Transcatheter Tricuspid Valve-in-Valve Procedure—An Illustrative Case Report and Review
Abstract
:1. Introduction
2. Imaging Assessment of the TV
2.1. Echocardiography
2.2. Cardiac Computed Tomography
2.3. Cardiac Magnetic Resonance
3. Transcatheter Tricuspid Anatomic Challenges
4. Transcatheter Tricuspid Valve Repair or Replacement
5. Transcatheter Tricuspid Valve Replacement
5.1. Orthotopic Valve Replacement
5.2. Heterotopic Valve Replacement
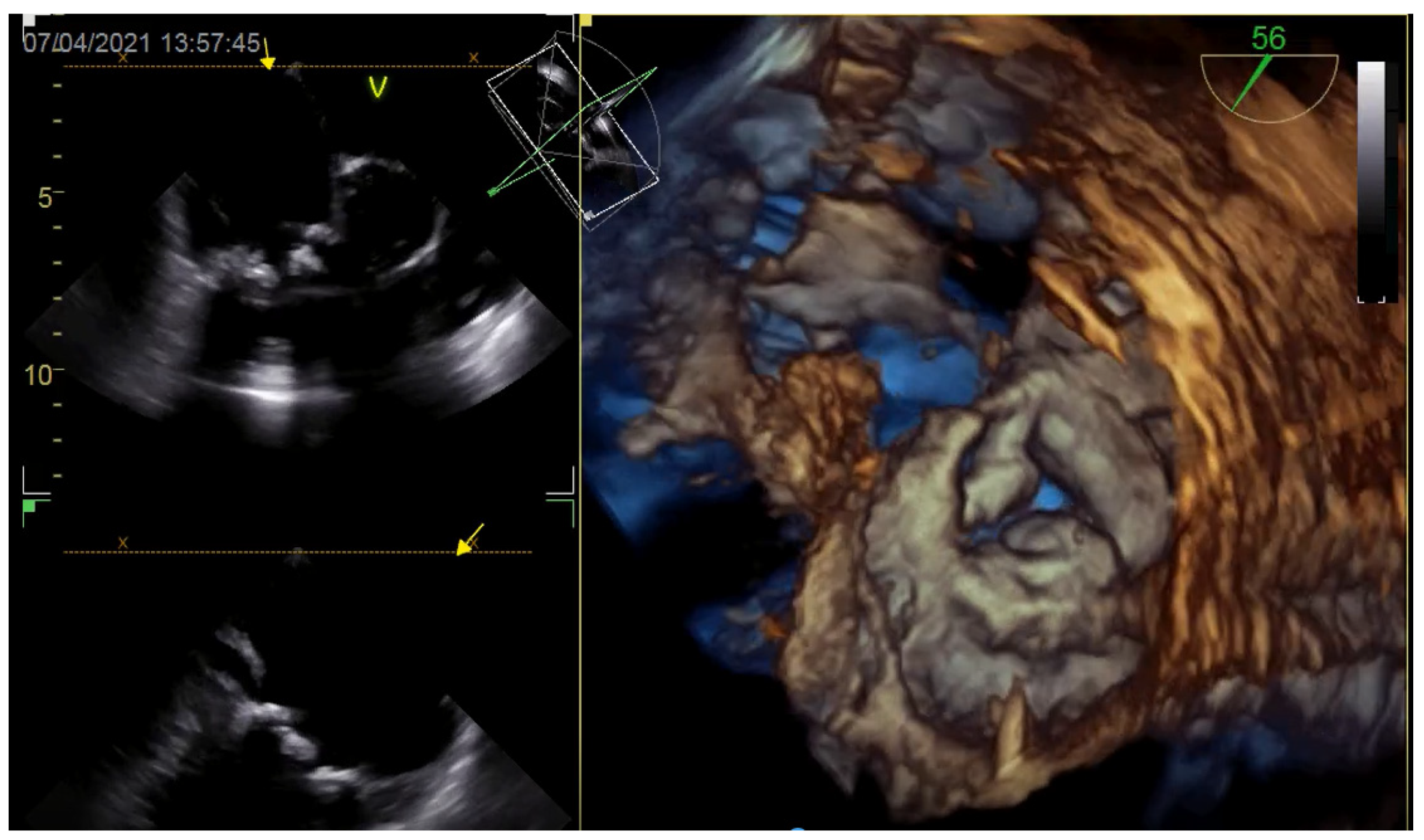
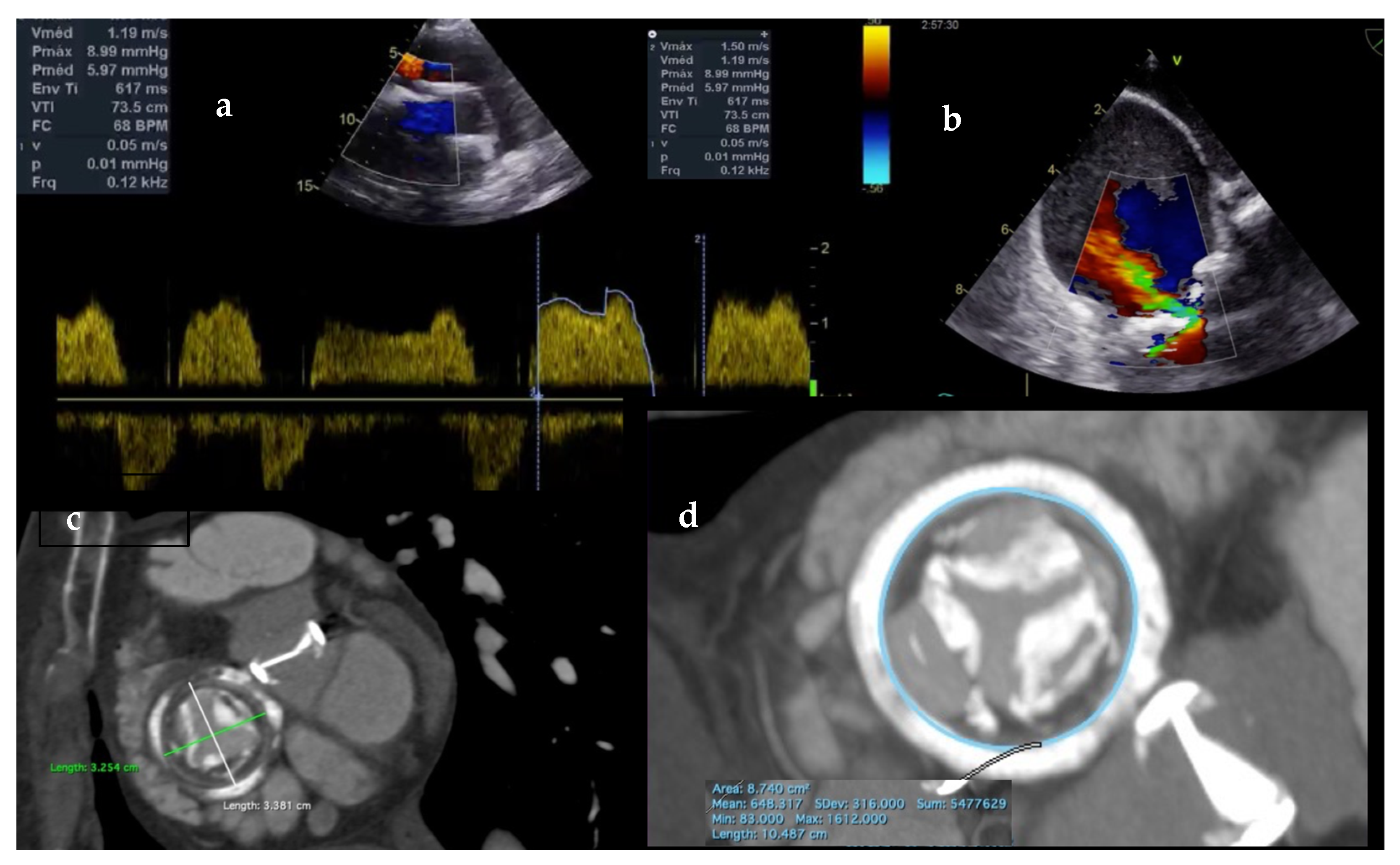
6. Case Report
6.1. Procedure
6.2. Clinical Course
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dahou, A.; Levin, D. Anatomy and physiology of the tricuspid valve. JACC Cardiovasc. Imaging 2019, 12, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T. State-of-the-art review of echocardiographic imaging in the evaluation and treatment of functional tricuspid regurgitation. Circ Cardiovasc. Imaging 2016, 9, e005332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, P.M.; Raney, A.A. Tricuspid Valve Disease. Curr. Probl. Cardiol. 2008, 33, 47–84. [Google Scholar] [CrossRef]
- Taramasso, M.; Gavazzoni, M.; Pozzoli, A.; Dreyfus, G.D.; Bolling, S.F.; George, I.; Kapos, I.; Tanner, F.C.; Zuber, M.; Maisano, F.; et al. Tricuspid regurgitation: Predicting the need for intervention, procedural success, and recurrence of disease. JACC Cardiovasc. Imaging 2019, 12, 605–621. [Google Scholar] [CrossRef]
- Golamari, R.; Bhattacharya, P.T. Tricuspid Stenosis. StatPearls. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499990/ (accessed on 12 July 2021).
- Sadeghpour, A.; Hassanzadeh, M. Impact of severe tricuspid regurgitation on long term survival. Res. Cardiovasc. Med. 2013, 2, 121. [Google Scholar] [CrossRef]
- Zack, C.J.; Fender, E.A. National Trends and Outcomes in Isolated Tricuspid Valve Surgery. J. Am. Coll. Cardiol. 2017, 70, 2953–2960. [Google Scholar] [CrossRef]
- Sánchez-Espín, G. Rodríguez-Capitán. Outcomes of Isolated Tricuspid Valve Surgery. Heart Surg. Forum 2020, 23, E763–E769. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.; Berzingi, C.O. Contemporary trends in the use and outcomes of surgical treatment of tricuspid regurgitation. J. Am. Heart Assoc. 2017, 6, e007597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Axtell, A.L.; Bhambhani, V. Surgery does not improve survival in patients with isolated severe tricuspid regurgitation. JACC 2019, 74, 715–725. [Google Scholar] [CrossRef]
- Leurent, G.; Collet, J.P. The Tricuspid Valve: No Longer the Forgotten Valve! JACC Cardiovasc. Interv. 2019, 2, 2496–2498. [Google Scholar] [CrossRef]
- Nath, J.; Foster, E. Impact of tricuspid regurgitation on long-term survival. J. Am. Coll. Cardiol. 2004, 43.3, 405–409. [Google Scholar] [CrossRef] [Green Version]
- Neuhold, S.; Huelsmann, M. Impact of tricuspid regurgitation on survival in patients with chronic heart failure: Unexpected findings of a long-term observational study. Eur. Heart J. 2013, 34, 844–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taramasso, M.; Maisano, F. Prognostic impact and late evolution of untreated moderate (2/4+) functional tricuspid regurgitation in patients undergoing aortic valve replacement. J. Card. Surg. 2016, 31, 9–14. [Google Scholar] [CrossRef] [Green Version]
- Benfari, G.; Antoine, C. Excess mortality associated with functional tricuspid regurgitation complicating heart failure with reduced ejection fraction. Circulation 2019, 140, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Dreyfus, J.; Ghalem, N. Timing of referral of patients with severe isolated tricuspid valve regurgitation to surgeons (from a French Nationwide Database). Am. J. Cardiol. 2018, 122, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Nishimura, R.A. ACC/AHA guideline for the management of patients with valvular heart disease: Executive summary: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 77, 450–500. [Google Scholar] [CrossRef]
- Falk, V. Baumgartner. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. J. Cardiothorac. Surg. 2017, 52, 616–664. [Google Scholar] [CrossRef]
- Hamandi, M.; Smith, R.L. Outcomes of isolated tricuspid valve surgery have improved in the modern era. Ann. Thorac. Surg. 2019, 108, 11–15. [Google Scholar] [CrossRef]
- Chen, J.; Hu, K. Isolated reoperation for tricuspid regurgitation after left-sided valve surgery: Technique evolution. Eur. J. Cardiothorac. Surg. 2020, 57, 142–150. [Google Scholar] [CrossRef]
- Buzzatti, N.; Iaci, G. Long-term outcomes of tricuspid valve replacement after previous left-side heart surgery. Eur. J. Cardiothorac. Surg. 2014, 46, 713–719. [Google Scholar] [CrossRef] [Green Version]
- Asmarats, L.; Puri, R. Transcatheter tricuspid valve interventions: Landscape, challenges, and future directions. J. Am. Coll. Cardiol. 2018, 71, 2935–2956. [Google Scholar] [CrossRef]
- Calvert, P.A.; Himbert, D. Transfemoral implantation of an Edwards SAPIEN valve in a tricuspid bio-prosthesis without fluoroscopic landmarks. EuroIntervention 2012, 7, 1336–1339. [Google Scholar] [CrossRef]
- Hoendermis, E.S.; Douglas, Y.L. Percutaneous Edwards SAPIEN valve implantation in the tricuspid position: Case report and review of literature. EuroIntervention 2012, 8, 628–633. [Google Scholar] [CrossRef]
- Taramasso, M.; Nietlispach, F. Transfemoral tricuspid valve-in-valve implantation: Snare it to make it simpler! EuroIntervention 2016, 12, 402. [Google Scholar] [CrossRef] [PubMed]
- McElhinney, D.B.; Cabalka, A.K. Transcatheter Tricuspid Valve-in-Valve Implantation for the Treatment of Dysfunctional Surgical Bioprosthetic Valves: An International, Multicenter Registry Study. Valve-in-Valve International Database (VIVID) Registry. Circulation 2016, 133, 1582–1593. [Google Scholar] [CrossRef] [PubMed]
- Taramasso, M.; Maisano, F. Transcatheter tricuspid valve intervention: State of the art. EuroIntervention 2017, 13, AA40–AA50. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, W.A.; Adams, D. Recommendations for noninvasive evaluation of native valvular regurgitation: A report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Vieitez, J.M.; Monteagudo, J.M. New insights of tricuspid regurgitation: A large-scale prospective cohort study. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 196–202. [Google Scholar] [CrossRef]
- Taramasso, M.; Alessandrini, H. Outcomes after current transcatheter tricuspid valve intervention: Mid-term results from the international TriValve registry. JACC Cardiovasc. Interv. 2019, 12, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Hahn, R.T.; Zamorano, J.L. The need for a new tricuspid regurgitation grading scheme. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1342–1343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, C.C.; Veen, K.M. Uncertainties and challenges in surgical and transcatheter tricuspid valve therapy: A state-of-the-art expert review. Eur. Heart J. 2020, 41, 1932–1940. [Google Scholar] [CrossRef]
- Ancona, F.; Stella, S. Multimodality imaging of the tricuspid valve with implication for percutaneous repair approaches. Heart 2017, 103, 1073–1081. [Google Scholar] [CrossRef]
- Naoum, C.; Blanke, P. Cardiac computed tomography and magnetic resonance imaging in the evaluation of mitral and tricuspid valve disease: Implications for transcatheter interventions. Circ. Cardiovasc. Imaging. 2017, 10, e005331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozzoli, A.; Maisano, F. Fluoroscopic anatomy of the tricuspid valve: Implications for transcatheter procedures. Int. J. Cardiol. 2017, 244, 119–120. [Google Scholar] [CrossRef] [PubMed]
- Besler, C.; Orban, M. Predictors of procedural and clinical outcomes in patients with symptomatic tricuspid regurgitation undergoing transcatheter edge-to-edge repair. JACC Cardiovasc. Interv. 2018, 11, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, Y.H.; Ho, E. Update on Transcatheter Tricuspid Valve Replacement Therapies. Front. Cardiovasc. Med. 2021, 8, 619558. [Google Scholar] [CrossRef]
- Fam, N.P.; von Bardeleben, R.S. Transfemoral transcatheter tricuspid valve replacement with the EVOQUE system: A multicenter, observational, first-in-human experience. JACC Cardiovasc. Interv. 2021, 14, 501–511. [Google Scholar] [CrossRef]
- Lauten, A.; Figulla, H.R. Percutaneous caval stent valve implantation: Investigation of an interventional approach for treatment of tricuspid regurgitation. Eur. Heart J. 2010, 31, 1274–1281. [Google Scholar] [CrossRef] [Green Version]
- Sanon, S.; Cabalka, A.K. Transcatheter tricuspid valve-in-valve and valve-in-ring implantation for degenerated surgical prosthesis. JACC Cardiovasc. Interv. 2019, 12, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Lauten, A.; Ferrari, M. Heterotopic transcatheter tricuspid valve implantation: First-in-man application of a novel approach to tricuspid regurgitation. Eur. Heart J. 2011, 32, 1207–1213. [Google Scholar] [CrossRef] [Green Version]
- Lauten, A.; Figulla, H.R. Interventional treatment of severe tricuspid regurgitation: Early clinical experience in a multicenter, observational, first-in-man study. Circ. Cardiovasc. Interv. 2018, 11, e006061. [Google Scholar] [CrossRef] [PubMed]

| Potential Contraindications to Transcatheter Tricuspid Valve Repair |
|---|
| Primary valve abnormalities |
| Tricuspid stenosis |
| Permanent ventricular pacing lead that interacts with the tricuspid leaflet |
| Severe leaflet tenting and very large tricuspid annular diameter |
| Large coaptation gap > 6–8 mm and non-central regurgitant jets |
| Calcification in the potential grasping target and immobile or severely retracted leaflets (especially the septal leaflet) |
| Potential Contraindications to Transcatheter Tricuspid Valve Replacement |
|---|
| Large tricuspid annulus |
| Iliac veins, inferior vena cava or right ventricle not fitting delivery system |
| High bleeding risk |
| Right ventricle and tricuspid annulus incompatible geometry |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro da Costa, M.J.; Quintella, E.F.; Kohn, L.; Lacoste, M.O.; Leite, G.L.; Hadid, L.; Kruczan, D.D.; Zajdenverg, R.; de Castro Sabino, H.; da Motta, P.A.M. Transcatheter Tricuspid Valve-in-Valve Procedure—An Illustrative Case Report and Review. J. Clin. Med. 2021, 10, 4004. https://doi.org/10.3390/jcm10174004
Montenegro da Costa MJ, Quintella EF, Kohn L, Lacoste MO, Leite GL, Hadid L, Kruczan DD, Zajdenverg R, de Castro Sabino H, da Motta PAM. Transcatheter Tricuspid Valve-in-Valve Procedure—An Illustrative Case Report and Review. Journal of Clinical Medicine. 2021; 10(17):4004. https://doi.org/10.3390/jcm10174004
Chicago/Turabian StyleMontenegro da Costa, Márcio José, Edgard Freitas Quintella, Luiz Kohn, Maximiliano Otero Lacoste, Gustavo Lycurgo Leite, Leonardo Hadid, Dany David Kruczan, Ricardo Zajdenverg, Hugo de Castro Sabino, and Paulo Antônio Marra da Motta. 2021. "Transcatheter Tricuspid Valve-in-Valve Procedure—An Illustrative Case Report and Review" Journal of Clinical Medicine 10, no. 17: 4004. https://doi.org/10.3390/jcm10174004
APA StyleMontenegro da Costa, M. J., Quintella, E. F., Kohn, L., Lacoste, M. O., Leite, G. L., Hadid, L., Kruczan, D. D., Zajdenverg, R., de Castro Sabino, H., & da Motta, P. A. M. (2021). Transcatheter Tricuspid Valve-in-Valve Procedure—An Illustrative Case Report and Review. Journal of Clinical Medicine, 10(17), 4004. https://doi.org/10.3390/jcm10174004





