Comparative Analysis of Lenvatinib and Hepatic Arterial Infusion Chemotherapy in Unresectable Hepatocellular Carcinoma: A Multi-Center, Propensity Score Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Population
2.2. Treatment Protocol
2.3. Response Evaluation
2.4. Propensity-Score Matching (PSM)
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Treatment Responses
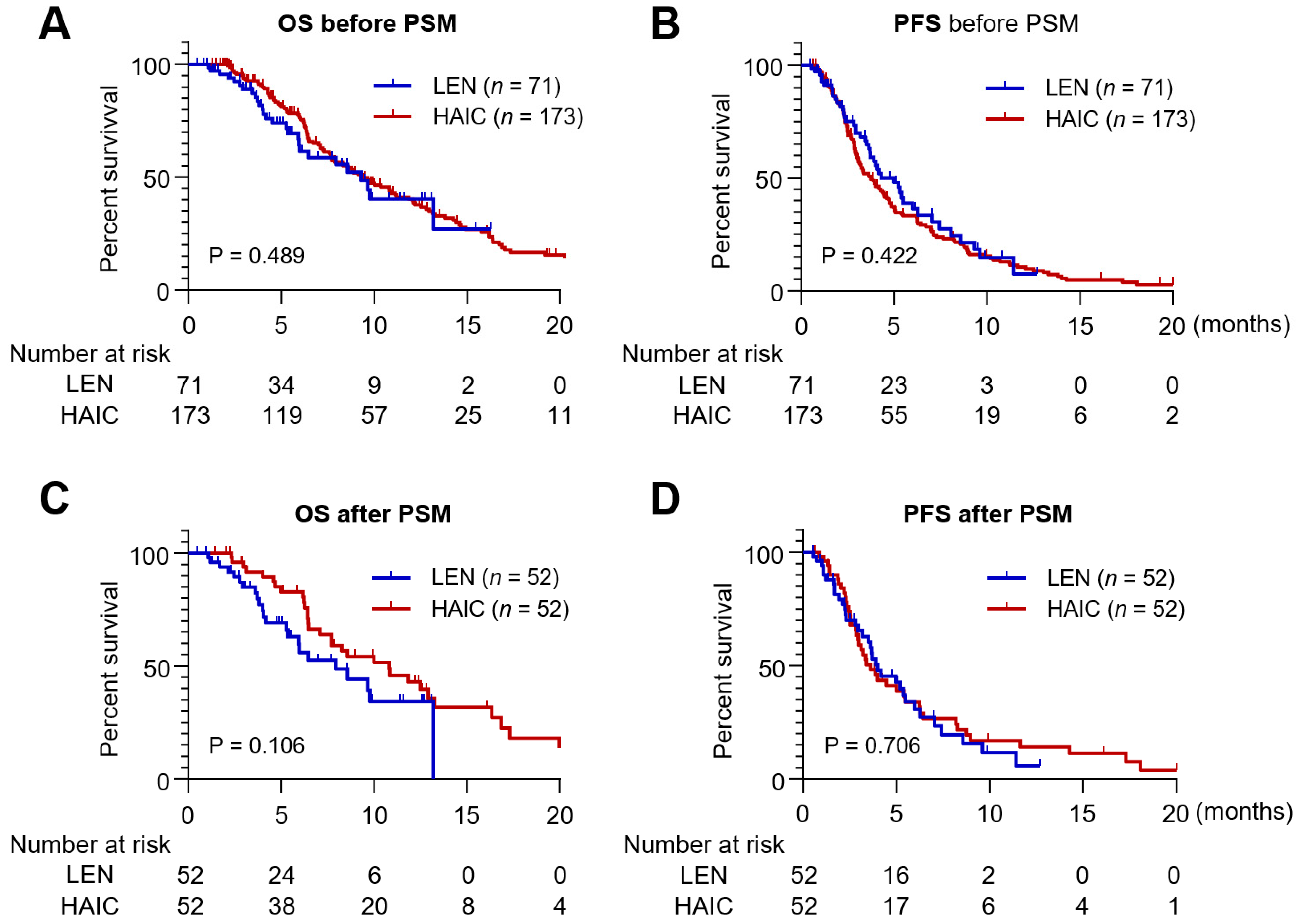
3.3. Survival Outcomes
3.4. Factors Contributing to Survival Outcomes
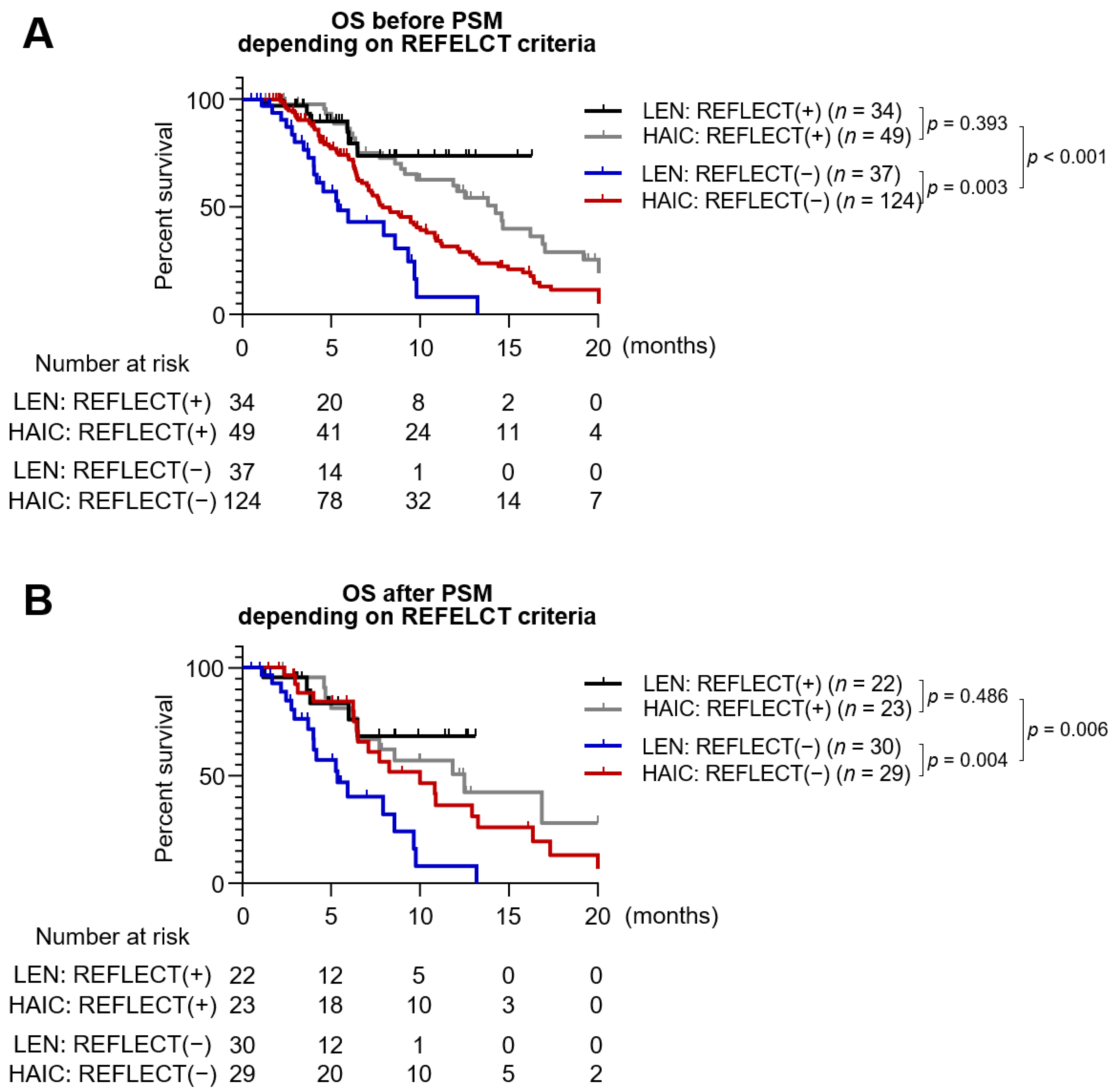
3.5. Patients with Tumor Burden beyond the REFLECT Eligibility Criteria
3.6. Treatment-Related Toxicity
3.7. Liver Function after Lenvatinib or HAIC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Villanueva, A. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2012, 56, 908–943. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Lee, J.; Sung, P.S.; Yang, H.; Lee, S.K.; Nam, H.C.; Yoo, S.H.; Lee, H.L.; Kim, H.Y.; Lee, S.W.; Kwon, J.H.; et al. A Real-World Comparative Analysis of Lenvatinib and Sorafenib as a Salvage Therapy for Transarterial Treatments in Unresectable HCC. J. Clin. Med. 2020, 9, 4121. [Google Scholar] [CrossRef] [PubMed]
- Hoshi, T.; Watanabe Miyano, S.; Watanabe, H.; Sonobe, R.M.K.; Seki, Y.; Ohta, E.; Nomoto, K.; Matsui, J.; Funahashi, Y. Lenvatinib induces death of human hepatocellular carcinoma cells harboring an activated FGF signaling pathway through inhibition of FGFR-MAPK cascades. Biochem. Biophys. Res. Commun. 2019, 513, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Duran, S.R.; Jaquiss, R.D.B. Hepatocellular Carcinoma. N. Engl. J. Med. 2019, 381, e2. [Google Scholar] [CrossRef] [PubMed]
- Sho, T.; Suda, G.; Ogawa, K.; Shigesawa, T.; Suzuki, K.; Nakamura, A.; Ohara, M.; Umemura, M.; Kawagishi, N.; Natsuizaka, M.; et al. Lenvatinib in patients with unresectable hepatocellular carcinoma who do not meet the REFLECT trial eligibility criteria. Hepatol. Res. 2020, 50, 966–977. [Google Scholar] [CrossRef]
- Kudo, M.; Matsui, O.; Izumi, N.; Iijima, H.; Kadoya, M.; Imai, Y.; Okusaka, T.; Miyayama, S.; Tsuchiya, K.; Ueshima, K.; et al. JSH Consensus-Based Clinical Practice Guidelines for the Management of Hepatocellular Carcinoma: 2014 Update by the Liver Cancer Study Group of Japan. Liver Cancer 2014, 3, 458–468. [Google Scholar] [CrossRef]
- Sung, P.S.; Choi, M.H.; Yang, H.; Lee, S.K.; Chun, H.J.; Jang, J.W.; Choi, J.Y.; Yoon, S.K.; Choi, J.I.; Lee, Y.J.; et al. Diffusion-Weighted Magnetic Resonance Imaging in Hepatocellular Carcinoma as a Predictor of a Response to Cisplatin-Based Hepatic Arterial Infusion Chemotherapy. Front. Oncol. 2020, 10, 600233. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, B.K.; Kim, S.U.; Park, J.Y.; Ahn, S.H.; Seong, J.S.; Han, K.H.; Kim, D.Y. A survey on transarterial chemoembolization refractoriness and a real-world treatment pattern for hepatocellular carcinoma in Korea. Clin. Mol. Hepatol. 2020, 26, 24–32. [Google Scholar] [CrossRef]
- Moriya, K.; Namisaki, T.; Sato, S.; Furukawa, M.; Douhara, A.; Kawaratani, H.; Kaji, K.; Shimozato, N.; Sawada, Y.; Saikawa, S.; et al. Bi-monthly hepatic arterial infusion chemotherapy as a novel strategy for advanced hepatocellular carcinoma in decompensated cirrhotic patients. Clin. Mol. Hepatol. 2019, 25, 381–389. [Google Scholar] [CrossRef]
- Kokudo, N.; Takemura, N.; Hasegawa, K.; Takayama, T.; Kubo, S.; Shimada, M.; Nagano, H.; Hatano, E.; Izumi, N.; Kaneko, S.; et al. Clinical practice guidelines for hepatocellular carcinoma: The Japan Society of Hepatology 2017 (4th JSH-HCC guidelines) 2019 update. Hepatol. Res. 2019, 49, 1109–1113. [Google Scholar] [CrossRef]
- Sung, P.S.; Yang, K.; Bae, S.H.; Oh, J.S.; Chun, H.J.; Nam, H.C.; Jang, J.W.; Choi, J.Y.; Yoon, S.K. Reduction of Intrahepatic Tumour by Hepatic Arterial Infusion Chemotherapy Prolongs Survival in Hepatocellular Carcinoma. Anticancer Res. 2019, 39, 3909–3916. [Google Scholar] [CrossRef]
- Lin, C.C.; Hung, C.F.; Chen, W.T.; Lin, S.M. Hepatic Arterial Infusion Chemotherapy for Advanced Hepatocellular Carcinoma with Portal Vein Thrombosis: Impact of Early Response to 4 Weeks of Treatment. Liver Cancer 2015, 4, 228–240. [Google Scholar] [CrossRef]
- Kudo, M.; Ueshima, K.; Yokosuka, O.; Ogasawara, S.; Obi, S.; Izumi, N.; Aikata, H.; Nagano, H.; Hatano, E.; Sasaki, Y.; et al. Sorafenib plus low-dose cisplatin and fluorouracil hepatic arterial infusion chemotherapy versus sorafenib alone in patients with advanced hepatocellular carcinoma (SILIUS): A randomised, open label, phase 3 trial. Lancet Gastroenterol. Hepatol. 2018, 3, 424–432. [Google Scholar] [CrossRef]
- He, M.; Li, Q.; Zou, R.; Shen, J.; Fang, W.; Tan, G.; Zhou, Y.; Wu, X.; Xu, L.; Wei, W.; et al. Sorafenib Plus Hepatic Arterial Infusion of Oxaliplatin, Fluorouracil, and Leucovorin vs Sorafenib Alone for Hepatocellular Carcinoma With Portal Vein Invasion: A Randomized Clinical Trial. JAMA Oncol. 2019, 5, 953–960. [Google Scholar] [CrossRef]
- Nakano, M.; Niizeki, T.; Nagamatsu, H.; Tanaka, M.; Kuromatsu, R.; Satani, M.; Okamura, S.; Iwamoto, H.; Shimose, S.; Shirono, T.; et al. Clinical effects and safety of intra-arterial infusion therapy of cisplatin suspension in lipiodol combined with 5-fluorouracil versus sorafenib, for advanced hepatocellular carcinoma with macroscopic vascular invasion without extra-hepatic spread: A prospective cohort study. Mol. Clin. Oncol. 2017, 7, 1013–1020. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Choi, J.H.; Chung, W.J.; Bae, S.H.; Song, D.S.; Song, M.J.; Kim, Y.S.; Yim, H.J.; Jung, Y.K.; Suh, S.J.; Park, J.Y.; et al. Randomized, prospective, comparative study on the effects and safety of sorafenib vs. hepatic arterial infusion chemotherapy in patients with advanced hepatocellular carcinoma with portal vein tumor thrombosis. Cancer Chemother. Pharmacol. 2018, 82, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.; Niizeki, T.; Nagamatsu, H.; Ueshima, K.; Nomura, T.; Kuzuya, T.; Kasai, K.; Kooka, Y.; Hiraoka, A.; Sugimoto, R.; et al. Survival Benefit of Hepatic Arterial Infusion Chemotherapy over Sorafenib in the Treatment of Locally Progressed Hepatocellular Carcinoma. Cancers 2021, 13, 646. [Google Scholar] [CrossRef]
- Song, D.S.; Song, M.J.; Bae, S.H.; Chung, W.J.; Jang, J.Y.; Kim, Y.S.; Lee, S.H.; Park, J.Y.; Yim, H.J.; Cho, S.B.; et al. A comparative study between sorafenib and hepatic arterial infusion chemotherapy for advanced hepatocellular carcinoma with portal vein tumor thrombosis. J. Gastroenterol. 2015, 50, 445–454. [Google Scholar] [CrossRef]
- Sung, P.S.; Park, H.L.; Yang, K.; Hwang, S.; Song, M.J.; Jang, J.W.; Choi, J.Y.; Yoon, S.K.; Yoo, I.R.; Bae, S.H. (18)F-fluorodeoxyglucose uptake of hepatocellular carcinoma as a prognostic predictor in patients with sorafenib treatment. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 384–391. [Google Scholar] [CrossRef]
- Rhee, H.; Chung, T.; Yoo, J.E.; Nahm, J.H.; Woo, H.Y.; Choi, G.H.; Han, D.H.; Park, Y.N. Gross type of hepatocellular carcinoma reflects the tumor hypoxia, fibrosis, and stemness-related marker expression. Hepatol. Int. 2020, 14, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Kumada, T.; Tsuji, K.; Takaguchi, K.; Itobayashi, E.; Kariyama, K.; Ochi, H.; Tajiri, K.; Hirooka, M.; Shimada, N.; et al. Validation of Modified ALBI Grade for More Detailed Assessment of Hepatic Function in Hepatocellular Carcinoma Patients: A Multicenter Analysis. Liver Cancer 2019, 8, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Dueck, A.C.; Mendoza, T.R.; Mitchell, S.A.; Reeve, B.B.; Castro, K.M.; Rogak, L.J.; Atkinson, T.M.; Bennett, A.V.; Denicoff, A.M.; O’Mara, A.M.; et al. Validity and Reliability of the US National Cancer Institute’s Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 2015, 1, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Peng, W.; Liu, X.; Li, C.; Li, X.; Wen, T.F. Post-treatment alpha-fetoprotein response predicts prognosis of patients with hepatocellular carcinoma: A meta-analysis. Medicine (Baltimore) 2019, 98, e16557. [Google Scholar] [CrossRef] [PubMed]
- Goh, M.J.; Oh, J.H.; Park, Y.; Kim, J.; Kang, W.; Sinn, D.H.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; et al. Efficacy and Safety of Lenvatinib Therapy for Unresectable Hepatocellular Carcinoma in a Real-World Practice in Korea. Liver Cancer 2021, 10, 52–62. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Early Relative Change in Hepatic Function with Lenvatinib for Unresectable Hepatocellular Carcinoma. Oncology 2019, 97, 334–340. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; Takaguchi, K.; Kariyama, K.; Itobayashi, E.; Tajiri, K.; et al. Important Clinical Factors in Sequential Therapy Including Lenvatinib against Unresectable Hepatocellular Carcinoma. Oncology 2019, 97, 277–285. [Google Scholar] [CrossRef]
- Korean Liver Cancer, A.; National Cancer, C. 2018 Korean Liver Cancer Association-National Cancer Center Korea Practice Guidelines for the Management of Hepatocellular Carcinoma. Gut Liver 2019, 13, 227–299. [Google Scholar] [CrossRef]
- Surveillance Group; Diagnosis Group; Staging Group; Surgery Group; Local ablation Group; TACE/TARE/HAI Group; Target therapy/systemic therapy Group; Radiotherapy Group; Prevention Group; Drafting Group; et al. Management consensus guideline for hepatocellular carcinoma: 2016 updated by the Taiwan Liver Cancer Association and the Gastroenterological Society of Taiwan. J. Formos. Med. Assoc. 2018, 117, 381–403. [Google Scholar] [CrossRef]
- Terashima, T.; Yamashita, T.; Arai, K.; Kawaguchi, K.; Kitamura, K.; Yamashita, T.; Sakai, Y.; Mizukoshi, E.; Honda, M.; Kaneko, S. Beneficial Effect of Maintaining Hepatic Reserve during Chemotherapy on the Outcomes of Patients with Hepatocellular Carcinoma. Liver Cancer 2017, 6, 236–249. [Google Scholar] [CrossRef]
- Terashima, T.; Yamashita, T.; Arai, K.; Kawaguchi, K.; Kitamura, K.; Yamashita, T.; Sakai, Y.; Mizukoshi, E.; Honda, M.; Kaneko, S. Response to chemotherapy improves hepatic reserve for patients with hepatocellular carcinoma and Child-Pugh B cirrhosis. Cancer Sci. 2016, 107, 1263–1269. [Google Scholar] [CrossRef] [PubMed]
- Saeki, I.; Yamasaki, T.; Maeda, M.; Hisanaga, T.; Iwamoto, T.; Matsumoto, T.; Hidaka, I.; Ishikawa, T.; Takami, T.; Sakaida, I. Evaluation of the "assessment for continuous treatment with hepatic arterial infusion chemotherapy" scoring system in patients with advanced hepatocellular carcinoma. Hepatol. Res. 2018, 48, E87–E97. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Shimizu, S.; Sato, T.; Morimoto, M.; Kojima, Y.; Inaba, Y.; Hagihara, A.; Kudo, M.; Nakamori, S.; Kaneko, S.; et al. Sorafenib plus hepatic arterial infusion chemotherapy with cisplatin versus sorafenib for advanced hepatocellular carcinoma: Randomized phase II trial. Ann. Oncol. 2016, 27, 2090–2096. [Google Scholar] [CrossRef] [PubMed]
- He, M.-K.; Liang, R.-B.; Zhao, Y.; Xu, Y.-J.; Chen, H.-W.; Zhou, Y.-M.; Lai, Z.-C.; Xu, L.; Wei, W.; Zhang, Y.-J. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther. Adv. Med. Oncol. 2021, 13, 17588359211002720. [Google Scholar] [CrossRef]
- Miyaki, D.; Aikata, H.; Kan, H.; Fujino, H.; Urabe, A.; Masaki, K.; Fukuhara, T.; Kobayashi, T.; Naeshiro, N.; Nakahara, T.; et al. Clinical outcome of sorafenib treatment in patients with advanced hepatocellular carcinoma refractory to hepatic arterial infusion chemotherapy. J. Gastroenterol. Hepatol. 2013, 28, 1834–1841. [Google Scholar] [CrossRef]
- Kondo, M.; Numata, K.; Hara, K.; Nozaki, A.; Fukuda, H.; Chuma, M.; Maeda, S.; Tanaka, K. Treatment of Advanced Hepatocellular Carcinoma after Failure of Sorafenib Treatment: Subsequent or Additional Treatment Interventions Contribute to Prolonged Survival Postprogression. Gastroenterol. Res. Pract. 2017, 2017, 5728946. [Google Scholar] [CrossRef]
- Terashima, T.; Yamashita, T.; Horii, R.; Arai, K.; Kawaguchi, K.; Kitamura, K.; Yamashita, T.; Sakai, Y.; Mizukoshi, E.; Honda, M.; et al. Potential efficacy of therapies targeting intrahepatic lesions after sorafenib treatment of patients with hepatocellular carcinoma. BMC Cancer 2016, 16, 338. [Google Scholar] [CrossRef]
- Lee, C.H.; Lee, Y.B.; Kim, M.A.; Jang, H.; Oh, H.; Kim, S.W.; Cho, E.J.; Lee, K.H.; Lee, J.H.; Yu, S.J.; et al. Effectiveness of nivolumab versus regorafenib in hepatocellular carcinoma patients who failed sorafenib treatment. Clin. Mol. Hepatol. 2020, 26, 328–339. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Tada, T.; Kariyama, K.; Tani, J.; Fukunishi, S.; Atsukawa, M.; Hirooka, M.; Tsuji, K.; Ishikawa, T.; et al. What Can Be Done to Solve the Unmet Clinical Need of Hepatocellular Carcinoma Patients following Lenvatinib Failure? Liver Cancer 2021. [Google Scholar] [CrossRef]
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Treatment | Lenvatinib (n = 71) | HAIC (n = 173) | p-Value | Lenvatinib (n = 52) | HAIC (n = 52) | p-Value |
| Male sex | 62 (87.3) | 150 (86.7) | 1.000 | 47 (90.4) | 45 (86.5) | 0.760 |
| Age (years) | 63.1 ± 11.5 | 58.3 ± 10.2 | 0.001 | 61.0 ± 11.2 | 61.2 ± 11.6 | 0.939 |
| Child-Pugh | <0.001 | 1.000 | ||||
| A | 53 (74.6) | 82 (47.4) | 34 (65.4) | 33 (63.5) | ||
| B | 18 (25.4) | 91 (52.6) | 18 (34.6) | 19 (36.5) | ||
| Etiology | 0.019 | 0.585 | ||||
| HBV | 42 (59.2) | 134 (77.5) | 32 (61.5) | 37 (71.2) | ||
| HCV | 7 (9.9) | 13 (7.5) | 4 (7.7) | 5 (9.6) | ||
| Alcohol | 16 (22.5) | 16 (9.2) | 12 (23.1) | 8 (15.4) | ||
| Others | 6 (8.5) | 10 (5.8) | 4 (7.7) | 2 (3.8) | ||
| AFP (ng/mL) | 662.2 (37.5–8000.2) | 976 (57.2–13,670) | 0.037 | 1479.3 (66.5–11,987) | 308.51 (29–12,979.5) | 0.458 |
| PIVKA (mAU/mL) | 1648.5 (107.9–20,154.9) | 1725 (353–14,845) | 0.673 | 5850.5 (130.8–25,629.3) | 872 (405.5–4796.7) | 0.046 |
| Albumin (g/dL) | 3.7 ± 0.5 | 3.4 ± 0.5 | 0.001 | 3.5 ± 0.5 | 3.6 ± 0.5 | 0.527 |
| Platelet (109/L) | 168.8 ± 97.7 | 174.9 ± 104.7 | 0.678 | 164.2 ± 100.1 | 176.0 ± 105.8 | 0.560 |
| Maximal tumor size (cm) | 7.7 ± 5.3 | 9.7 ± 4.8 | 0.005 | 8.0 ± 5.0 | 8.1 ± 4.8 | 0.934 |
| Tumor type | <0.001 | 0.549 | ||||
| Nodular | 51 (71.8) | 64 (37.0) | 33 (63.5) | 29 (55.8) | ||
| Non-nodular | 20 (28.2) | 109 (63.0) | 19 (36.5) | 23 (44.2) | ||
| PVTT | 33 (46.5) | 131 (75.7) | <0.001 | 29 (55.8) | 29 (55.8) | 1.000 |
| Extrahepatic metastasis | 37 (52.1) | 47 (27.2) | <0.001 | 20 (38.5) | 24 (46.2) | 0.552 |
| BCLC | 0.408 | 0.625 | ||||
| B | 14 (19.7) | 25 (14.5) | 12 (23.1) | 9 (17.3) | ||
| C | 57 (80.3) | 148 (85.5) | 40 (76.9) | 43 (82.7) | ||
| ECOG | 0.160 | 0.578 | ||||
| 0 | 28 (39.4) | 80 (46.2) | 19 (36.5) | 18 (34.6) | ||
| 1 | 39 (54.9) | 74 (42.8) | 30 (57.7) | 28 (53.8) | ||
| 2 | 4 (5.6) | 19 (11.0) | 3 (5.8) | 6 (11.5) | ||
| Previous treatment history | 59 (83.1) | 89 (51.4) | <0.001 | 40 (76.9) | 30 (57.7) | 0.060 |
| Before PSM | After PSM | |||||
|---|---|---|---|---|---|---|
| Lenvatinib (n = 71) | HAIC (n = 173) | p-Value | Lenvatinib (n = 52) | HAIC (n = 52) | p-Value | |
| Treatment responses | 0.292 | 0.583 | ||||
| CR | 2 (2.8) | 6 (3.5) | 2 (3.8) | 4 (7.7) | ||
| PR | 15 (21.1) | 39 (22.5) | 10 (19.2) | 11 (21.2) | ||
| SD | 24 (33.8) | 89 (51.4) | 15 (28.8) | 23 (44.2) | ||
| PD | 20 (28.2) | 38 (22.0) | 16 (30.8) | 13 (25.0) | ||
| NA | 10 (14.1) | 1 (0.6) | 9 (17.3) | 1 (1.9) | ||
| ORR | 17 (23.9) | 45 (26.0) | 0.736 | 12 (23.1) | 15 (28.8) | 0.502 |
| DCR | 41 (57.7) | 134 (77.5) | 0.002 | 27 (51.9) | 38 (73.1) | 0.026 |
| Variables | Overall Survival | Progression-Free Survival | ||||
|---|---|---|---|---|---|---|
| Univariate (p-Value) | Multivariate (p-Value) | HR (95% CI) | Univariate (p-Value) | Multivariate (p-Value) | HR (95% CI) | |
| Lenvatinib vs. HAIC | 0.490 | 0.424 | ||||
| Age ≤ 60 years | 0.005 | 0.098 | 1.4 (0.9–1.9) | 0.010 | 0.105 | 1.3 (0.9–1.7) |
| HBV vs. non-HBV | 0.516 | 0.227 | ||||
| Tumor size ≤ 5 cm | 0.010 | 0.257 | 0.8 (0.5–1.2) | 0.026 | 0.379 | 0.9 (0.6–1.2) |
| Macrovascular invasion | 0.211 | 0.108 | ||||
| Extrahepatic metastasis | 0.017 | 0.014 | 1.6 (1.1–2.3) | 0.042 | 0.061 | 1.3 (0.9–1.8) |
| Child class A | 0.002 | 0.028 | 0.7 (0.5–0.9) | <0.001 | 0.010 | 0.7 (0.5–0.9) |
| AFP ≤ 1000 | 0.001 | 0.030 | 0.7 (0.5–0.9) | <0.001 | 0.026 | 0.7 (0.5–0.9) |
| PIVKA-II ≤ 1000 | 0.067 | 0.353 | 0.8 (0.6–1.2) | 0.137 | ||
| Adverse Event | HAIC (n = 52) | Lenvatinib (n = 52) | p-Value |
|---|---|---|---|
| AE grade ≥3 (overlapped) | 25 (48.1) | 23 (44.2) | 0.694 |
| HFSR | 0 (0) | 2 (3.8) | |
| Hypertension | 0 (0) | 5 (9.6) | |
| Nephrotoxicity | |||
| Proteinuria | 0 (0) | 3 (5.8) | |
| Elevated creatinine | 2 (3.8) | 0 (0) | |
| Hematologic | |||
| Anemia | 4 (7.7) | 1 (1.9) | |
| Neutropenia | 1 (1.9) | 0 (0) | |
| Thrombocytopenia | 2 (3.8) | 5 (9.6) | |
| Laboratory | |||
| Hyperbilirubinemia | 7 (13.5) | 3 (5.8) | |
| AST | 11 (21.2) | 2 (3.8) | |
| ALT | 7 (13.5) | 1 (1.9) | |
| Gastrointestinal | |||
| Nausea/vomiting | 3 (5.8) | 2 (3.8) | |
| Diarrhea | 2 (3.8) | 5 (9.6) | |
| Decreased appetite | 3 (5.8) | 2 (3.8) | |
| Hepatic encephalopathy | 3 (5.8) | 5 (9.6) | |
| Fatigue | 3 (5.8) | 2 (3.8) | |
| Dyspnea | 0 (0) | 1 (1.9) | |
| Abdominal pain | 1 (1.9) | 0 (0) |
| Lenvatinib (n = 52) | HAIC (n = 52) | p | |
|---|---|---|---|
| Child-Pugh class A | 23 (44.2) | 35 (67.3) | 0.018 |
| mALBI grade ≤2a | 13 (25.0) | 25 (48.1) | 0.015 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.; Han, J.-W.; Sung, P.-S.; Lee, S.-K.; Yang, H.; Nam, H.-C.; Yoo, S.-H.; Lee, H.-L.; Kim, H.-Y.; Lee, S.-W.; et al. Comparative Analysis of Lenvatinib and Hepatic Arterial Infusion Chemotherapy in Unresectable Hepatocellular Carcinoma: A Multi-Center, Propensity Score Study. J. Clin. Med. 2021, 10, 4045. https://doi.org/10.3390/jcm10184045
Lee J, Han J-W, Sung P-S, Lee S-K, Yang H, Nam H-C, Yoo S-H, Lee H-L, Kim H-Y, Lee S-W, et al. Comparative Analysis of Lenvatinib and Hepatic Arterial Infusion Chemotherapy in Unresectable Hepatocellular Carcinoma: A Multi-Center, Propensity Score Study. Journal of Clinical Medicine. 2021; 10(18):4045. https://doi.org/10.3390/jcm10184045
Chicago/Turabian StyleLee, Jaejun, Ji-Won Han, Pil-Soo Sung, Soon-Kyu Lee, Hyun Yang, Hee-Chul Nam, Sun-Hong Yoo, Hae-Lim Lee, Hee-Yeon Kim, Sung-Won Lee, and et al. 2021. "Comparative Analysis of Lenvatinib and Hepatic Arterial Infusion Chemotherapy in Unresectable Hepatocellular Carcinoma: A Multi-Center, Propensity Score Study" Journal of Clinical Medicine 10, no. 18: 4045. https://doi.org/10.3390/jcm10184045
APA StyleLee, J., Han, J.-W., Sung, P.-S., Lee, S.-K., Yang, H., Nam, H.-C., Yoo, S.-H., Lee, H.-L., Kim, H.-Y., Lee, S.-W., Kwon, J.-H., Jang, J.-W., Kim, C.-W., Nam, S.-W., Oh, J.-S., Chun, H.-J., Bae, S.-H., Choi, J.-Y., & Yoon, S.-K. (2021). Comparative Analysis of Lenvatinib and Hepatic Arterial Infusion Chemotherapy in Unresectable Hepatocellular Carcinoma: A Multi-Center, Propensity Score Study. Journal of Clinical Medicine, 10(18), 4045. https://doi.org/10.3390/jcm10184045







