The Acute Effects of Aerobic Exercise on Nocturnal and Pre-Sleep Arousal in Patients with Unipolar Depression: Preplanned Secondary Analysis of a Randomized Controlled Trial
Abstract
:1. Introduction
2. Methods
2.1. Participants
2.2. Patient Characteristics
2.3. Randomization
2.4. Graded Exercise Test, Intervention, and Control
2.5. Baseline and Follow-Up Assessments
2.5.1. Polysomnography and Heart Rate Variability
2.5.2. Pre-Sleep Arousal
2.6. Statistical Methods
3. Results
3.1. Sleep Period
3.2. Sleep Stages
3.3. Pre-Sleep
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alvaro, P.K.; Roberts, R.M.; Harris, J.K. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep 2013, 36, 1059–1068. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.-P.; Han, Y.; Ma, J.; Wang, R.-J.; Shi, L.; Wang, T.-Y.; He, J.; Yue, J.-L.; Shi, J.; Tang, X.-D.; et al. Cooccurrence and bidirectional prediction of sleep disturbances and depression in older adults: Meta-analysis and systematic review. Neurosci. Biobehav. Rev. 2017, 75, 257–273. [Google Scholar] [CrossRef]
- Li, L.; Wu, C.; Gan, Y.; Qu, X.; Lu, Z. Insomnia and the risk of depression: A meta-analysis of prospective cohort studies. BMC Psychiatry 2016, 16, 375. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.; Tu, S.; Sheng, J.; Shao, A. Depression in sleep disturbance: A review on a bidirectional relationship, mechanisms and treatment. J. Cell Mol. Med. 2019, 23, 2324–2332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Mill, J.G.; Hoogendijk, W.J.; Vogelzangs, N.; van Dyck, R.; Penninx, B.W. Insomnia and sleep duration in a large cohort of patients with major depressive disorder and anxiety disorders. J. Clin. Psychiatry 2010, 71, 239–246. [Google Scholar] [CrossRef]
- Spiegelhalder, K.; Regen, W.; Nanovska, S.; Baglioni, C.; Riemann, D. Comorbid sleep disorders in neuropsychiatric disorders across the life cycle. Curr. Psychiatry Rep. 2013, 15, 1–6. [Google Scholar] [CrossRef]
- Tsuno, N.; Besset, A.; Ritchie, K. Sleep and depression. J. Clin. Psychiatry 2005, 66, 1254–1269. [Google Scholar] [CrossRef]
- Geoffroy, P.A.; Hoertel, N.; Etain, B.; Bellivier, F.; Delorme, R.; Limosin, F.; Peyre, H. Insomnia and hypersomnia in major depressive episode: Prevalence, sociodemographic characteristics and psychiatric comorbidity in a population-based study. J. Affect. Disord. 2018, 226, 132–141. [Google Scholar] [CrossRef]
- Boland, E.M.; Vittengl, J.R.; Clark, L.A.; Thase, M.E.; Jarrett, R.B. Is sleep disturbance linked to short- and long-term outcomes following treatments for recurrent depression? J. Affect. Disord. 2019, 262, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Troxel, W.M.; Kupfer, D.J.; Reynolds, C.F.; Frank, E.; Thase, M.; Miewald, J.; Buysse, D.J. Insomnia and objectively measured sleep disturbances predict treatment outcome in depressed patients treated with psychotherapy or psychotherapy-pharmacotherapy combinations. J. Clin. Psychiatry 2011, 73, 478–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bei, B.; Asarnow, L.D.; Krystal, A.; Edinger, J.D.; Buysse, D.J.; Manber, R. Treating insomnia in depression: Insomnia related factors predict long-term depression trajectories. J. Consult. Clin. Psychol. 2018, 86, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Mason, B.L.; Davidov, A.; Minhajuddin, A.; Trivedi, M.H. Focusing on insomnia symptoms to better understand depression: A STAR*D report. J. Affect. Disord. 2019, 260, 183–186. [Google Scholar] [CrossRef]
- Paunio, T.; Korhonen, T.; Hublin, C.; Partinen, M.; Koskenvuo, K.; Koskenvuo, M.; Kaprio, J. Poor sleep predicts symptoms of depression and disability retirement due to depression. J. Affect. Disord. 2015, 172, 381–389. [Google Scholar] [CrossRef]
- Carney, C.E.; Segal, Z.V.; Edinger, J.D.; Krystal, A.D. A comparison of rates of residual insomnia symptoms following pharmacotherapy or cognitive-behavioral therapy for major depressive disorder. J. Clin. Psychiatry 2007, 68, 254–260. [Google Scholar] [CrossRef]
- Perlis, M.L.; Giles, D.E.; Buysse, D.J.; Tu, X.; Kupfer, D.J. Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J. Affect. Disord. 1997, 42, 209–212. [Google Scholar] [CrossRef]
- Nierenberg, A.A.; Husain, M.M.; Trivedi, M.H.; Fava, M.; Warden, D.; Wisniewski, S.R.; Miyahara, S.; Rush, A.J. Residual symptoms after remission of major depressive disorder with citalopram and risk of relapse: A STAR*D report. Psychol. Med. 2009, 40, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Riemann, D.; Spiegelhalder, K.; Feige, B.; Voderholzer, U.; Berger, M.; Perlis, M.; Nissen, C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med. Rev. 2010, 14, 19–31. [Google Scholar] [CrossRef]
- Harvey, A.G. A cognitive model of insomnia. Behav. Res. Ther. 2002, 40, 869–893. [Google Scholar] [CrossRef]
- Hegerl, U.; Hensch, T. The vigilance regulation model of affective disorders and ADHD. Neurosci. Biobehav. Rev. 2012, 44, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.H.; Arand, D.L. Hyperarousal and insomnia: State of the science. Sleep Med. Rev. 2010, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Karlson, C.W.; Stevens, N.R.; Olson, C.A.; Hamilton, N.A. Depression, fatigue, and pre-sleep arousal: A mediation model. J. Coll. Stud. Psychother. 2010, 24, 307–327. [Google Scholar] [CrossRef]
- Kim, A.Y.; Jang, E.H.; Choi, K.W.; Jeon, H.J.; Byun, S.; Sim, J.Y.; Choi, J.H.; Yu, H.Y. Skin conductance responses in Major Depressive Disorder (MDD) under mental arithmetic stress. PLoS ONE 2019, 14, e0213140. [Google Scholar] [CrossRef]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar] [CrossRef]
- Ulke, C.; Sander, C.; Jawinski, P.; Mauche, N.; Huang, J.; Spada, J.; Wittekind, D.; Mergl, R.; Luck, T.; Riedel-Heller, S.; et al. Sleep disturbances and upregulation of brain arousal during daytime in depressed versus non-depressed elderly subjects. World J. Biol. Psychiatry 2016, 18, 633–640. [Google Scholar] [CrossRef]
- Hegerl, U.; Wilk, K.; Olbrich, S.; Schoenknecht, P.; Sander, C. Hyperstable regulation of vigilance in patients with major depressive disorder. World J. Biol. Psychiatry 2011, 13, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Kalmbach, D.A.; Cheng, P.; Drake, C.L. A pathogenic cycle between insomnia and cognitive arousal fuels perinatal depression: Exploring the roles of nocturnal cognitive arousal and perinatal-focused rumination. Sleep 2021, 44. [Google Scholar] [CrossRef] [PubMed]
- Bassett, D. A literature review of heart rate variability in depressive and bipolar disorders. Aust. N. Z. J. Psychiatry 2015, 50, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Kemp, A.H.; Quintana, D.S.; Gray, M.A.; Felmingham, K.L.; Brown, K.; Gatt, J.M. Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biol. Psychiatry 2010, 67, 1067–1074. [Google Scholar] [CrossRef]
- Stapelberg, N.J.; Hamilton-Craig, I.; Neumann, D.L.; Shum, D.H.K.; McConnell, H. Mind and heart: Heart rate variability in major depressive disorder and coronary heart disease—A review and recommendations. Aust. N. Z. J. Psychiatry 2012, 46, 946–957. [Google Scholar] [CrossRef]
- Koch, C.; Wilhelm, M.; Salzmann, S.; Rief, W.; Euteneuer, F. A meta-analysis of heart rate variability in major depression. Psychol. Med. 2019, 49, 1948–1957. [Google Scholar] [CrossRef]
- Brown, L.; Karmakar, C.; Gray, R.; Jindal, R.; Lim, T.; Bryant, C. Heart rate variability alterations in late life depression: A meta-analysis. J. Affect. Disord. 2018, 235, 456–466. [Google Scholar] [CrossRef]
- Yang, A.C.; Tsai, S.-J.; Yang, C.-H.; Kuo, C.-H.; Chen, T.-J.; Hong, C.-J. Reduced physiologic complexity is associated with poor sleep in patients with major depression and primary insomnia. J. Affect. Disord. 2011, 131, 179–185. [Google Scholar] [CrossRef]
- Leistedt, S.J.-J.; Linkowski, P.; Lanquart, J.-P.; Mietus, J.E.; Davis, R.B.; Goldberger, A.L.; Costa, M.D. Decreased neuroautonomic complexity in men during an acute major depressive episode: Analysis of heart rate dynamics. Transl. Psychiatry 2011, 1, e27. [Google Scholar] [CrossRef]
- Saad, M.; Ray, L.B.; Bujaki, B.; Parvaresh, A.; Palamarchuk, I.; De Koninck, J.; Douglass, A.; Lee, E.K.; Soucy, L.J.; Fogel, S.; et al. Using heart rate profiles during sleep as a biomarker of depression. BMC Psychiatry 2019, 19, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pawlowski, M.A.; Gazea, M.; Wollweber, B.; Dresler, M.; Holsboer, F.; Keck, M.E.; Steiger, A.; Adamczyk, M.; Mikoteit, T. Heart rate variability and cordance in rapid eye movement sleep as biomarkers of depression and treatment response. J. Psychiatr. Res. 2017, 92, 64–73. [Google Scholar] [CrossRef]
- Kwon, H.B.; Yoon, H.; Choi, S.H.; Choi, J.-W.; Lee, Y.J.; Park, K.S. Heart rate variability changes in major depressive disorder during sleep: Fractal index correlates with BDI score during REM sleep. Psychiatry Res. 2018, 271, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Herbsleb, M.; Schumann, A.; Lehmann, L.; Gabriel, H.H.W.; Bär, K.-J. Cardio-respiratory fitness and autonomic function in patients with major depressive disorder. Front. Psychiatry 2020, 10. [Google Scholar] [CrossRef]
- Huang, M.; Shah, A.; Su, S.; Goldberg, J.; Lampert, R.J.; Levantsevych, O.M.; Shallenberger, L.; Pimple, P.; Bremner, J.D.; Vaccarino, V. Association of depressive symptoms and heart rate variability in Vietnam War-Era Twins: A longitudinal twin difference study. JAMA Psychiatry 2018, 75, 705–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jandackova, V.K.; Britton, A.; Malik, M.; Steptoe, A. Heart rate variability and depressive symptoms: A cross-lagged analysis over a 10-year period in the whitehall II study. Psychol. Med. 2016, 46, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Penninx, B.W.J.H. Depression and cardiovascular disease: Epidemiological evidence on their linking mechanisms. Neurosci. Biobehav. Rev. 2017, 74, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, V.; Caterino, A.L.; Bianco, F.; Caputi, C.G.; Salerni, S.; Sciomer, S.; Maffei, S.; Gallina, S. Depression and cardiovascular disease: The deep blue sea of women’s heart. Trends Cardiovasc. Med. 2019, 30, 170–176. [Google Scholar] [CrossRef]
- Kop, W.J.; Stein, P.K.; Tracy, R.P.; Barzilay, J.I.; Schulz, R.; Gottdiener, J.S. Autonomic nervous system dysfunction and inflammation contribute to the increased cardiovascular mortality risk associated with depression. Psychosom. Med. 2010, 72, 626–635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sgoifo, A.; Carnevali, L.; Alfonso, M.D.L.A.P.; Amore, M. Autonomic dysfunction and heart rate variability in depression. Stress 2015, 18, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Chen, D.; Yang, Y.; Zheng, Y.; Hui, R. Depression increases the risk of hypertension incidence: A meta-analysis of prospective cohort studies. J. Hypertens. 2012, 30, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Gan, Y.; Gong, Y.; Tong, X.; Sun, H.; Cong, Y.; Dong, X.; Wang, Y.; Xu, X.; Yin, X.; Deng, J.; et al. Depression and the risk of coronary heart disease: A meta-analysis of prospective cohort studies. BMC Psychiatry 2014, 14, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Kling, J.M. Depression and the risk of myocardial infarction and coronary death. Medicine 2016, 95. [Google Scholar] [CrossRef]
- Alvares, G.A.; Quintana, D.S.; Hickie, I.B.; Guastella, A.J. Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: A systematic review and meta-analysis. J. Psychiatry Neurosci. 2016, 41, 89–104. [Google Scholar] [CrossRef] [Green Version]
- Kemp, A.; Brunoni, A.R.; Santos, I.; Nunes, M.A.; Dantas, E.; De Figueiredo, R.C.; Pereira, A.C.; Ribeiro, A.; Mill, J.G.; Andreão, R.V.; et al. Effects of depression, anxiety, comorbidity, and antidepressants on resting-state heart rate and its variability: An ELSA-Brasil cohort baseline study. Am. J. Psychiatry 2014, 171, 1328–1334. [Google Scholar] [CrossRef] [Green Version]
- O’Regan, C.; Kenny, R.A.; Cronin, H.; Finucane, C.; Kearney, P.M. Antidepressants strongly influence the relationship between depression and heart rate variability: Findings from the Irish longitudinal study on ageing (TILDA). Psychol. Med. 2015, 45, 623–636. [Google Scholar] [CrossRef] [Green Version]
- Noordam, R.; van den Berg, M.E.; Niemeijer, M.N.; Aarts, N.; Hofman, A.; Tiemeier, H.; Kors, J.A.; Stricker, B.H.; Eijgelsheim, M.; Visser, L.E.; et al. Antidepressants and heart-rate variability in older adults: A population-based study. Psychol. Med. 2016, 46, 1239–1247. [Google Scholar] [CrossRef]
- Licht, C.M.M.; de Geus, E.J.C.; van Dyck, R.; Penninx, B.W.J.H. Longitudinal evidence for unfavorable effects of antidepressants on heart rate variability. Biol. Psychiatry 2010, 68, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Neyer, S.; Witthöft, M.; Cropley, M.; Pawelzik, M.; Lugo, R.G.; Sütterlin, S. Reduction of depressive symptoms during inpatient treatment is not associated with changes in heart rate variability. PLoS ONE 2021, 16, e0248686. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, Y.T.; Steffen, P.R. Adding HRV biofeedback to psychotherapy increases heart rate variability and improves the treatment of major depressive disorder. Int. J. Psychophysiol. 2018, 131, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Lin, I.-M.; Fan, S.-Y.; Yen, C.-F.; Yeh, Y.-C.; Tang, T.-C.; Huang, M.-F.; Liu, T.-L.; Wang, P.-W.; Lin, H.-C.; Tsai, H.-Y.; et al. Heart rate variability biofeedback increased autonomic activation and improved symptoms of depression and insomnia among patients with major depression disorder. Clin. Psychopharmacol. Neurosci. 2019, 17, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Pinter, A.; Szatmari, S., Jr.; Horvath, T.; Penzlin, A.I.; Barlinn, K.; Siepmann, M.; Siepmann, T. Cardiac dysautonomia in depression—heart rate variability biofeedback as a potential add-on therapy. Neuropsychiatr. Dis. Treat. 2019, 15, 1287–1310. [Google Scholar] [CrossRef] [Green Version]
- Chien, H.-C.; Chung, Y.-C.; Yeh, M.-L.; Lee, J.-F. Breathing exercise combined with cognitive behavioural intervention improves sleep quality and heart rate variability in major depression. J. Clin. Nurs. 2015, 24, 3206–3214. [Google Scholar] [CrossRef]
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [CrossRef]
- Gartlehner, G.; Hansen, R.A.; Morgan, L.C.; Thaler, K.; Lux, L.J.; Van Noord, M.; Mager, U.; Gaynes, B.N.; Thieda, P.; Strobelberger, M.; et al. Second-Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: An. Update of the 2007 Comparative Effectiveness Review, AHRQ Comparative Effectiveness Reviews; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2011. [Google Scholar]
- Pigott, H.E.; Leventhal, A.M.; Alter, G.S.; Boren, J.J. Efficacy and effectiveness of antidepressants: Current status of research. Psychother. Psychosom. 2010, 79, 267–279. [Google Scholar] [CrossRef]
- Li, H.; Qian, F.; Hou, C.; Li, X.; Gao, Q.; Luo, Y.; Tao, L.; Yang, X.; Wang, W.; Zheng, D.; et al. Longitudinal changes in depressive symptoms and risks of cardiovascular disease and all-cause mortality: A nationwide population-based cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 2200–2206. [Google Scholar] [CrossRef]
- Gilsanz, P.; Kubzansky, L.D.; Tchetgen, E.J.T.; Wang, Q.; Kawachi, I.; Patton, K.K.; Fitzpatrick, A.L.; Kop, W.J.; Longstreth, W.; Glymour, M.M. Changes in depressive symptoms and subsequent risk of stroke in the cardiovascular health study. Stroke 2017, 48, 43–48. [Google Scholar] [CrossRef]
- Gilsanz, P.; Walter, S.; Tchetgen, E.J.T.; Patton, K.K.; Moon, J.R.; Capistrant, B.D.; Marden, J.R.; Kubzansky, L.D.; Kawachi, I.; Glymour, M.M. Changes in depressive symptoms and incidence of first stroke among middle-aged and older US adults. J. Am. Heart Assoc. 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Carlsen, T.; Salvesen, Ø.; Sui, X.; Lavie, C.J.; Blair, S.N.; Wisløff, U.; Ernstsen, L. Long-term changes in depressive symptoms and estimated cardiorespiratory fitness and risk of all-cause mortality: The nord-trøndelag health study. Mayo Clin. Proc. 2018, 93, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Mirza, S.S.; Ikram, M.A.; Freak-Poli, R.; Hofman, A.; Rizopoulos, D.; Tiemeier, H. 12 year trajectories of depressive symptoms in community-dwelling older adults and the subsequent risk of death over 13 years. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xie, H.; Liu, M.; Wang, Z.; Zou, L.; Yeung, A.S.; Hui, S.S.; Yang, Q. The effects of Tai Chi on heart rate variability in older Chinese individuals with depression. Int. J. Environ. Res. Public Health 2018, 15, 2771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, I.-H.; Wu, W.-L.; Lin, I.-M.; Chang, Y.-K.; Lin, Y.-J.; Yang, P.-C. Effects of Yoga on heart rate variability and depressive symptoms in women: A randomized controlled trial. J. Altern. Complement. Med. 2017, 23, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Toni, G.; Murri, M.B.; Piepoli, M.; Zanetidou, S.; Cabassi, A.; Squatrito, S.; Bagnoli, L.; Piras, A.; Mussi, C.; Senaldi, R.; et al. Physical exercise for late-life depression: Effects on heart rate variability. Am. J. Geriatr. Psychiatry 2016, 24, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, J.A.; Sherwood, A.; Babyak, M.A.; Watkins, L.L.; Smith, P.J.; Hoffman, B.M.; O’Hayer, C.V.F.; Mabe, S.; Johnson, J.; Doraiswamy, P.M.; et al. Exercise and pharmacological treatment of depressive symptoms in patients with coronary heart disease. J. Am. Coll. Cardiol. 2012, 60, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Sandercock, G.R.H.; Bromley, P.D.; Brodie, D.A. Effects of exercise on heart rate variability: Inferences from meta-analysis. Med. Sci. Sports Exerc. 2005, 37, 433–439. [Google Scholar] [CrossRef]
- Raffin, J.; Barthélémy, J.-C.; Dupré, C.; Pichot, V.; Berger, M.; Féasson, L.; Busso, T.; Da Costa, A.; Colvez, A.; Montuy-Coquard, C.; et al. Exercise frequency determines heart rate variability gains in older people: A meta-analysis and meta-regression. Sports Med. 2019, 49, 719–729. [Google Scholar] [CrossRef]
- Nolan, R.P.; Jong, P.; Barry-Bianchi, S.M.; Tanaka, T.H.; Floras, J.S. Effects of drug, biobehavioral and exercise therapies on heart rate variability in coronary artery disease: A systematic review. Eur. J. Cardiovasc. Prev. Rehabilitation 2008, 15, 386–396. [Google Scholar] [CrossRef]
- Routledge, F.S.; Campbell, T.S.; McFetridge-Durdle, J.A.; Bacon, S.L. Improvements in heart rate variability with exercise therapy. Can. J. Cardiol. 2010, 26, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Brupbacher, G.; Gerger, H.; Zander-Schellenberg, T.; Straus, D.; Porschke, H.; Gerber, M.; von Känel, R.; Schmidt-Trucksäss, A. The effects of exercise on sleep in unipolar depression: A systematic review and network meta-analysis. Sleep Med. Rev. 2021, 59, 101452. [Google Scholar] [CrossRef] [PubMed]
- American Sleep Association Sleep Hygiene Tips. Available online: https://www.sleepassociation.org/about-sleep/sleep-hygiene-tips/ (accessed on 18 May 2021).
- Myllymäki, T.; Rusko, H.; Syväoja, H.; Juuti, T.; Kinnunen, M.-L.; Kyröläinen, H. Effects of exercise intensity and duration on nocturnal heart rate variability and sleep quality. Eur. J. Appl. Physiol. 2012, 112, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Shirakawa, K. Sleep onset is disrupted following pre-sleep exercise that causes large physiological excitement at bedtime. Eur. J. Appl. Physiol. 2014, 114, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Campo, D.J.; Ávila-Gandía, V.; Luque, A.J.; Rubio-Arias, J.Á. Effects of hour of training and exercise intensity on nocturnal autonomic modulation and sleep quality of amateur ultra-endurance runners. Physiol. Behav. 2018, 198, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Jones, H.; Whitworth-Turner, C.; Louis, J. High-intensity exercise in the evening does not disrupt sleep in endurance runners. Eur. J. Appl. Physiol. 2020, 120, 359–368. [Google Scholar] [CrossRef] [Green Version]
- Yuda, E.; Moriyama, Y.; Mori, T.; Yoshida, Y.; Kawahara, M.; Hayano, J. Acute effects of endurance exercise on nocturnal autonomic functions in sedentary subjects: A pilot study. J. Exerc. Rehabil. 2018, 14, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, A.R.L.; de Souza, K.A.; Dos Santos, K.M.; de Miranda, R.M.; Serra, A.J.; Peçanha, T.; Ferreira, J.C.; Cambri, L.T.; Arsa, G. Ambulatory heart rate variability in overweight and obese men after high-intensity interval exercise versus moderate-intensity continuous exercise. Eur. J. Sport Sci. 2021, 1–9. [Google Scholar] [CrossRef]
- Peçanha, T.; Prodel, E.; Bartels, R.; Nasario-Junior, O.; Paula, R.B.; Silva, L.P.; Laterza, M.C.; Lima, J.R.P. 24-h cardiac autonomic profile after exercise in sedentary subjects. Int. J. Sports Med. 2014, 35, 245–252. [Google Scholar] [CrossRef]
- Hynynen, E.; Vesterinen, V.; Rusko, H.; Nummela, A. Effects of moderate and heavy endurance exercise on nocturnal HRV. Int. J. Sports Med. 2010, 31, 428–432. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Brupbacher, G.; Straus, D.; Porschke, H.; Zander-Schellenberg, T.; Gerber, M.; von Känel, R.; Schmidt-Trucksäss, A. The acute effects of aerobic exercise on sleep in patients with depression: Study protocol for a randomized controlled trial. Trials 2019, 20, 352. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [Green Version]
- Quintana, D.S.; Alvares, G.A.; Heathers, J.a.J. Guidelines for reporting articles on psychiatry and heart rate variability (GRAPH): Recommendations to advance research communication. Transl. Psychiatry 2016, 6, e803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brupbacher, G.; Zander-Schellenberg, T.; Straus, D.; Porschke, H.; Infanger, D.; Gerber, M.; von Känel, R.; Schmidt-Trucksäss, A. The acute effects of aerobic exercise on sleep in patients with unipolar depression: A randomized controlled trial. Sleep 2021, zsab177. [Google Scholar] [CrossRef] [PubMed]
- American College of Sports Medicine. ACSM’s Guidelines for Exercise Testing and Prescription, 8th ed.; LWW: Philadelphia, PA, USA, 2009; ISBN 978-0-7817-6902-0. [Google Scholar]
- Stiasny-Kolster, K.; Möller, J.C.; Heinzel-Gutenbrunner, M.; Baum, E.; Ries, V.; Oertel, W.H. Validation of the Restless Legs Syndrome Screening Questionnaire (RLSSQ). Somnologie 2009, 13, 37–42. [Google Scholar] [CrossRef]
- Kroenke, K.; Spitzer, R.L.; Williams, J.B. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosom. Med. 2002, 64, 258–266. [Google Scholar] [CrossRef] [Green Version]
- Obbarius, A.; van Maasakkers, L.; Baer, L.; Clark, D.M.; Crocker, A.G.; de Beurs, E.; Emmelkamp, P.M.G.; Furukawa, T.A.; Hedman-Lagerlöf, E.; Kangas, M.; et al. Standardization of health outcomes assessment for depression and anxiety: Recommendations from the ICHOM depression and anxiety working group. Qual. Life Res. 2017, 26, 3211–3225. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, C.; Buss, U.; Snaith, R.P. HADS-D—Hospital Anxiety and Depression Scale-Deutsche Version (HADS-D—Hospital Anxiety and Depression Scale—German Version); Huber: Bern, Switzerland, 2011. [Google Scholar]
- Brennan, C.; Worrall-Davies, A.; McMillan, D.; Gilbody, S.; House, A. The hospital anxiety and depression scale: A diagnostic meta-analysis of case-finding ability. J. Psychosom. Res. 2010, 69, 371–378. [Google Scholar] [CrossRef]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Backhaus, J.; Riemann, D. Schlafstörungen Bewältigen [Coping with Sleep Disorders]; Beltz Psychology Verlags Union: Weinheim, Germany, 1996. [Google Scholar]
- Chiu, H.-Y.; Chang, L.-Y.; Hsieh, Y.-J.; Tsai, P.-S. A meta-analysis of diagnostic accuracy of three screening tools for insomnia. J. Psychosom Res. 2016, 87, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.; Richardson, G.; Roehrs, T.; Scofield, H.; Roth, T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep 2004, 27, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Kalmbach, D.A.; Pillai, V.; Arnedt, J.T.; Drake, C.L. Identifying at-risk individuals for insomnia using the ford insomnia response to stress test. Sleep 2016, 39, 449–456. [Google Scholar] [CrossRef] [Green Version]
- Dieck, A.; Helbig, S.; Drake, C.L.; Backhaus, J. Validation of the German version of the ford insomnia response to stress test. J. Sleep Res. 2018, 27, e12621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, C.; Brand, S.; Holsboer-Trachsler, E.; Pühse, U.; Colledge, F.; Gerber, M. Validation of the German version of the short form of the dysfunctional beliefs and attitudes about sleep scale (DBAS-16). Neurol. Sci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Dickhuth, H.H.; Yin, L.; Niess, A.; Röcker, K.; Mayer, F.; Heitkamp, H.C.; Horstmann, T. Ventilatory, lactate-derived and catecholamine thresholds during incremental treadmill running: Relationship and reproducibility. Int J. Sports Med. 1999, 20, 122–127. [Google Scholar] [CrossRef] [PubMed]
- O’Callaghan, C.A. OxMaR: Open source free software for online minimization and randomization for clinical trials. PLoS ONE 2014, 9, e110761. [Google Scholar] [CrossRef] [Green Version]
- Kredlow, M.A.; Capozzoli, M.C.; Hearon, B.A.; Calkins, A.W.; Otto, M.W. The effects of physical activity on sleep: A meta-analytic review. J. Behav. Med. 2015, 38, 427–449. [Google Scholar] [CrossRef]
- Reis, C.; Dias, S.; Rodrigues, A.M.; Sousa, R.D.; Gregório, M.J.; Branco, J.; Canhão, H.; Paiva, T. Sleep duration, lifestyles and chronic diseases: A cross-sectional population-based study. Sleep Sci. 2018, 11, 217–230. [Google Scholar] [CrossRef]
- Buman, M.P.; Hekler, E.B.; King, A.C.; Bliwise, D.L. Moderators and mediators of exercise-induced objective sleep improvements in midlife and older adults with sleep complaints. Health Psychol. 2011, 30, 579–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, A.-W.; Tetzlaff, J.M.; Altman, D.G.; Laupacis, A.; Gøtzsche, P.C.; Krleža-Jerić, K.; Hróbjartsson, A.; Mann, H.; Dickersin, K.; Berlin, J.A.; et al. SPIRIT 2013 statement: Defining standard protocol items for clinical trials. Ann. Intern. Med. 2013, 158, 200–207. [Google Scholar] [CrossRef] [Green Version]
- Haskell, W.L.; Lee, I.-M.; Pate, R.R.; Powell, K.E.; Blair, S.N.; Franklin, B.A.; Macera, C.A.; Heath, G.W.; Thompson, P.D.; Bauman, A.; et al. Physical activity and public health: Updated recommendation for adults from the American college of sports medicine and the American Heart Association. Circulation 2007, 116, 1081–1093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Höchsmann, C.; Knaier, R.; Eymann, J.; Hintermann, J.; Infanger, D.; Schmidt-Trucksäss, A. Validity of activity trackers, smartphones, and phone applications to measure steps in various walking conditions. Scand. J. Med. Sci Sports 2018. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.L.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events; American Academy of Sleep Medicine: Darien, IL, USA, 2012. [Google Scholar]
- Kendrick, A.H.; Hardyman, M.; Pickersgill, R.; Pillinger, N.; Santos, B. Quality assurance of full polysomnography scoring using the American Academy of Sleep Medicine (AASM) Inter-Scorer Reliability (ISR) program. Eur. Respir. J. 2017, 50. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef]
- Rajalakshmi, R.; Akila, B.; Tharion, E. Intra-class correlation among heart rate variability analysis softwares across different physiological postures. Indian J. Physiol. Pharmacol. 2015, 59, 2–8. [Google Scholar] [PubMed]
- Pan, J.; Tompkins, W.J. A real-time QRS detection algorithm. IEEE Trans. Biomed. Eng. 1985, 32, 230–236. [Google Scholar] [CrossRef]
- Lipponen, J.A.; Tarvainen, M.P. A robust algorithm for heart rate variability time series artefact correction using novel beat classification. J. Med. Eng. Technol. 2019, 43, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Tarvainen, M.P.; Ranta-aho, P.O.; Karjalainen, P.A. An advanced detrending method with application to HRV analysis. IEEE Trans. Biomed. Eng. 2002, 49, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Lomb, N.R. Least-squares frequency analysis of unequally spaced data. Astrophys. Space Sci. 1976, 39, 447–462. [Google Scholar] [CrossRef]
- Scargle, J.D. Studies in astronomical time series analysis. II-statistical aspects of spectral analysis of unevenly spaced data. Astrophys. J. 1982, 263, 835–853. [Google Scholar] [CrossRef]
- Laguna, P.; Moody, G.B.; Mark, R.G. Power spectral density of unevenly sampled data by least-square analysis: Performance and application to heart rate signals. IEEE Trans. Biomed. Eng. 1998, 45, 698–715. [Google Scholar] [CrossRef]
- Saini, B.S.; Singh, D.; Uddin, M.; Kumar, V. Improved power spectrum estimation for RR-interval time series. World Acad. Sci. Eng. Tech. 2008, 46, 44–48. [Google Scholar]
- Singh, D.; Vinod, K.; Saxena, S.C.; Deepak, K.K. Effects of RR segment duration on HRV spectrum estimation. Physiol. Meas. 2004, 25, 721–735. [Google Scholar] [CrossRef]
- Burr, R.L.; Cowan, M.J. Autoregressive spectral models of heart rate variability. Practical issues. J. Electrocardiol. 1992, 25, 224–233. [Google Scholar] [CrossRef]
- Moody, G.B. Spectral analysis of heart rate without resampling. In Proceedings of the Computers in Cardiology Conference, London, UK, 5–8 September 1993; pp. 715–718. [Google Scholar]
- Chang, K.L.; Monahan, K.J.; Griffin, M.P.; Lake, D.; Moorman, J.R. Comparison and clinical application of frequency domain methods in analysis of neonatal heart rate time series. Ann. Biomed. Eng. 2001, 29, 764–774. [Google Scholar] [CrossRef] [PubMed]
- Clifford, G.D.; Tarassenko, L. Quantifying errors in spectral estimates of HRV due to beat replacement and resampling. IEEE Trans. Biomed. Eng. 2005, 52, 630–638. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Yadav, R.; Yung, I.; Zajdel, D.P.; Oken, B.S. Sensitivity to mental effort and test-retest reliability of heart rate variability measures in healthy seniors. Clin. Neurophysiol. 2011, 122, 2059–2066. [Google Scholar] [CrossRef]
- Delane, A.; Bohorquez, J.; Gupta, S.; Schiavenato, M. Lomb algorithm versus fast fourier transform in heart rate variability analyses of pain in premature infants. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2016, 2016, 944–947. [Google Scholar] [CrossRef]
- Thong, T.; Yung, I.O.; Zajdel, D.P.; Ellingson, R.M.; McNames, J.; Aboy, M.; Oken, B.S. Heart rate variability analysis of effect of nicotine using periodograms. Conf. Proc. IEEE Eng. Med. Biol Soc. 2004, 1, 294–297. [Google Scholar] [CrossRef]
- Electrophysiology, Task Force of the European Society of Cardiology the North American Society of Pacing. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Circulation 1996, 93, 1043–1065. [Google Scholar] [CrossRef] [Green Version]
- Burr, R.L. Interpretation of normalized spectral heart rate variability indices in sleep research: A critical review. Sleep 2007, 30, 913–919. [Google Scholar] [CrossRef] [Green Version]
- Laborde, S.; Mosley, E.; Thayer, J.F. Heart rate variability and cardiac vagal tone in psychophysiological research—Recommendations for experiment planning, data analysis, and data reporting. Front. Psychol. 2017, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicassio, P.M.; Mendlowitz, D.R.; Fussell, J.J.; Petras, L. The phenomenology of the pre-sleep state: The development of the pre-sleep arousal scale. Behav. Res. Ther. 1985, 23, 263–271. [Google Scholar] [CrossRef]
- Gieselmann, A.; de Jong-Meyer, R.; Pietrowsky, R. Kognitive und körperliche erregung in der phase vor dem einschlafen. Z. Klinische Psychol. Psychother. 2012, 41, 73–80. [Google Scholar] [CrossRef]
- Vickers, A.J.; Altman, D.G. Analysing controlled trials with baseline and follow up measurements. BMJ 2001, 323, 1123–1124. [Google Scholar] [CrossRef] [Green Version]
- Kahan, B.C.; Morris, T.P. Reporting and analysis of trials using stratified randomisation in leading medical journals: Review and reanalysis. BMJ 2012, 345, e5840. [Google Scholar] [CrossRef] [Green Version]
- Long, J.S.; Ervin, L.H. Using heteroscedasticity consistent standard errors in the linear regression model. Ame. Stat. 2000, 54, 217–224. [Google Scholar] [CrossRef]
- Van Buuren, S.; Groothuis-Oudshoorn, K. Mice: Multivariate imputation by chained equations in R. J. Stat. Softw. 2011, 45, 1–67. [Google Scholar] [CrossRef] [Green Version]
- Little, R.J.A. A test of missing completely at random for multivariate data with missing values. J. Am. Stat. Assoc. 1988, 83, 1198–1202. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Myllymäki, T.; Kyröläinen, H.; Savolainen, K.; Hokka, L.; Jakonen, R.; Juuti, T.; Martinmäki, K.; Kaartinen, J.; Kinnunen, M.-L.; Rusko, H. Effects of vigorous late-night exercise on sleep quality and cardiac autonomic activity. J. Sleep Res. 2011, 20, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Al Haddad, H.; Laursen, P.B.; Ahmaidi, S.; Buchheit, M. Nocturnal heart rate variability following supramaximal intermittent exercise. Int. J. Sports Physiol. Perform. 2009, 4, 435–447. [Google Scholar]
- Yamanaka, Y.; Hashimoto, S.; Takasu, N.N.; Tanahashi, Y.; Nishide, S.-Y.; Honma, S.; Honma, K.-I. Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 309, R1112–R1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hautala, A.; Tulppo, M.P.; Mäkikallio, T.H.; Laukkanen, R.; Nissilä, S.; Huikuri, H.V. Changes in cardiac autonomic regulation after prolonged maximal exercise. Clin. Physiol. 2001, 21, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, V.P.; de Oliveira, N.A.; Silveira, H.; Mello, R.G.T.; Deslandes, A.C. Heart rate variability indexes as a marker of chronic adaptation in athletes: A systematic review. Ann. Noninvasive Electrocardiol. 2014, 20, 108–118. [Google Scholar] [CrossRef]
- Riemann, D.; Baglioni, C.; Bassetti, C.; Bjorvatn, B.; Dolenc Groselj, L.; Ellis, J.G.; Espie, C.A.; Garcia-Borreguero, D.; Gjerstad, M.; Gonçalves, M.; et al. European guideline for the diagnosis and treatment of insomnia. J. Sleep Res. 2017, 26, 675–700. [Google Scholar] [CrossRef]
- Jansson-Fröjmark, M.; Norell-Clarke, A. The cognitive treatment components and therapies of cognitive behavioral therapy for insomnia: A systematic review. Sleep Med. Rev. 2018, 42, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, M.; Hayano, J.; Oikawa, L.O.; Katsamanis, M.; Lehrer, P. Heart rate variability biofeedback improves cardiorespiratory resting function during sleep. Appl. Psychophysiol. Biofeedback 2013, 38, 265–271. [Google Scholar] [CrossRef]
- De Zambotti, M.; Sizintsev, M.; Claudatos, S.; Barresi, G.; Colrain, I.M.; Baker, F.C. Reducing bedtime physiological arousal levels using immersive audio-visual respiratory bio-feedback: A pilot study in women with insomnia symptoms. J. Behav. Med. 2019, 42, 973–983. [Google Scholar] [CrossRef]
- Ebben, M.R.; Kurbatov, V.; Pollak, C.P. Moderating laboratory adaptation with the use of a heart-rate variability biofeedback device (StressEraser). Appl. Psychophysiol. Biofeedback 2009, 34, 245–249. [Google Scholar] [CrossRef]
- Stutz, J.; Eiholzer, R.; Spengler, C.M. Effects of evening exercise on sleep in healthy participants: A systematic review and meta-analysis. Sports Med. 2019, 49, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Appelhans, B.M.; Luecken, L.J. Heart rate variability as an index of regulated emotional responding. Rev. Gen. Psychol. 2006, 10, 229–240. [Google Scholar] [CrossRef] [Green Version]
- Beauchaine, T.P.; Thayer, J.F. Heart rate variability as a transdiagnostic biomarker of psychopathology. Int. J. Psychophysiol. 2015, 98, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Khalsa, S.S.; Lapidus, R.C. Can interoception improve the pragmatic search for biomarkers in psychiatry? Front. Psychiatry 2016, 7, 121. [Google Scholar] [CrossRef] [PubMed]
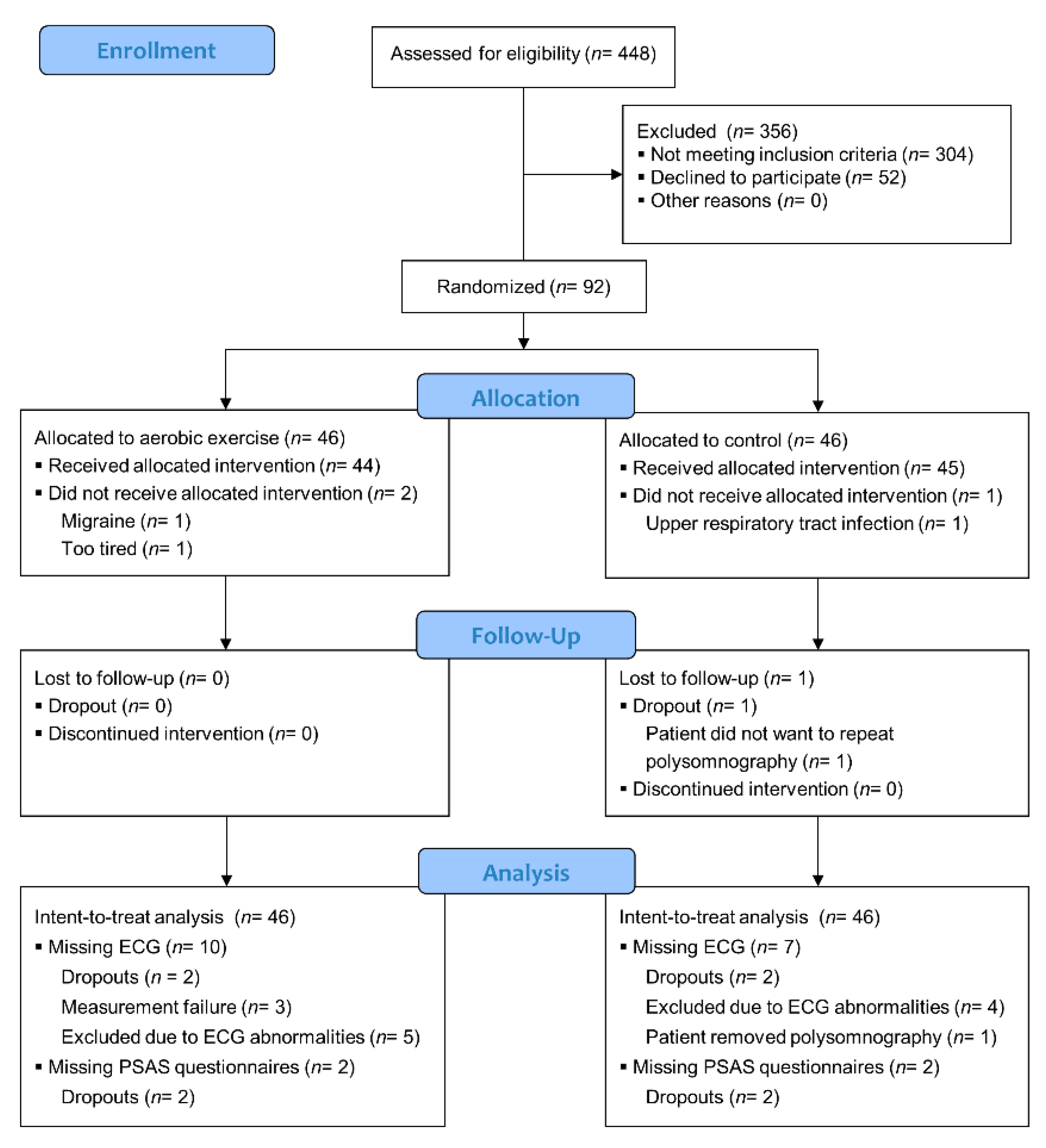
| Intervention Group (N = 46) | Control Group (N = 46) | ||
|---|---|---|---|
| Age | 46 (37, 53) | 48 (43, 51) | |
| Sex | female | 32 (70) | 33 (72) |
| male | 14 (30) | 13 (28) | |
| BMI | 25.0 (21.8, 29.2) | 24.4 (22.2, 27.7) | |
| Systolic blood pressure (mmHg) | 121 (115, 131) | 121 (112, 134) | |
| Diastolic blood pressure (mmHg) | 80 (74, 88) | 81 (72, 87) | |
| Smoking | never smoked | 26 (57) | 24 (52) |
| stopped smoking since ≥12 months | 8 (17) | 10 (22) | |
| currently smoking or stopped since <12 months | 12 (26) | 12 (26) | |
| Alcohol | less than 1 day/week | 5 (15) | 6 (18) |
| 1–2 days/week | 14 (41) | 6 (18) | |
| 3–6 days/week | 7 (21) | 12 (37) | |
| daily | 8 (23) | 9 (27) | |
| PHQ15 | 12.5 (8.3, 15.8) | 12.5 (8.0, 14.0) | |
| PHQ9 | 14.0 (12.0, 17.0) | 15.0 (12.0, 17.0) | |
| HADS anxiety | 11.0 (9.3, 14.0) | 11.5 (9.0, 14.0) | |
| Antidepressant medication | 19 (41) | 21 (50) | |
| DBAS | 4.6 (3.6, 5.7) | 4.8 (3.8, 5.4) | |
| FIRST | 27.0 (24.0, 29.8) | 27.5 (21.3, 29.8) | |
| PSQI | 10.0 (7.00, 13.8) | 9.5 (6.3, 12.0) | |
| Sleep efficiency a | 91.3 (84.4, 93.5) | 88.8 (82.5, 94.0) | |
| Total sleep time a | 439.0 (393.3, 479.5) | 416.7 (382.3, 463.1) | |
| Sleep onset latency a | 14.0 (5.5, 23.3) | 14.5 (6.8, 27.2) | |
| Wake after sleep onset a | 30.8 (18.0, 43.3) | 37.8 (19.1, 62.6) | |
| Number of awakenings a | 17.0 (13.5, 23.5) | 15.8 (11.4, 20.8) | |
| RMSSD | 32.1 (20.7, 49.0) | 34.4 (24.5, 51.4) | |
| SDNN | 37.8 (27.2, 55.0) | 34.7 (29.5, 52.6) | |
| LF (ms2) | 858 (474, 1659) | 780 (436, 1852) | |
| HF (ms2) | 382 (159, 925) | 410 (253, 810) | |
| Somatic pre-sleep arousal | 10.0 (9.0, 12.8) | 12.0 (9.0, 15.0) | |
| Cognitive pre-sleep arousal | 15.0 (13.3, 18.0) | 15.0 (11.0, 20.0) | |
| Term | 95% Confidence Interval | p-Value | |||
|---|---|---|---|---|---|
| Intercept | 6.78 | 6.03 | −5.42 | 18.98 | 0.27 |
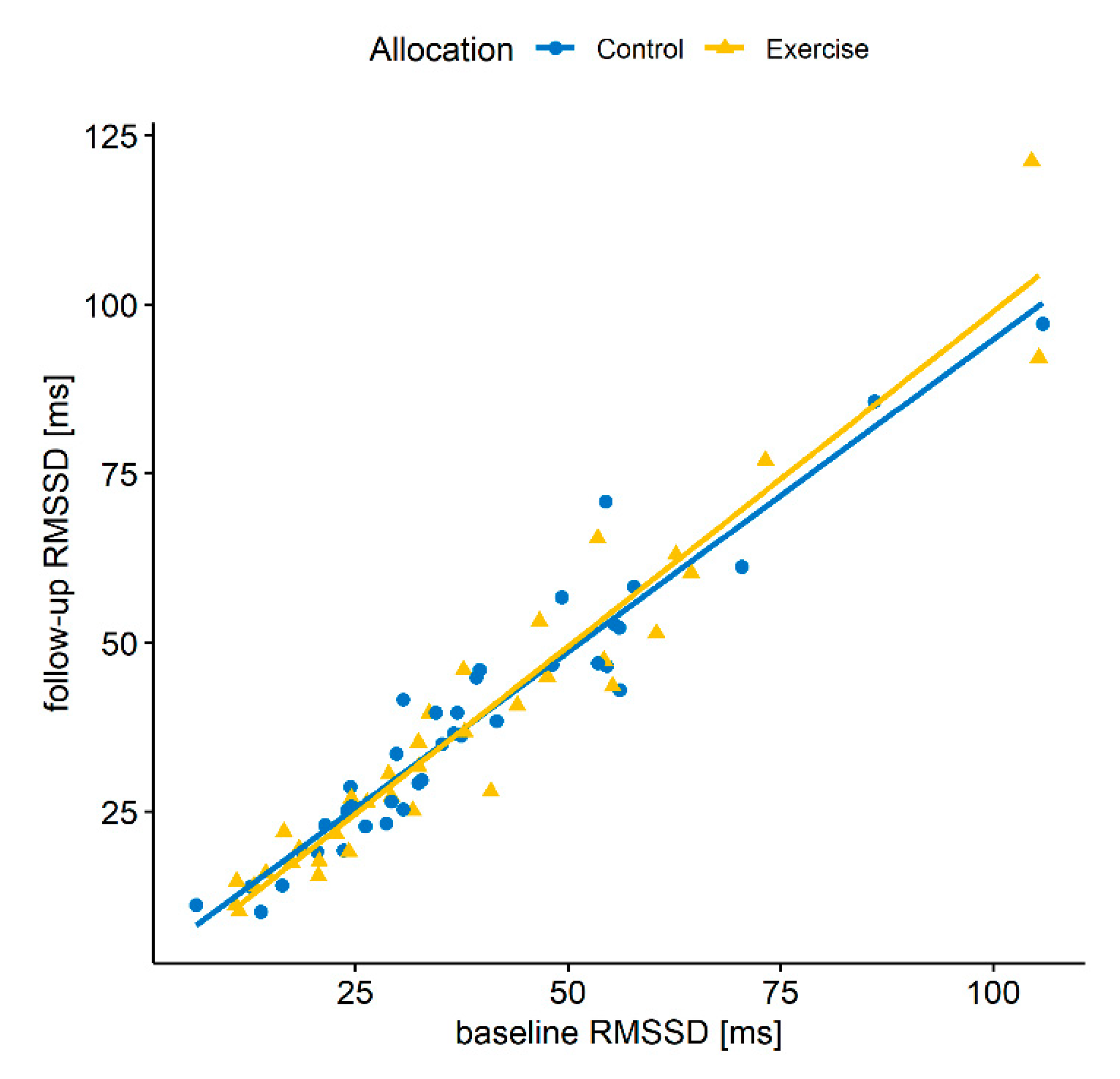
| Baseline RMSSD | 0.93 | 0.04 | 0.84 | 0.84 | 0.001 |
| Age | −0.10 | 0.10 | −0.30 | 0.10 | 0.61 |
| Sex (male a) | 1.21 | 1.70 | −2.22 | 4.64 | 0.48 |
| PHQ9 | 0.08 | 0.17 | −0.26 | 0.42 | 0.64 |
| PSQI | −0.16 | 0.18 | −0.51 | 0.19 | 0.37 |
| Allocation (exercise b) | 0.12 | 1.53 | −2.98 | 3.22 | 0.94 |
| Outcome | 95% Confidence Interval | p-Value | |||
|---|---|---|---|---|---|
| SDNN | −0.27 | 1.78 | −4.08 | 3.53 | 0.88 |
| LF | −130 | 260 | −791 | 531 | 0.64 |
| HF | 16 | 75 | −135 | 167 | 0.83 |
| LF/HF-ratio | −0.17 | 0.34 | −0.85 | 0.51 | 0.62 |
| Sleep Stage | 95% Confidence Interval | p-Value | |||
|---|---|---|---|---|---|
| N2 | −0.69 | 4.75 | −10.41 | 9.02 | 0.88 |
| N3 | −7.15 | 7.11 | −23.10 | 8.81 | 0.34 |
| non-REM | 0.29 | 1.81 | −3.32 | 3.90 | 0.87 |
| REM | −4.93 | 8.04 | −22.42 | 12.55 | 0.55 |
| Outcome | 95% Confidence Interval | p-Value | |||
|---|---|---|---|---|---|
| RMSSD | 0.21 | 2.61 | −4.99 | 5.41 | 0.94 |
| SDNN | −1.00 | 2.58 | −6.15 | 4.15 | 0.70 |
| LF | 34 | 155 | −274 | 343 | 0.83 |
| HF | 100 | 130 | −158 | 358 | 0.44 |
| LF/HF-ratio | 1.33 | 1.05 | −0.76 | 3.43 | 0.21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brupbacher, G.; Zander-Schellenberg, T.; Straus, D.; Porschke, H.; Infanger, D.; Gerber, M.; von Känel, R.; Schmidt-Trucksäss, A. The Acute Effects of Aerobic Exercise on Nocturnal and Pre-Sleep Arousal in Patients with Unipolar Depression: Preplanned Secondary Analysis of a Randomized Controlled Trial. J. Clin. Med. 2021, 10, 4028. https://doi.org/10.3390/jcm10174028
Brupbacher G, Zander-Schellenberg T, Straus D, Porschke H, Infanger D, Gerber M, von Känel R, Schmidt-Trucksäss A. The Acute Effects of Aerobic Exercise on Nocturnal and Pre-Sleep Arousal in Patients with Unipolar Depression: Preplanned Secondary Analysis of a Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(17):4028. https://doi.org/10.3390/jcm10174028
Chicago/Turabian StyleBrupbacher, Gavin, Thea Zander-Schellenberg, Doris Straus, Hildburg Porschke, Denis Infanger, Markus Gerber, Roland von Känel, and Arno Schmidt-Trucksäss. 2021. "The Acute Effects of Aerobic Exercise on Nocturnal and Pre-Sleep Arousal in Patients with Unipolar Depression: Preplanned Secondary Analysis of a Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 17: 4028. https://doi.org/10.3390/jcm10174028
APA StyleBrupbacher, G., Zander-Schellenberg, T., Straus, D., Porschke, H., Infanger, D., Gerber, M., von Känel, R., & Schmidt-Trucksäss, A. (2021). The Acute Effects of Aerobic Exercise on Nocturnal and Pre-Sleep Arousal in Patients with Unipolar Depression: Preplanned Secondary Analysis of a Randomized Controlled Trial. Journal of Clinical Medicine, 10(17), 4028. https://doi.org/10.3390/jcm10174028








