Inferior Pulmonary Ligament Division May Be Unnecessary during Left Upper Lobectomy: Effects on Lung Volume, Bronchial Angle and Bronchial Tortuosity
Abstract
1. Introduction
2. Materials and Methods
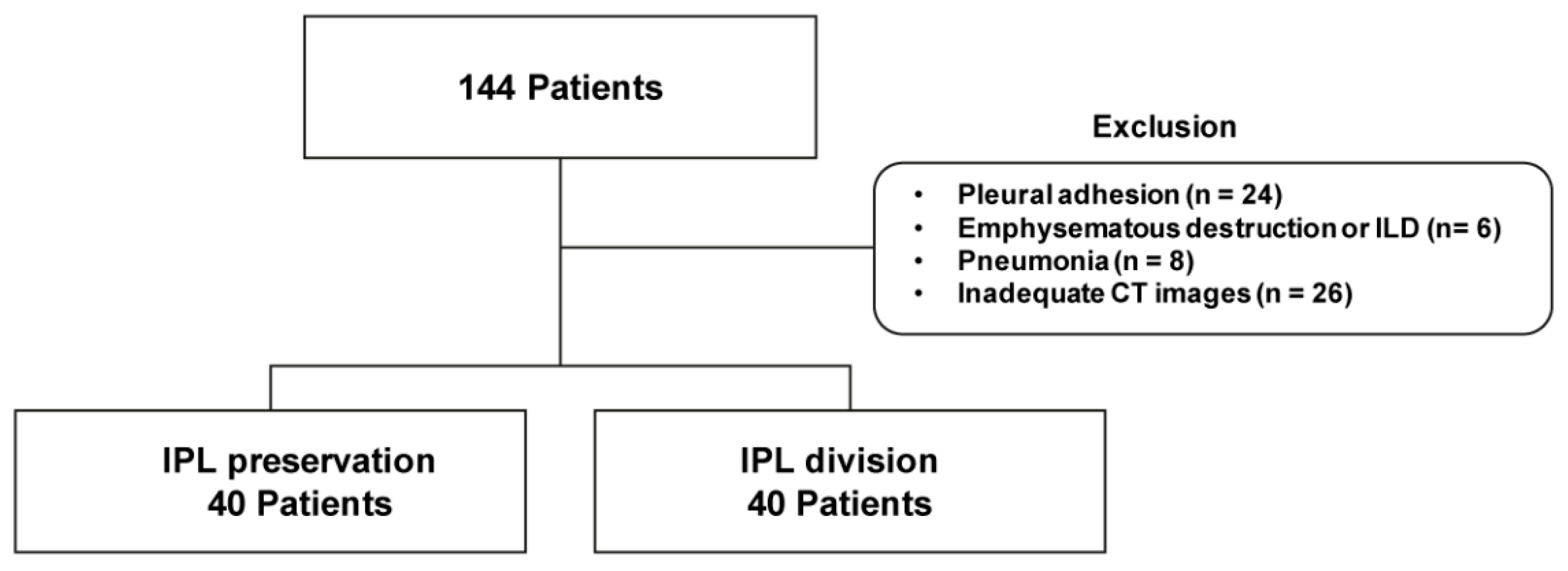
2.1. Patients
2.2. Surgery
2.3. Chest CT Protocol
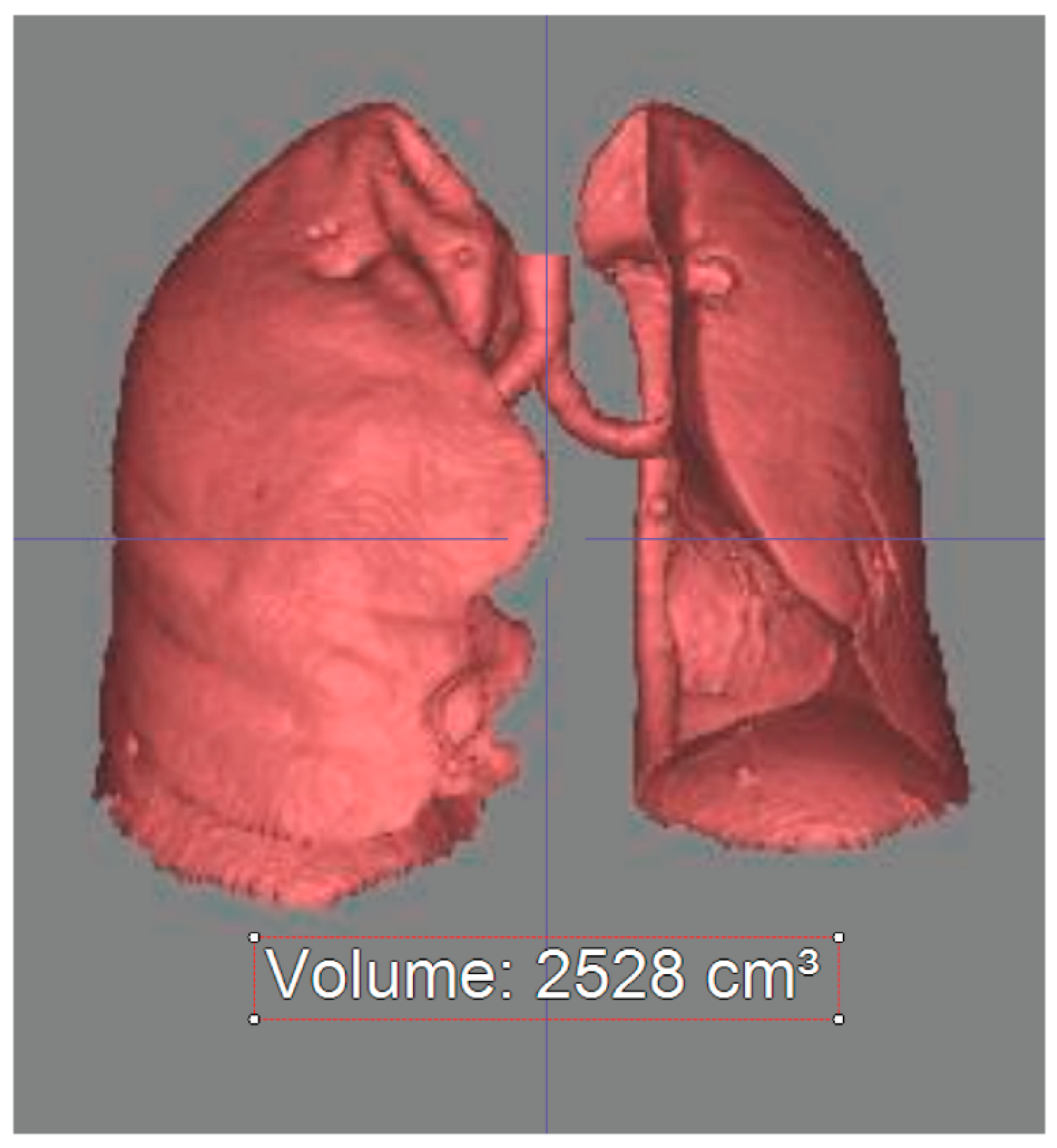
2.4. Chest CT Analysis—Lung Volume Measurement
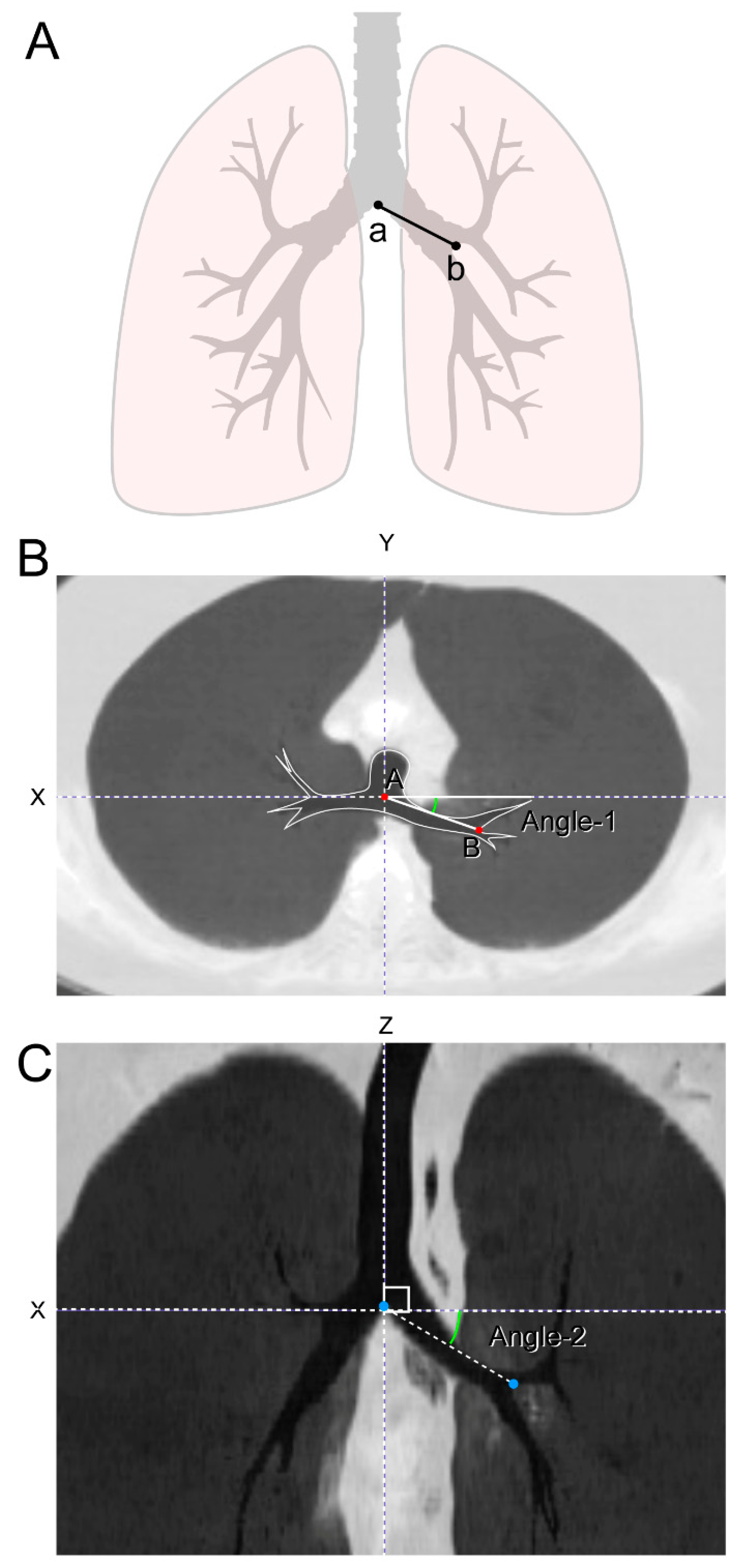
2.5. Chest CT Analysis—Bronchial Angle Measurement
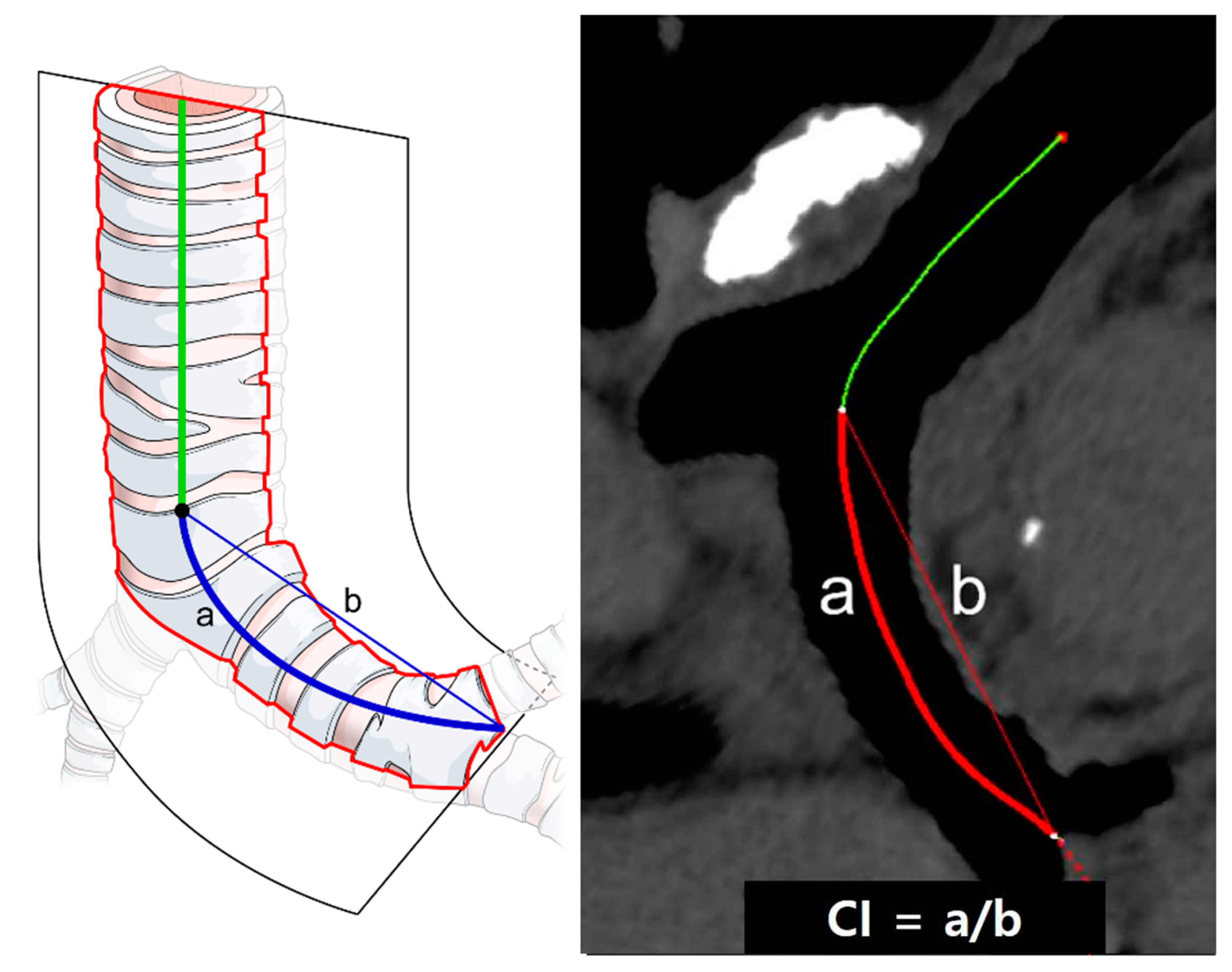
2.6. Chest CT Analysis—Bronchial Tortuosity Measurement
2.7. Statistical Analysis
3. Results
3.1. Right Lung Volume
3.2. Left Lung Volume
3.3. Left Bronchial Angles
3.4. Left Bronchial Tortuosity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Usuda, K.; Sagawa, M.; Aikawa, H.; Tanaka, M.; Machida, Y.; Ueno, M.; Sakuma, T. Do japanese thoracic surgeons think that dissection of the pulmonary ligament is necessary after an upper lobectomy? Surg. Today 2010, 40, 1097–1099. [Google Scholar] [CrossRef] [PubMed]
- Khanbhai, M.; Dunning, J.; Yap, K.H.; Rammohan, K.S. Dissection of the pulmonary ligament during upper lobectomy: Is it necessary? Interact. Cardiovasc. Thorac. Surg. 2013, 17, 403–406. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seok, Y.; Yi, E.; Cho, S.; Jheon, S.; Kim, K. Perioperative outcomes of upper lobectomy according to preservation or division of the inferior pulmonary ligament. J. Thorac. Dis. 2015, 7, 2033–2040. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Yang, A.-R.; Peng, H.; Xu, Z.-Y.; Wu, J.-Q.; Wang, P. Dividing inferior pulmonary ligament may change the bronchial angle. J. Surg. Res. 2015, 201, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Moon, D.H.; Kim, H.R.; Lee, S.M.; Chae, E.J.; Choi, C.-M.; Choi, S.H.; Kim, Y.-H.; Kim, D.K.; Park, S.-I. Effect of inferior pulmonary ligament division on residual lung volume and function after a right upper lobectomy. Interact. Cardiovasc. Thorac. Surg. 2019, 28, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.A.; Colley, D.P. Pulmonary lymphatics visualized during pedal lymphangiography. Radiology 1980, 136, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Cross, G.; Woodring, J.H. Inferior pulmonary ligament lymphadenopathy: Demonstration by computed tomography. J. Comput. Tomogr. 1987, 11, 303–306. [Google Scholar] [CrossRef]
- Murakami, G.; Adachi, N.; Sato, I.; Sato, T.; Hoshi, H. Venous Drainage of the Thoracic Esophagus toward the Pulmonary Vein. Okajimas Folia Anat. Jpn. 1994, 71, 13–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okiemy, G.; Foucault, C.; Avisse, C.; Hidden, G.; Riquet, M. Lymphatic drainage of the diaphragmatic pleura to the peritracheobronchial lymph nodes. Surg. Radiol. Anat. 2003, 25, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Rost, R.C., Jr.; Proto, A.V. Inferior pulmonary ligament: Computed tomographic appearance. Radiology 1983, 148, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Paling, M.R.; Griffin, G.K. Lower lobe collapse due to pleural effusion: A CT analysis. J. Comput. Assist. Tomogr. 1985, 9, 1079–1083. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Tanaka, T.; Hayashi, M.; Tanaka, N.; Li, T.-S.; Hamano, K. Clinical Ramifications of Bronchial Kink After Upper Lobectomy. Ann. Thorac. Surg. 2012, 93, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, M.; Maehara, T.; Nakayama, H.; Sakamoto, K. Decreased invasiveness via two methods of thoracoscopic lobectomy for lung cancer, compared with open thoracotomy. Respirology 2007, 12, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Altorki, N.K.; Sheng, S.; Lee, P.C.; Harpole, D.H.; Onaitis, M.W.; Stiles, B.M.; Port, J.L.; D’Amico, T.A. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: A propensity-matched analysis from the STS database. J. Thorac. Cardiovasc. Surg. 2010, 139, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhou, Y.; Wang, W. Total thoracoscopic high-position sleeve lobectomy of the right upper lobe of the lung. J. Thorac. Dis. 2018, 10, 4490–4497. [Google Scholar] [CrossRef] [PubMed]
- Rivera, C.; Bernard, A.; Falcoz, P.-E.; Thomas, P.; Schmidt, A.; Bénard, S.; Vicaut, E.; Dahan, M. Characterization and Prediction of Prolonged Air Leak After Pulmonary Resection: A Nationwide Study Setting Up the Index of Prolonged Air Leak. Ann. Thorac. Surg. 2011, 92, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Asamura, H.; Nakayama, H.; Kondo, H.; Tsuchiya, R.; Naruke, T. Lobe-specific extent of systematic lymph node dissection for non–small cell lung carcinomas according to a retrospective study of metastasis and prognosis. J. Thorac. Cardiovasc. Surg. 1999, 117, 1102–1111. [Google Scholar] [CrossRef]
- Ishiguro, F.; Matsuo, K.; Fukui, T.; Mori, S.; Hatooka, S.; Mitsudomi, T. Effect of selective lymph node dissection based on patterns of lobe-specific lymph node metastases on patient outcome in patients with resectable non–small cell lung cancer: A large-scale retrospective cohort study applying a propensity score. J. Thorac. Cardiovasc. Surg. 2010, 139, 1001–1006. [Google Scholar] [CrossRef] [PubMed]

| Variables | Group P, n = 40 | Group D, n = 40 | p-Value |
|---|---|---|---|
| Age, years | 63.6 ± 11.1 | 64.2 ± 9.7 | 0.825 |
| Male, n (%) | 24 (60%) | 26 (65%) | 0.817 |
| Height, cm | 162.9 ± 7.8 | 163.7 ± 8.2 | 0.677 |
| Weight, kg | 65.0 ± 8.4 | 64.7 ± 8.9 | 0.646 |
| Smoking, n (%) | 20 (50%) | 24 (60%) | 0.347 |
| COPD, n (%) | 0 | 2 (5%) | 0.494 |
| Preoperative FEV1, mL | 1.98 ± 0.56 | 1.89 ± 0.48 | 0.598 |
| Preoperative FVC, mL | 2.73 ± 0.78 | 2.67 ± 0.64 | 0.669 |
| Tumor size, cm | 2.8 ± 1.9 | 2.7 ± 1.7 | 0.623 |
| Histologic diagnosis, n (%) | 0.785 | ||
| Adenocarcinoma | 34 (85%) | 32 (80%) | |
| Squamous cell carcinoma | 5 (12.5%) | 6 (15%) | |
| Others | 1 (2.5%) | 2 (5%) | |
| Pathologic cancer stage, n (%) | 0.485 | ||
| I | 27 (67.5%) | 24 (60%) | |
| II | 13 (32.5%) | 16 (40%) | |
| Complete fissure, n (%) | 7 (17.5%) | 8 (20%) | 0.980 |
| CTD indwelling time, n (%) | 4.5 ± 3.8 | 4.8 ± 3.4 | 0.598 |
| Group P, n = 40 | Group D, n = 40 | p-Value | |
|---|---|---|---|
| Right lung | |||
| Preoperative volume, mL | 2409.8 ± 665.0 | 2475.0 ± 500.0 | 0.622 |
| Postoperative volume, mL | 2619.1 ± 654.0 | 2622.7 ± 505.8 | 0.978 |
| Volume difference, mL | 209.3 ± 454.2 | 147.6 ± 344.1 | 0.496 |
| Left lung | |||
| Preoperative volume, mL | 1970.9 ± 574.3 | 2061.9 ± 513.3 | 0.458 |
| Postoperative volume, mL | 1412.8 ± 420.6 | 1325.6 ± 383.3 | 0.335 |
| Volume difference, mL | −558.1 ± 410.0 | −736.3 ± 382.7 | 0.043 |
| Group P, n = 40 | Group D, n = 40 | p-Value | |
|---|---|---|---|
| Angle 1 | |||
| Preoperative angle 1, | 13.5 ± 5.1 | 14.3 ± 4.6 | 0.497 |
| Postoperative angle 1, | 10.6 ± 5.7 | 8.5 ± 8.2 | 0.198 |
| Angle 1 difference, | −2.9 ± 5.5 | −5.7 ± 7.7 | 0.065 |
| Angle 2 | |||
| Preoperative angle 2, | 27.9 ± 5.5 | 28.5 ± 6.9 | 0.689 |
| Postoperative angle 2, | 12.2 ± 9.0 | 9.3 ± 9.8 | 0.170 |
| Angle 2 difference, | −15.7 ± 8.4 | −19.2 ±11.0 | 0.114 |
| Group P, n = 40 | Group D, n = 40 | p-Value | |
|---|---|---|---|
| CI | |||
| Preoperative CI | 1.05 ± 0.02 | 1.04 ± 0.02 | 0.142 |
| Postoperative CI | 1.08 ± 0.03 | 1.11 ± 0.05 | 0.003 |
| CI difference | 0.03 ± 0.03 | 0.06 ± 0.03 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, D.H.; Park, C.H.; Jung, J.H.; Kim, T.H.; Haam, S.J.; Lee, S. Inferior Pulmonary Ligament Division May Be Unnecessary during Left Upper Lobectomy: Effects on Lung Volume, Bronchial Angle and Bronchial Tortuosity. J. Clin. Med. 2021, 10, 4033. https://doi.org/10.3390/jcm10184033
Moon DH, Park CH, Jung JH, Kim TH, Haam SJ, Lee S. Inferior Pulmonary Ligament Division May Be Unnecessary during Left Upper Lobectomy: Effects on Lung Volume, Bronchial Angle and Bronchial Tortuosity. Journal of Clinical Medicine. 2021; 10(18):4033. https://doi.org/10.3390/jcm10184033
Chicago/Turabian StyleMoon, Duk Hwan, Chul Hwan Park, Joon Ho Jung, Tae Hoon Kim, Seok Jin Haam, and Sungsoo Lee. 2021. "Inferior Pulmonary Ligament Division May Be Unnecessary during Left Upper Lobectomy: Effects on Lung Volume, Bronchial Angle and Bronchial Tortuosity" Journal of Clinical Medicine 10, no. 18: 4033. https://doi.org/10.3390/jcm10184033
APA StyleMoon, D. H., Park, C. H., Jung, J. H., Kim, T. H., Haam, S. J., & Lee, S. (2021). Inferior Pulmonary Ligament Division May Be Unnecessary during Left Upper Lobectomy: Effects on Lung Volume, Bronchial Angle and Bronchial Tortuosity. Journal of Clinical Medicine, 10(18), 4033. https://doi.org/10.3390/jcm10184033






