. Results
3.1. Study Characteristics
3.2. Population
3.3. Intervention
3.4. Comparator
3.5. Outcomes
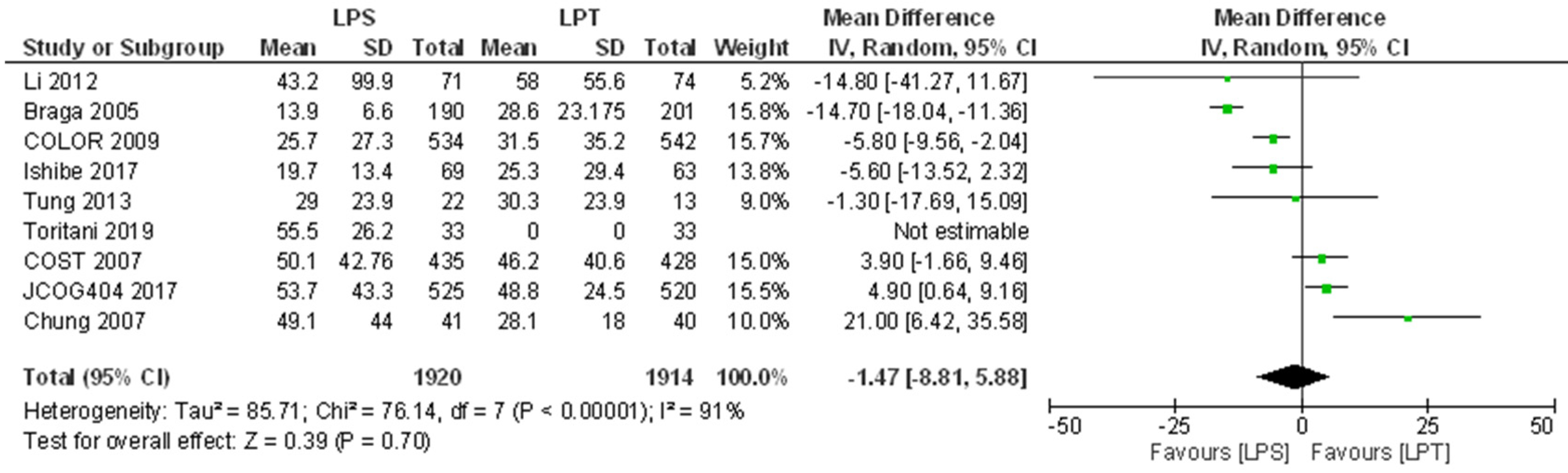
3.5.1. Overall Survival
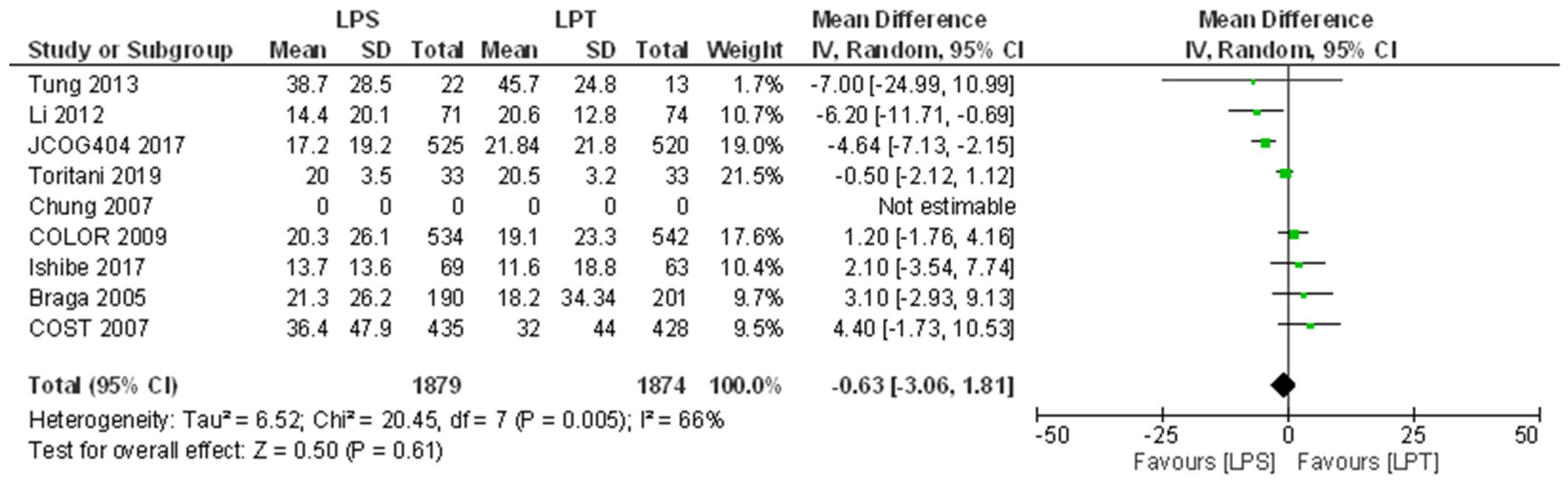
3.5.2. Disease-Free Survival
3.5.3. Meta-Analysis
3.6. Bias Assessment
3.6.1. Selection Bias
3.6.2. Performance Bias
3.6.3. Detection Bias
3.6.4. Attrition Bias
3.6.5. Reporting Bias
4. Discussion
4.1. Quality of Evidence (GRADE)
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Scottish Intercollegiate Guidelines Network. Diagnosis and Management of Colorectal Cancer: A National Clinical Guideline. 2016. Available online: https://www.sign.ac.uk/assets/sign126.pdf (accessed on 20 May 2020).
- Cancer Research UK. Cancer Research UK. Bowel Cancer Statistics. Available online: https://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/bowel-cancer#heading-Zero (accessed on 20 May 2020).
- National Institute for Health and Care Excellence. Colorectal Cancer: NICE Guideline. 2020. Available online: https://www.nice.org.uk/guidance/ng151/resources/colorectal-cancer-pdf-66141835244485 (accessed on 20 May 2020).
- Dragovich, T.; Tsikltis, V.L. Medscape. Colon Cancer. Available online: https://emedicine.medscape.com/article/277496-overview#a4 (accessed on 20 May 2020).
- Mayo Clinic. Mayo Clinic. Colectomy. Available online: https://www.mayoclinic.org/tests-procedures/colectomy/about/pac-20384631 (accessed on 20 May 2020).
- Macmillan Cancer Support. Macmillan Cancer Support. Surgery to Remove Colon Cancer. Available online: https://www.macmillan.org.uk/cancer-information-and-support/treatments-and-drugs/surgery-to-remove-colon-cancer (accessed on 20 May 2020).
- National Health Service (NHS). Treatment: Bowel Cancer. Available online: https://www.nhs.uk/conditions/bowel-cancer/treatment/ (accessed on 20 May 2020).
- Forget, P.; Simonet, O.; De Kock, M. Cancer surgery induces inflammation, immunosuppression and neo-angiogenesis, but is it influenced by analgesics? F1000Research 2013, 2, 102. [Google Scholar] [CrossRef]
- Higgins, J.P.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altaman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Tung, K.; Cheung, H.; Ng, L.; Chung, C.; Li, M. Endo-laparoscopic approach versus conventional open surgery in the treat-ment of obstructing left-sided colon cancer: Long-term follow-up of a randomized trial. Asian J. Endosc. Surg. 2013, 6, 78–81. [Google Scholar] [CrossRef]
- Braga, M.; Frasson, M.; Vignali, A.; Zuliani, W.; Civelli, V.; Di Carlo, V. Laparoscopic vs. Open Colectomy in Cancer Patients: Long-Term Complications, Quality of Life, and Survival. Dis. Colon Rectum 2005, 48, 2217–2223. [Google Scholar] [CrossRef]
- Braga, M.; Frasson, M.; Zuliani, W.; Vignali, A.; Pecorelli, N.; Di Carlo, V. Randomized clinical trial of laparoscopic versus open left colonic resection. BJS 2010, 97, 1180–1186. [Google Scholar] [CrossRef]
- Chung, C.C.; Ng, D.; Tsang, W.W.C.; Tang, W.L.; Yau, K.K.K.; Cheung, H.Y.S.; Wong, J.C.H.; Li, M.K.W. Hand-assisted Laparoscopic Versus Open Right Colectomy. Ann. Surg. 2007, 246, 728–733. [Google Scholar] [CrossRef] [PubMed]
- Fleshman, J.; Sargent, D.; Green, E.; Anvari, M.; Stryker, S.J.; Beart, R.W.; Hellinger, M.; Flanagan, R.; Peters, W.; Nelson, H. Laparoscopic Colectomy for Cancer Is Not Inferior to Open Surgery Based on 5-Year Data from the COST Study Group Trial. Ann. Surg. 2007, 246, 655–664. [Google Scholar] [CrossRef] [PubMed]
- 1Kitano, S.; Inomata, I.; Mizusawa, J.; Katayama, H.; Watanabe, M.; Yamamoto, S. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): A phase 3, randomised controlled trial. Lancet Gastroenterol. Hepatol. 2017, 2, 261–268. [Google Scholar] [CrossRef]
- Ishibe, A.; Ota, M.; Fujii, S.; Suwa, Y.; Suzuki, S.; Suwa, H.; Momiyama, M.; Watanabe, J.; Watanabe, K.; Taguri, M.; et al. Midterm follow-up of a randomized trial of open surgery versus laparoscopic surgery in elderly patients with colorectal cancer. Surg. Endosc. 2017, 31, 3890–3897. [Google Scholar] [CrossRef] [PubMed]
- Li, J.C.-M.; Leung, K.L.; Ng, S.S.-M.; Liu, S.Y.-W.; Lee, J.F.-Y.; Hon, S.S.-F. Laparoscopic-assisted versus open resection of right-sided colonic cancer—A prospective randomized controlled trial. Int. J. Color. Dis. 2011, 27, 95–102. [Google Scholar] [CrossRef] [PubMed]
- The Colon Cancer Laparoscopic or Open Resection Study Group. Survival after laparoscopic surgery versus open surgery for colon cancer: Long-term outcome of a randomised clinical trial. Lancet Oncol. 2009, 10, 44–52. [Google Scholar] [CrossRef]
- Toritani, K.; Watanabe, J.; Nakagawa, K.; Suwa, Y.; Suwa, H.; Ishibe, A. Randomized controlled trial to evaluate laparoscopic versus open surgery in transverse and descending colon cancer patients. Int. J. Colorectal Dis. 2019, 34, 1211–1220. [Google Scholar] [CrossRef]
- GetData Graph Digitizer—Graph Digitizing Software. Getdata-Graph-Digitizer.com. Available online: http://getdata-graph-digitizer.com/ (accessed on 17 May 2020).
- GRADE Working Group. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (Updated October 2013); Chünemann, H., Brożek, J., Guyatt, G., Oxman, A., Eds.; GRADE [Chapter 12.2.1]; The Cochrane Collaboration: London, UK, 2013; Available online: http://gdt.guidelinedevelopment.org/app/handbook/handbook.html (accessed on 20 May 2020).
- Chapter 7: Considering bias and conflicts of interest among the included studies. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (Updated September 2020); Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane: London, UK, 2020; Available online: https://www.training.cochrane.org/handbook (accessed on 20 May 2020).
- Chapter 6: Choosing effect measures and computing estimates of effect. In Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (Updated September 2020); Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane: London, UK, 2020; Available online: https://www.training.cochrane.org/handbook (accessed on 20 May 2020).
- Scottish Intercollegiate Guidelines Network (SIGN). Critical Appraisal Notes and Checklists. Available online: https://www.sign.ac.uk/checklists-and-notes.html (accessed on 15 May 2020).
- Dumville, J.C.; Torgerson, D.; Hewitt, C.E. Reporting attrition in randomised controlled trials. BMJ 2006, 332, 969–971. [Google Scholar] [CrossRef] [Green Version]
- Guner, A.; Kim, H.-I. Biomarkers for Evaluating the Inflammation Status in Patients with Cancer. J. Gastric Cancer 2019, 19, 254–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrenbruch, C.; Shembrey, C.; Paquet-Fifield, S.; Mølck, C.; Cho, H.-J.; Michael, M.; Thomson, B.N.J.; Heriot, A.G.; Hollande, F. Surgical stress response and promotion of metastasis in colorectal cancer: A complex and heterogeneous process. Clin. Exp. Metastasis 2018, 35, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Forget, P.; Aguirre, J.A.; Bencic, I.; Borgeat, A.; Cama, A.; Condron, C.; Eintrei, C.; Eroles, P.; Gupta, A.; Hales, T.G.; et al. How Anesthetic, Analgesic and Other Non-Surgical Techniques During Cancer Surgery Might Affect Postoperative Oncologic Outcomes: A Summary of Current State of Evidence. Cancers 2019, 11, 592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallat, J. Understanding the null hypothesis (H0) in non-inferiority trials. Crit. Care 2017, 21, 101. [Google Scholar] [CrossRef] [Green Version]
- Case, L.D.; Ambrosius, W.T. Power and Sample Size. Methods Mol. Biol. 2007, 404, 377–408. [Google Scholar] [CrossRef]
- Day, R. Comorbidities in Older People. GP Online. 2017. Available online: https://www.gponline.com/comorbidities-older-people/elderly-care/article/1440520 (accessed on 18 May 2020).
- Søgaard, M.; Thomsen, R.; Bossen, K.S.; Sørensen, H.H.T.; Nørgaard, M. The impact of comorbidity on cancer survival: A review. Clin. Epidemiology 2013, 5, 3–29. [Google Scholar] [CrossRef] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.-Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di, B.; Li, Y.; Wei, K.; Xiao, X.; Shi, J.; Zhang, Y.; Yang, X.; Gao, P.; Zhang, K.; Yuan, Y.; et al. Laparoscopic versus open surgery for colon cancer: A meta-analysis of 5-year follow-up outcomes. Surg. Oncol. 2013, 22, e39–e43. [Google Scholar] [CrossRef] [PubMed]
- Song, X.-J.; Liu, Z.-L.; Zeng, R.; Ye, W.; Liu, C.-W. A meta-analysis of laparoscopic surgery versus conventional open surgery in the treatment of colorectal cancer. Medicine 2019, 98, e15347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, C.; Wang, Q.; Li, Z.; Lin, J.; Wang, H. Differences in Stage of Cancer at Diagnosis, Treatment, and Survival by Race and Ethnicity Among Leading Cancer Types. JAMA Netw. Open 2020, 3, e202950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishore, J.; Goel, M.; Khanna, P. Understanding survival analysis: Kaplan-Meier estimate. Int. J. Ayurveda Res. 2010, 1, 274–278. [Google Scholar] [CrossRef] [Green Version]
- Hackshaw, A. Small studies: Strengths and limitations. Eur. Respir. J. 2008, 32, 1141–1143. [Google Scholar] [CrossRef]
| Patients | Adult population >18 years Colon cancer (all stages) | Paediatrics <18 years Metastatic disease Rectal cancer |
| Intervention | Intended curative laparoscopic surgery of primary tumour | |
| Control | Intended curative open surgery of primary tumour | |
| Outcomes | 3-year disease-free survival 3-year overall survival 5-year disease-free survival 5-year overall survival Survival period Disease-free period | |
| Study Design | Randomised controlled trials published in English | All other study types |
| Study | Intervention | Control | Population | Average Age (Year) | Sample Size Analysed | Male Sex (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Laparoscopic | Open Resection | Laparoscopic | Open Resection | Laparoscopic | Open Resection | 95% CI | ||||
| Braga et al., 2005 | Laparoscopic surgery | Open surgery | ≥18 years Colorectal cancer | 65 (13) mean (SD) | 67 (11) mean (SD) | 190 | 201 | 60 | 60 | −14.70 [−18.04,−11.36] |
| Braga et al., 2010 | Laparoscopic surgery | Open surgery | ≥18 years Carcinoma of left colon | 62.9 † | 64.9 † | 78 | 89 | 51 † | 53 † | |
| Chung et al., 2007 | Hand-assisted laparoscopic colectomy | Open colectomy | ≥18 years Carcinoma of cecum or ascending colon | 71 | 72.5 | 41 | 40 | 61 | 65 | 21.00 [6.42,35.58] |
| COLOR, 2009 | Laparoscopic surgery | Open resection | ≥18 years Carcinoma of caecum, ascending colon, descending colon or sigmoid colon | 71 | 71 | 534 | 542 | 52 | 53 | −5.80 [−9.56,−2.04] |
| COST, 2007 | Laparoscopically assisted colectomy | Open colectomy | ≥18 years Carcinoma of right, left or sigmoid colon | 70 | 69 | 435 | 428 | 51 | 49 | 3.90 [−1.66,9.46] |
| Ishibe et al., 2017 | Minimally invasive laparoscopic resection | Conventional open resection | ≥75 years Adenocarcinoma of colon and rectum | NI | NI | 69 | 63 | 50 | 60 | −5.60 [−13.52,2.32] |
| JCOG404, 2017 | Laparoscopic surgery | Open surgery | 20–75 years Carcinoma of caecum, ascending colon, sigmoid colon | 64 | 64 | 525 | 520 | 54 | 60 | 4.90 [0.64,9.16] |
| Li et al., 2012 | Laparoscopic assisted right hemicolectomy | Open right hemicolectomy | All ages ‡ carcinomas of caecum, ascending colon, hepatic flexure or transverse colon | 68 | 68 | 71 | 74 | 46 | 43 | −14.80 [−41.27,11.67] |
| Toritani et al., 2019 | Laparoscopic surgery | Conventional open surgery | ≥20 years Transverse and descending colon cancer | 64 | 67 | 33 | 33 | 73 | 48 | |
| Tung et al., 2013 | Endolaparoscopic resection | Conventional open surgery | ≥18 years Obstructed left sided colon cancer. | 64.5 | 68.5 | 22 | 13 | 58 | 50 | −1.30 [−17.69,15.09] |
| Overall Survival | 3-Year Follow-Up (%) | 5-Year Follow-Up (%) | LPS | LPT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | LPS | LPT | p | LPS | LPT | p | Mean | SD | Patients | Mean | SD | Patients | Weight | 95% CI | Quality of Evidence (GRADE) | Comments |
| Li 2012 | 83.4 | 86.1 | NA | 74.2 | 75.0 | 0.835 | 43.2 | 99.9 | 71 | 58.0 | 55.6 | 74 | 5.2% | −14.80 [−41.27,11.67] | ⊕⊕⊝⊝ Low a,e | There may have been no difference in 5-year OS between laparoscopic and open surgery. |
| Braga 2005 | NI | NI | NA | 72 | 66 | 0.321 | 13.9 | 6.6 | 190 | 28.6 | 23.175 | 201 | 15.8% | −14.70 [−18.04,−11.36] | ⊕⊕⊝⊝ Low a,e | There may have been no difference in 5-year OS between laparoscopic and open surgery. |
| Braga 2010 | NI | NI | NA | 61.1 | 56.5 | 0.16–0.65 according to stage | NA | NA | NA | NA | NA | NA | 0 | Not estimable | ⊕⊕⊝⊝ Low a,e | There may have been no difference in 5-year OS between laparoscopic and open surgery. |
| COLOR 2009 | 81.8 | 84.2 | 0.45 | 73.8 | 74.2 | NI | 25.7 | 27.3 | 534 | 31.5 | 35.2 | 542 | 15.7% | −5.80 [−9.56,−2.04] | ⊕⊕⊕⊝ Moderate a | There is probably no difference in 3-year OS after laparoscopic surgery compared to open surgery. |
| Ishibe 2017 | 93.9 | 93.5 | 0.901 | NI | NI | NA | 19.7 | 13.4 | 69 | 25.3 | 29.4 | 63 | 13.8% | −5.60 [−13.52,2.32] | ⊕⊕⊕⊝ Moderate a | There is probably no difference in 5-year OS after laparoscopic surgery compared to open surgery. |
| Tung 2013 | 71 | 46 | NA | 48 | 27 | 0.076 | 29.0 | 23.9 | 22 | 30.3 | 23.9 | 13 | 9.0% | −1.30 [−17.69,15.09] | ⊕⊝⊝⊝ Very Low b,d,e | There may have been no difference in 3-year OS after laparoscopic compared to open surgery, but the evidence is uncertain. |
| Toritani 2019 | 97.1 | 100.0 | NA | 93.3 | 100.0 | 0.543 | 55.5 | 26.2 | 33 | 0.0 | 0.0 | 33 | 0 | Not estimable | ⊕⊕⊝⊝ Low a,e | 5-year OS after Laparoscopic surgery may not have been non-inferior to open surgery. |
| COST 2007 | 86.8 | 86.8 | NA | 76.4 | 74.6 | 0.93 | 50.1 | 42.76 | 435 | 46.2 | 40.6 | 428 | 15.0% | 3.90 [−1.66,9.46] | ⊕⊕⊕⊝ Moderate e | There is probably no difference in 5-year OS after laparoscopic surgery compared to open surgery. |
| JCOG404 2017 | 96 | 95.8 | NA | 91.8 | 90.4 | 0.073† | 53.7 | 43.3 | 525 | 48.8 | 24.5 | 520 | 15.5% | 4.90 [0.64,9.16] | ⊕⊝⊝⊝ Very Low b,d,e | There may have been no difference in 5-year OS after laparoscopic compared to open surgery, but the evidence is uncertain. |
| Chung 2007 | 95 | 86 | NA | 83 | 74 | 0.90 | 49.1 | 44.0 | 41 | 28.1 | 18.0 | 40 | 10.0% | 21.00 [6.42,35.58] | ⊕⊝⊝⊝ Very Low a,d,e | There may have been no difference in 5-year OS after laparoscopic compared to open surgery, but the evidence is uncertain. |
| Disease-Free Survival | 3-Year Follow-Up (%) | 5-Year Follow-Up (%) | LPS | LPT | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study | LPS | LPT | p | LPS | LPT | p | Mean | SD | Patients | Mean | SD | Patients | Weight | 95% CI | Quality of Evidence (GRADE) | Comments |
| Tung 2013 | 77 | 78 | NA | 52 | 48 | 0.63 | 38.7 | 28.5 | 22 | 45.7 | 24.8 | 13 | 1.7% | −7.00 [−24.99,10.99] | ⊕⊝⊝⊝ Very Low a,d,e | There may have been no difference in 5-year DFS after laparoscopic compared to open surgery but the evidence is uncertain. |
| Li 2012 | 84.3 | 86.3 | NA | 82.3 | 84.1 | 0.78 | 14.4 | 20.1 | 71 | 20.6 | 12.8 | 74 | 10.7% | −6.20 [−11.71,−0.69] | ⊕⊕⊕⊝ Moderate e | There is probably no difference in 5-year DFS after laparoscopic surgery compared to open surgery. |
| JCOG0404 2017 | 80.1 | 82 | NA | 80 | 79 | NI | 17.2 | 19.2 | 525 | 21.84 | 21.8 | 520 | 19.0% | −4.64 [−7.13,−2.15] | ⊕⊕⊝⊝ Low a,e | NA |
| Toritani 2019 | 90.5 | 87.2 | NA | 90.5 | 87.3 | 0.752 | 20.0 | 3.5 | 33 | 20.5 | 3.2 | 33 | 21.5% | −0.50 [−2.12,1.12] | ⊕⊝⊝⊝ Very Low b,d,e | There may have been no difference in 5-year DFS after laparoscopic compared to open surgery but the evidence is uncertain. |
| COLOR 2009 | 74.2 | 76.2 | 0.70, 0.030 † | 66.5 | 67.9 | NI | 20.3 | 26.1 | 534 | 19.1 | 23.3 | 542 | 17.6% | 1.20 [−1.76,4.16] | ⊕⊕⊕⊝ Moderate a | 3-year DFS after laparoscopic surgery is probably not non-inferior to open surgery. |
| Ishibe 2017 | 89.6 | 91.5 | 0.73 | NI | NI | NA | 13.7 | 13.6 | 69 | 11.6 | 18.8 | 63 | 10.4% | 2.10 [−3.54,7.74] | ⊕⊝⊝⊝ Very Low b,d,e | There may have been no difference in 3-year DFS after laparoscopic compared to open surgery but the evidence is uncertain. |
| Braga 2005 | NI | NI | NA | 64.5 | 60.2 | 0.55–0.81 according to stage | 21.3 | 26.2 | 190 | 18.2 | 34.34 | 201 | 9.7% | 3.10 [−2.93,9.13] | ⊕⊕⊝⊝ Low a,e | There may have been no difference in 5-year DFS between laparoscopic and open surgery. |
| Braga 2010 | NI | NI | NA | 63 | 63 | 0.405 | NA | NA | NA | NA | NA | NA | 0 | Not estimable | ⊕⊕⊝⊝ Low a,e | There may have been no difference in 5-year DFS between laparoscopic and open surgery. |
| COST 2007 | 80.4 | 79.2 | NA | 69.2 | 68.4 | 0.94 | 36.4 | 47.9 | 435 | 32.0 | 44.0 | 428 | 9.5% | 4.40 [−1.73,10.53] | ⊕⊕⊕⊝ Moderate a | There is probably no difference in 5-year DFS after laparoscopic surgery compared to open surgery. |
| Chung 2007 | NI | NI | NA | NI | NI | NA | NA | NA | NA | NA | NA | NA | 0 | Not estimable | ⊕⊕⊝⊝ Low a,e | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lilley, R.; Chan, E.; Ng, N.; Orr, A.; Szostok, M.; Yeh, G.T.T.; Tulloch, R.; Ramsay, G.; Mokini, Z.; Forget, P. Recurrence Kinetics after Laparoscopic Versus Open Surgery in Colon Cancer. A Meta-Analysis. J. Clin. Med. 2021, 10, 4163. https://doi.org/10.3390/jcm10184163
Lilley R, Chan E, Ng N, Orr A, Szostok M, Yeh GTT, Tulloch R, Ramsay G, Mokini Z, Forget P. Recurrence Kinetics after Laparoscopic Versus Open Surgery in Colon Cancer. A Meta-Analysis. Journal of Clinical Medicine. 2021; 10(18):4163. https://doi.org/10.3390/jcm10184163
Chicago/Turabian StyleLilley, Ross, Evangeline Chan, Nicklaus Ng, Amber Orr, Marcin Szostok, Gloria Ting Ting Yeh, Ross Tulloch, George Ramsay, Zhirajr Mokini, and Patrice Forget. 2021. "Recurrence Kinetics after Laparoscopic Versus Open Surgery in Colon Cancer. A Meta-Analysis" Journal of Clinical Medicine 10, no. 18: 4163. https://doi.org/10.3390/jcm10184163
APA StyleLilley, R., Chan, E., Ng, N., Orr, A., Szostok, M., Yeh, G. T. T., Tulloch, R., Ramsay, G., Mokini, Z., & Forget, P. (2021). Recurrence Kinetics after Laparoscopic Versus Open Surgery in Colon Cancer. A Meta-Analysis. Journal of Clinical Medicine, 10(18), 4163. https://doi.org/10.3390/jcm10184163










