Lung Ultrasound in the Screening of Pulmonary Interstitial Involvement Secondary to Systemic Connective Tissue Disease: A Prospective Pilot Study Involving 180 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Ethics Committee
2.3. Imaging Examinations: Methodology
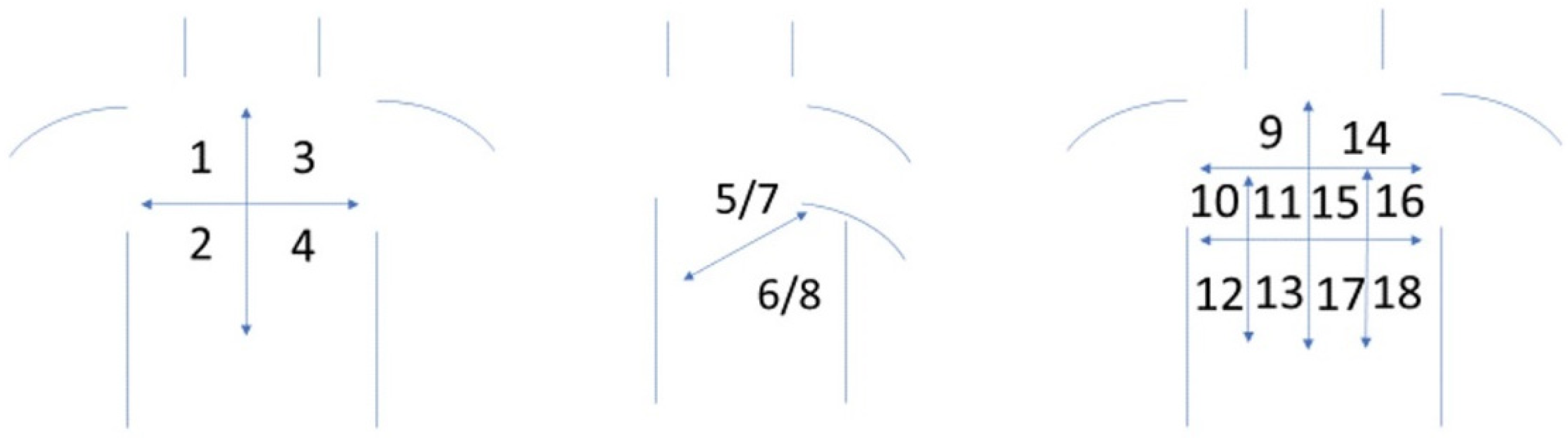
2.3.1. LUS Protocol
2.3.2. HRCT Protocol
2.3.3. ECHO Protocol
2.3.4. Statistical Analysis
3. Results
3.1. Study Group
3.2. Results of Imaging Examinations
3.2.1. ECHO Results
3.2.2. LUS and HRCT Results
3.2.3. Correlation of Findings in LUS and HRCT in the Group with Positive HRCT Results
3.2.4. Number of Particular Abnormalities Detected in LUS in Specific Localizations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Mira-Avendano, I.; Abril, A.; Burger, C.D.; Dellaripa, P.F.; Fischer, A.; Gotway, M.B.; Lee, A.S.; Lee, J.S.; Matteson, E.L.; Yi, E.S.; et al. Interstitial Lung Disease and Other Pulmonary Manifestations in Connective Tissue Diseases. Mayo Clin. Proc. 2019, 94, 309–325. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, R.P.; Ribeiro, R.; Melo, L.; Grima, B.; Oliveira, S.; Alvesa, J.D. Connective tissue disease-associated interstitial lung disease. Pulmonology 2020. [Google Scholar] [CrossRef] [PubMed]
- Vij, R.; Strek, M.E. Diagnosis and Treatment of Connective Tissue Disease-Associated Interstitial Lung Disease. Chest 2013, 143, 814–824. [Google Scholar] [CrossRef] [Green Version]
- Manolescu, D.; Oancea, C.; Timar, B.; Traila, D.; Malita, D.; Birsasteanu, F.; Tudorache, V. Ultrasound mapping of lung changes in idiopathic pulmonary fibrosis. Clin. Respir. J. 2019, 14, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Tardella, M.; Di Carlo, M.; Carotti, M.; Filippucci, E.; Grassi, W.; Salaffi, F. Ultrasound B-lines in the evaluation of interstitial lung disease in patients with systemic sclerosis: Cut-off point definition for the presence of significant pulmonary fibrosis. Medicine 2018, 97, e0566. [Google Scholar] [CrossRef] [PubMed]
- Doveri, M.; Frassi, F.; Consensi, A.; Vesprini, E.; Gargani, L.; Tafuri, M.; Picano, E.; Della Rossa, A.; Delle Sedie, A.; d’Ascanio, A.; et al. Le comete ultrasoniche polmonari (ULC): Un nuovo segno ecografico di fibrosi polmonare nella sclerodermia [Ultrasound lung comets: New echographic sign of lung interstitial fibrosis in systemic sclerosis]. Reumatismo 2008, 60, 180–184. (In Italian) [Google Scholar] [PubMed] [Green Version]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 233–271. [Google Scholar] [CrossRef] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for noninvasive evaluation of native valvular regurgitation A report from the american society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef] [Green Version]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Recommendations on the echocardiographic assessment of aortic valve stenosis: A focused update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 254–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathai, S.C.; Danoff, S. Management of interstitial lung disease associated with connective tissue disease. BMJ 2016, 352, h6819. [Google Scholar] [CrossRef] [PubMed]
- Kocheril, S.V.; Appleton, B.E.; Somers, E.; Kazerooni, E.A.; Flaherty, K.R.; Martinez, F.J.; Gross, B.H.; Crofford, L.J. Comparison of disease progression and mortality of connective tissue disease-related interstitial lung disease and idiopathic interstitial pneumonia. Arthritis Rheum. 2005, 53, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sperandeo, M.; De Cata, A.; Molinaro, F.; Trovato, F.M.; Catalano, D.; Simeone, A.; Varriale, A.; Martines, G.; Trovato, G.M. Ultrasound signs of pulmonary fibrosis in systemic sclerosis as timely indicators for chest computed tomography. Scand. J. Rheumatol. 2015, 44, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Barskova, T.; Gargani, L.; Guiducci, S.; Randone, S.B.; Bruni, C.; Carnesecchi, G.; Conforti, M.L.; Porta, F.; Pignone, A.; Caramella, D.; et al. Lung ultrasound for the screening of interstitial lung disease in very early systemic sclerosis. Ann. Rheum. Dis. 2013, 72, 390–395. [Google Scholar] [CrossRef]
- Xie, H.Q.; Zhang, W.W.; Sun, D.S.; Chen, X.M.; Yuan, S.F.; Gong, Z.H.; Liu, L. A simplified lung ultrasound for the diagnosis of interstitial lung disease in connective tissue disease: A meta-analysis. Arthritis Res. 2019, 21, 93. [Google Scholar] [CrossRef] [Green Version]
- Song, G.G.; Bae, S.-C.; Lee, Y.H. Diagnostic accuracy of lung ultrasound for interstitial lung disease in patients with connective tissue diseases: A meta-analysis. Clin. Exp. Rheumatol. 2016, 34, 11–16. [Google Scholar]
- Mohammadi, A.; Oshnoei, S.; Ghasemi-rad, M. Comparison of a new, modified lung ultrasonography technique with high-resolution CT in the diagnosis of the alveolo-interstitial syndrome of systemic scleroderma. Med. Ultrason. 2014, 16, 27–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lichtenstein, D.A. Lung ultrasound in the critically ill. Ann. Intensive Care. 2014, 4, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buda, N.; Kosiak, W.; Wełnicki, M.; Skoczylas, A.; Olszewski, R.; Piotrkowski, J.; Skoczyński, S.; Radzikowska, E.; Jassem, E.; Grabczak, E.M.; et al. Recommendations for Lung Ultrasound in Internal Medicine. Diagnostics 2020, 10, 597. [Google Scholar] [CrossRef]
- Wang, Y.; Gargani, L.; Barskova, T.; Furst, D.E.; Cerinic, M.M. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: A literature review. Arthritis Res. 2017, 19, 206. [Google Scholar] [CrossRef] [Green Version]
- Gargani, L.; Bruni, C.; Romei, C.; Frumento, P.; Moreo, A.; Agoston, G.; Guiducci, S.; Bellando-Randone, S.; Lepri, G.; Belloli, L.; et al. Prognostic Value of Lung Ultrasound B-Lines in Systemic Sclerosis. Chest 2020, 158, 1515–1525. [Google Scholar] [CrossRef]
- Hassan, R.I.; Lubertino, L.I.; Barth, M.A.; Quaglia, M.F.; Montoya, S.F.; Kerzberg, E.; Binda, M.D.C. Lung Ultrasound as a Screening Method for Interstitial Lung Disease in Patients With Systemic Sclerosis. JCR J. Clin. Rheumatol. 2019, 25, 304–307. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Lin, J.; Xie, X.; Hu, S.; Lin, Q.; Zheng, K.; Du, G.; Huang, X.; Zhang, G.; et al. Lung ultrasound B-lines and serum KL-6 correlate with the severity of idiopathic inflammatory myositis-associated interstitial lung disease. Rheumatology 2020, 59, 2024–2029. [Google Scholar] [CrossRef] [Green Version]
- Gasperini, M.L.; Gigante, A.; Iacolare, A.; Pellicano, C.; Lucci, S.; Rosato, E. The predictive role of lung ultrasound in progression of scleroderma interstitial lung disease. Clin. Rheumatol. 2020, 39, 119–123. [Google Scholar] [CrossRef]
- Singh, A.K.; Mayo, P.H.; Koenig, S.; Talwar, A.; Narasimhan, M. The Use of M-Mode Ultrasonography to Differentiate the Causes of B Lines. Chest 2018, 153, 689–696. [Google Scholar] [CrossRef]
- Soldati, G.; Demi, M.; Inchingolo, R.; Smargiassi, A.; Demi, L. On the Physical Basis of Pulmonary Sonographic Interstitial Syndrome. J. Ultrasound Med. 2016, 35, 2075–2086. [Google Scholar] [CrossRef]
- Buda, N.; Kosiak, W. Is a linear probe helpful in diagnosing diseases of pulmonary interstitial spaces? J. Ultrason. 2017, 17, 136–141. [Google Scholar] [CrossRef]
- Buda, N.; Piskunowicz, M.; Porzezińska, M.; Kosiak, W.; Zdrojewski, Z. Lung Ultrasonography in the Evaluation of Interstitial Lung Disease in Systemic Connective Tissue Diseases: Criteria and Severity of Pulmonary Fibrosis—Analysis of 52 Patients. Ultraschall Med. 2016, 37, 379–385. [Google Scholar] [CrossRef]
- Buda, N.; Skoczylas, A.; Demi, M.; Wojteczek, A.; Cylwik, J.; Soldati, G. Clinical Impact of Vertical Artifacts Changing with Frequency in Lung Ultrasound. Diagnostics 2021, 11, 401. [Google Scholar] [CrossRef]
| Parameter | n (%) |
|---|---|
| Gender | |
| Females | 130 (73.9) |
| Males | 46 (26.1) |
| Rheumatoid arthritis (RA) | 22 (12.5) |
| Eosinophilic granulomatosis with polyangiitis (EGPA) | 10 (5.7) |
| Granulomatosis with Polyangiitis (GPA) and Microscopic Polyangiitis (MPA) | 25 (14.2) |
| Myositis | 18 (10.2) |
| Systemic lupus erythematosus (SLE) | 25 (14.2) |
| Systemic sclerosis (SSC) | 30 (17) |
| Sjögren syndrome | 46 (26.1) |
| n | % | |
|---|---|---|
| LVEF < 50% | 3 | 1.7 |
| Left ventricle diastolic disfunction, grade III | 1 | 0.57 |
| IVC > 21 mm | 11 | 6.3 |
| IVC width expiration/inspiration <50% | 22 | 12.5 |
| RV systolic function: TAPSE < 17 mm | 6 | 3.4 |
| RV systolic function: s′ < 9.5 cm/s | 3 | 1.7 |
| Serious mitral valve defect (MR ≥ moderate or stenosis) | 4 | 2.3 |
| Serious aortic valve defect (AR ≥ moderate or stenosis) | 3 | 1.7 |
| ILD in CT | 28 | 15.9 |
| n | % | |
|---|---|---|
| LUS | ||
| Pleural line—irregular | 34 | 19.3 |
| Pleural line—coarse | 29 | 16.5 |
| Pleural line—fragmented | 18 | 10.2 |
| Pleural line—blurred | 7 | 4.0 |
| Pleural line—thickened | 1 | 0.6 |
| B-line artifacts—single | 28 | 15.9 |
| B-line artifacts—multiple | 17 | 9.7 |
| B-line artifacts—white lung | 4 | 2.3 |
| Am artifacts | 1 | 0.6 |
| Consolidations < 5 mm | 7 | 4.0 |
| Consolidations > 5 mm | 2 | 1.2 |
| HRCT | ||
| Reticular pattern | 29 | 16.5 |
| Small-nodular pattern | 3 | 1.7 |
| Interlobular septal thickening | 5 | 2.8 |
| Bronchiectasis, changed by inflammation | 25 | 14.2 |
| Cysts | 20 | 11.4 |
| Honeycombing | 7 | 4.0 |
| Ground-glass | 8 | 4.5 |
| Interstitial Disease in LUS | |||||
|---|---|---|---|---|---|
| No | Yes | ||||
| n | % | n | % | ||
| Interstitial disease in HRCT | no | 147 | 99.3 | 1 | 0.7 |
| yes | 1 | 3.6 | 27 | 96.4 | |
| LUS | HRCT | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reticular Pattern | Small-Nodular Pattern | Interlobular Septal Thickening | Bronchiectasis, Changed by Inflammation | Cysts | Honeycombing | Ground-Glass | ||||||||
| n (%) | φ | n (%) | φ | n (%) | φ | n (%) | φ | n (%) | φ | n (%) | φ | n (%) | φ | |
| Irregular pleural line | 250 (93.3) | 0.81 | 4 (100.0) | 0.10 | 31 (96.9) | 0.28 | 168 (90.3) | 0.64 | 94 (83.2) | 0.45 | 33 (97.1) | 0.29 | 37 (90.2) | 0.29 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||||
| Coarse pleural line | 183 (68.3) | 0.71 | 4 (100.0) | 0.13 | 29 (90.6) | 0.32 | 139 (74.7) | 0.65 | 68 (60.2) | 0.39 | 30 (88.2) | 0.32 | 34 (82.9) | 0.33 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||||
| Fragmented pleural line | 91 (34.0) | 0.50 | 1 (25.0) | 0.04 | 24 (75.0) | 0.39 | 75 (40.3) | 0.50 | 41 (36.3) | 0.34 | 25 (73.5) | 0.39 | 21 (51.2) | 0.29 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||||
| Blurred pleural line | 34 (12.7) | 0.33 | 0 (0) | −0.004 | 12 (37.5) | 0.34 | 33 (17.7) | 0.38 | 12 (10.6) | 0.17 | 22 (64.7) | 0.61 | 2 (4.9) | 0.04 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.030 | ||||||||
| Thickened pleural line | 1 (0.4) | 0.06 | 0 (0) | −0.001 | 0 (0) | −0.002 | 0 (0) | −0.004 | 0 (0) | −0.003 | 0 (0) | −0.002 | 0 (0) | −0.002 |
| p = 0.001 | p = 0.972 | p = 0.919 | p = 0.802 | p = 0.847 | p = 0.917 | p = 0.909 | ||||||||
| Single B-line artifacts | 103 (38.4) | 0.51 | 2 (50.0) | 0.08 | 6 (18.8) | 0.07 | 68 (36.6) | 0.40 | 33 (29.2) | 0.24 | 7 (20.6) | 0.08 | 16 (39.0) | 0.20 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||||
| Multiple B-line artifacts | 79 (29.6) | 0.50 | 2 (50.0) | 0.10 | 14 (43.8) | 0.25 | 62 (33.3) | 0.47 | 33 (29.2) | 0.31 | 9 (26.5) | 0.15 | 18 (43.9) | 0.29 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||||
| B-line artifacts—white lung | 14 (5.2) | 0.21 | 0 (0) | −0.002; | 11 (34.4) | 0.48 | 15 (8.1) | 0.27 | 7 (6.3) | 0.15 | 15 (44.1) | 0.64 | 0 (0) | −0.008 |
| p < 0.001 | p = 0.890 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||||
| AM artifacts | 1 (0.4) | 0.06 | 0 (0) | −0.001; | 0 (0) | −0.002 | 1 (0.5) | 0.07 | 0 (0) | −0.003 | 1 (2.9) | 0.17 | 0 (0) | −0.002 |
| p = 0.001 | p = 0.972 | p = 0.919 | p < 0.001 | p = 0.847 | p < 0.001 | p = 0.909 | ||||||||
| Consolidations < 5 mm | 31 (11.6) | 0.30 | 0 (0) | −0.004; | 13 (40.6) | 0.37 | 30 (16.2) | 0.35 | 9 (8.0) | 0.12 | 18 (54.5) | 0.51 | 4 (9.8) | 0.09 |
| p < 0.001 | p = 0.830 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | ||||||||
| Consolidations > 5 mm | 5 (1.9) | 0.13 | 0 (0) | −0.001; | 1 (3.1) | 0.07 | 5 (2.7) | 0.16 | 4 (3.5) | 0.16 | 1 (3.0) | 0.07 | 0 (0) | −0.005 |
| p < 0.001 | p = 0.936 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p = 0.797 | ||||||||
| ILD in LUS | ||||||
|---|---|---|---|---|---|---|
| No | Yes | |||||
| n | % | n | % | p | RR | |
| Pleural line—irregular | 7 | 4.7 | 27 | 96.4 | <0.001 | 4.82 |
| Pleural line—coarse | 2 | 1.4 | 27 | 96.4 | <0.001 | 14.40 |
| Pleural line—fragmented | 2 | 1.4 | 16 | 57.1 | <0.001 | 8.32 |
| Pleural line—blurred | 0 | 0 | 7 | 25.0 | <0.001 | - |
| Pleural line—thickened | 0 | 0 | 1 | 3.6 | 0.159 | - |
| B-line artifacts—single | 2 | 1.4 | 26 | 92.9 | <0.001 | 13.81 |
| B-line artifacts—multiple | 1 | 0.7 | 16 | 57.1 | <0.001 | 15.72 |
| B-line artifacts—white lung | 0 | 0 | 4 | 14.3 | 0.001 | - |
| Am artifacts | 0 | 0 | 1 | 3.6 | 0.159 | - |
| Consolidations < 5 mm | 0 | 0 | 7 | 25.0 | <0.001 | - |
| Consolidations > 5 mm | 0 | 0 | 2 | 7.1 | 0.025 | - |
| R-Anterior-Superior | L-Anterior-Superior | R-Anterior-Inferior | L-Anterior Inferior | R-Lateral Superior | L-Lateral Superior | R-Lateral Inferior | L-Lateral Inferior | R-Posterior-Superior K | L-Posterior Superior | R Posterior Middle-K | L-Posterior Middle-K | R-Posterior Inferior-K | L-Posterior Inferior-K | R Posterior-Middle-P | L-Posterior Middle-P | R-Posterior Inferior P | L-Posterior Inferior P | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pleural line—irregular | 8 (4.5) | 8 (4.5) | 22 (12.5) | 2 (12.5) | 5 (2.8) | 6 (3.4) | 25 (14.2) | 24 (13.6) | 10 (5.7) | 8 (4.5) | 20 (11.4) | 20 (11.4) | 32 (18.2) | 32 (18.2) | 19 (10.8) | 18 (10.2) | 31 (17.6) | 30 (17.0) |
| Pleural line—coarse | 4 (2.3) | 3 (1.7) | 14 (8.0) | 12 (6.9) | 3 (1.7) | 4 (2.3) | 20 (11.4) | 18 (10.2) | 6 (3.4) | 3 (1.7) | 9 (5.1) | 11 (6.3) | 29 (16.5) | 29 (16.5) | 7 (4.0) | 8 (4.5) | 26 (14.8) | 25 (14.2) |
| Pleural line—fragmented | 2 (1.1) | 1 (0.6) | 6 (3.4) | 6 (3.4) | 1 (0.6) | 3 (1.7) | 9 (5.1) | 10 (5.7) | 1 (0.6) | 1 (0.6) | 5 (2.8) | 9 (5.1) | 13 (7.4) | 13 (7.4) | 3 (1.7) | 2 (1.1) | 15 (8.5) | 13 (7.4) |
| Pleural line—blurred | 0 | 0 | 3 (1.7) | 2 (1.1) | 1 (0.6) | 1 (0.6) | 3 (1.7) | 3 (1.7) | 0 | 0 | 0 | 0 | 6 (3.4) | 7 (4.0) | 1 (0.6) | 0 | 5 (2.8) | 6 (3.4) |
| Pleural line—thickened | 0 | 1 (0.6) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| B-lines—single | 3 (1.7) | 2 (1.1) | 10 (5.7) | 10 (5.7) | 1 (0.6) | 2 (1.1) | 13 (7.4) | 11 (6.3) | 5 (2.8) | 3 (1.7) | 5 (2.8) | 6 (3.4) | 15 (8.5) | 14 (8.0) | 3 (1.7) | 5 (2.8) | 15 (8.5) | 13 (7.4) |
| B-lines-multiple | 1 (0.6) | 2 (1.1) | 4 (2.3) | 4 (2.3) | 3 (1.7) | 3 (1.7) | 6 (3.4) | 8 (4.5) | 1 (0.6) | 1 (0.6) | 4 (2.3) | 5 (2.8) | 10 (5.7) | 11 (6.3) | 3 (1.7) | 3 (1.7) | 9 (5.1) | 9 (5.1) |
| B-lines—white lung | 0 | 0 | 1 (0.6) | 1 (0.6) | 0 | 0 | 1 (0.6) | 1 (0.6) | 0 | 0 | 0 | 1 (0.6) | 2 (1.1) | 2 (1.1) | 0 | 0 | 3 (1.7) | 4 (2.3) |
| Am-lines | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.6) |
| Consolidations < 5 mm | 0 | 0 | 3 (1.7) | 3 (1.7) | 0 | 1 (0.6) | 2 (1.4) | 3 (1.7) | 0 | 0 | 2 (1.1) | 3 (1.7) | 4 (2.3) | 5 (2.8) | 2 (1.1) | 1 (0.6) | 4 (2.3) | 4 (2.3) |
| Consolidations > 5 mm | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (1.1) | 1 (0.6) | 0 | 0 | 1 (0.6) | 1 (0.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buda, N.; Wojteczek, A.; Masiak, A.; Piskunowicz, M.; Batko, W.; Zdrojewski, Z. Lung Ultrasound in the Screening of Pulmonary Interstitial Involvement Secondary to Systemic Connective Tissue Disease: A Prospective Pilot Study Involving 180 Patients. J. Clin. Med. 2021, 10, 4114. https://doi.org/10.3390/jcm10184114
Buda N, Wojteczek A, Masiak A, Piskunowicz M, Batko W, Zdrojewski Z. Lung Ultrasound in the Screening of Pulmonary Interstitial Involvement Secondary to Systemic Connective Tissue Disease: A Prospective Pilot Study Involving 180 Patients. Journal of Clinical Medicine. 2021; 10(18):4114. https://doi.org/10.3390/jcm10184114
Chicago/Turabian StyleBuda, Natalia, Anna Wojteczek, Anna Masiak, Maciej Piskunowicz, Wojciech Batko, and Zbigniew Zdrojewski. 2021. "Lung Ultrasound in the Screening of Pulmonary Interstitial Involvement Secondary to Systemic Connective Tissue Disease: A Prospective Pilot Study Involving 180 Patients" Journal of Clinical Medicine 10, no. 18: 4114. https://doi.org/10.3390/jcm10184114
APA StyleBuda, N., Wojteczek, A., Masiak, A., Piskunowicz, M., Batko, W., & Zdrojewski, Z. (2021). Lung Ultrasound in the Screening of Pulmonary Interstitial Involvement Secondary to Systemic Connective Tissue Disease: A Prospective Pilot Study Involving 180 Patients. Journal of Clinical Medicine, 10(18), 4114. https://doi.org/10.3390/jcm10184114






