Comparing Outcomes in Asymptomatic and Symptomatic Atrial Fibrillation: A Systematic Review and Meta-Analysis of 81,462 Patients
Abstract
:1. Introduction
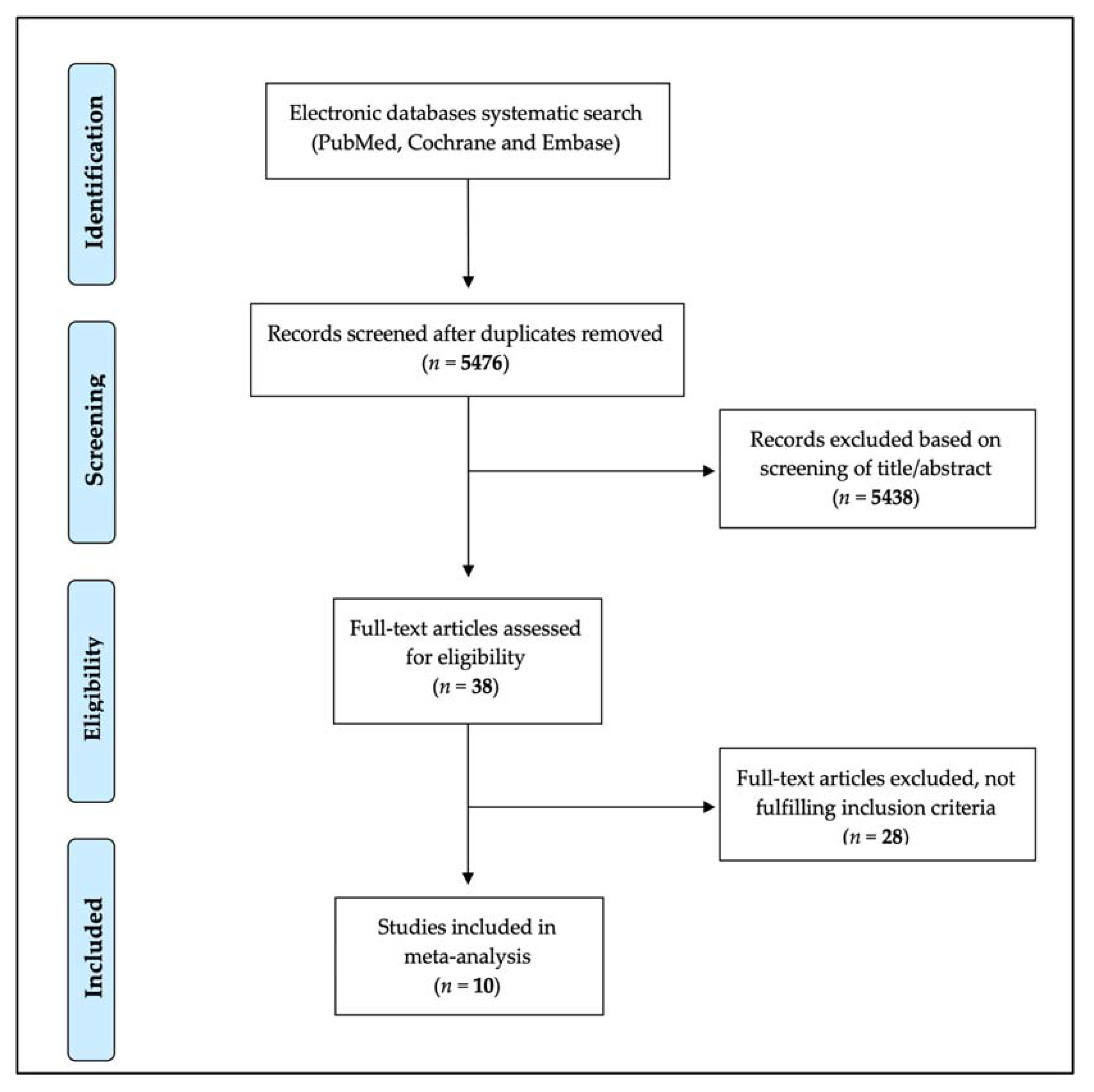
2. Materials and Methods
2.1. Study Selection
2.2. Data Extraction and Management
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
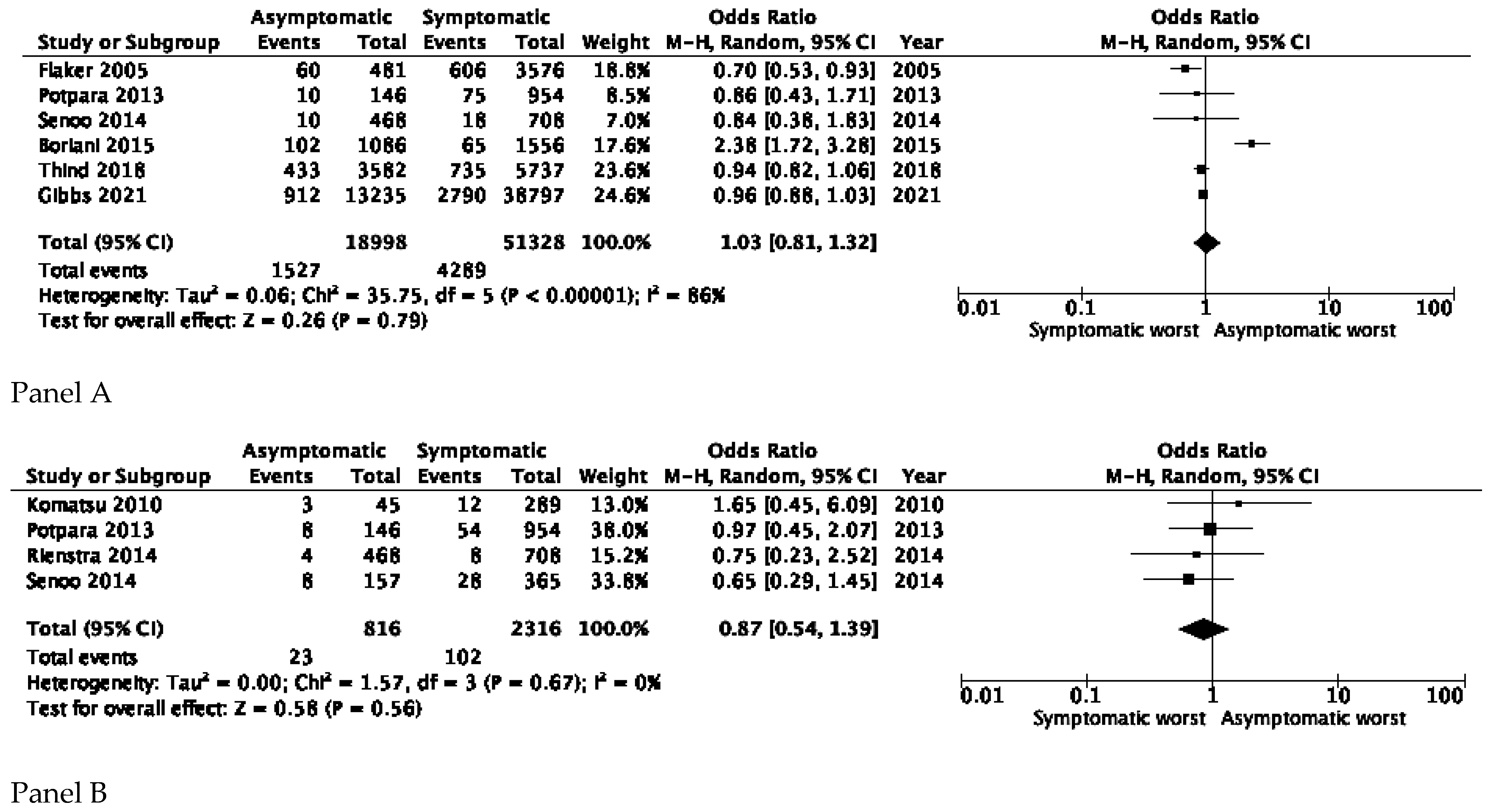
3.1. All-Cause and Cardiovascular Mortality
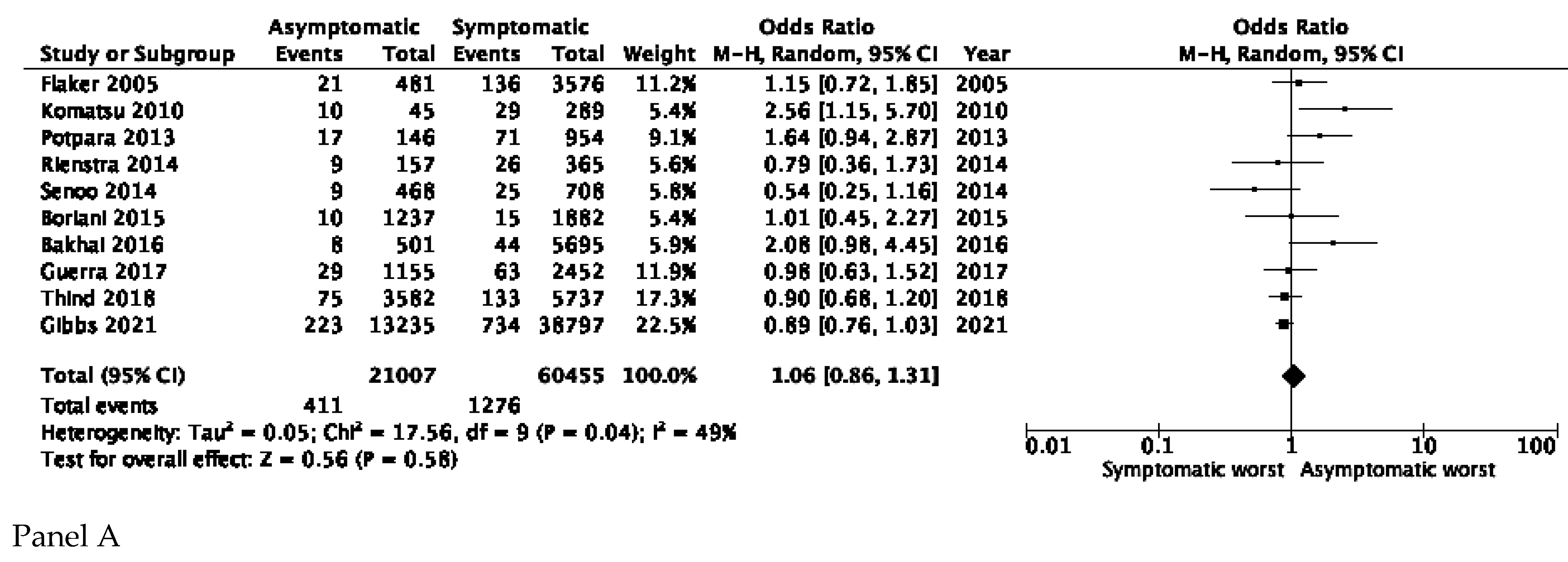
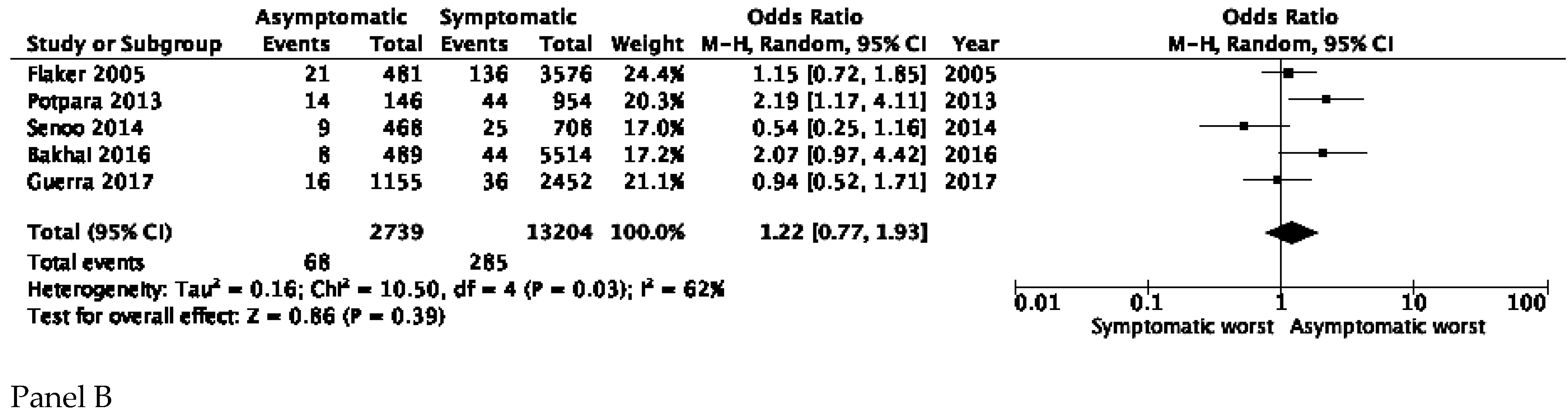
3.2. Stroke and Thromboembolic Events
3.3. Risk of Bias of Included Studies
3.4. Sensitivity Analysis
3.5. Inspection of Heterogeneity
4. Discussion
4.1. Major Findings
4.2. Importance of Integrated Approach to AF Care
4.3. Role of AF Screening
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomström-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.-A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur. Heart J. 2021, 42, 373–498. [Google Scholar]
- Boriani, G.; Diemberger, I.; Ziacchi, M.; Valzania, C.; Gardini, B.; Cimaglia, P.; Martignani, C.; Biffi, M. AF burden is important—Fact or fiction? Int. J. Clin. Pract. 2014, 68, 444–452. [Google Scholar] [CrossRef]
- Gladstone, D.; Spring, M.; Dorian, P.; Panzov, V.; Thorpe, K.; Hall, J.; Vaid, H.; O’Donnell, M.; Laupacis, A.; Côté, R.; et al. Atrial Fibrillation in Patients with Cryptogenic Stroke. N. Engl. J. Med. 2014, 370, 2467–2477. [Google Scholar] [CrossRef] [Green Version]
- Sanna, T.; Diener, H.-C.; Passman, R.S.; Di Lazzaro, V.; Bernstein, R.A.; Morillo, C.; Rymer, M.M.; Thijs, V.; Rogers, T.; Beckers, F.; et al. Cryptogenic Stroke and Underlying Atrial Fibrillation. N. Engl. J. Med. 2014, 370, 2478–2486. [Google Scholar] [CrossRef] [Green Version]
- Boriani, G.; Glotzer, T.V.; Santini, M.; West, T.M.; De Melis, M.; Sepsi, M.; Gasparini, M.; Lewalter, T.; Camm, J.A.; Singer, D.E. Device-detected atrial fibrillation and risk for stroke: An analysis of >10,000 patients from the SOS AF project (Stroke prevention Strategies based on Atrial Fibrillation information from implanted devices). Eur. Heart J. 2013, 35, 508–516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strano, S.; Toni, D.; Ammirati, F.; Sanna, T.; Tomaino, M.; Brignole, M.; Mazza, A.; Nguyen, B.L.; Di Bonaventura, C.; Ricci, R.P.; et al. Neuro-arrhythmology: A challenging field of action and research: A review from the Task Force of Neuro-arrhythmology of Italian Association of Arrhythmias and Cardiac Pacing. J. Cardiovasc. Med. 2019, 20, 731–744. [Google Scholar] [CrossRef] [PubMed]
- Albini, A.; Malavasi, V.L.; Vitolo, M.; Imberti, J.F.; Marietta, M.; Lip, G.Y.; Boriani, G. Long-term outcomes of postoperative atrial fibrillation following non cardiac surgery: A systematic review and metanalysis. Eur. J. Intern. Med. 2021, 85, 27–33. [Google Scholar] [CrossRef] [PubMed]
- De Moraes, E.R.F.L.; Cirenza, C.; Lopes, R.D.; Carvalho, A.C.; Guimaraes, P.O.; Rodrigues, A.A.E.; de Paola, A.A.V. Prevalence of atrial fibrillation and stroke risk assessment based on telemedicine screening tools in a primary healthcare setting. Eur. J. Intern. Med. 2019, 67, 36–41. [Google Scholar] [CrossRef]
- Vitolo, M.; Imberti, J.F.; Maisano, A.; Albini, A.; Bonini, N.; Valenti, A.C.; Malavasi, V.L.; Proietti, M.; Healey, J.S.; Lip, G.Y.; et al. Device-detected atrial high rate episodes and the risk of stroke/thrombo-embolism and atrial fibrillation incidence: A systematic review and meta-analysis. Eur. J. Intern. Med. 2021. [Google Scholar] [CrossRef]
- Magnussen, C.; Niiranen, T.J.; Ojeda, F.M.; Gianfagna, F.; Blankenberg, S.; Njølstad, I.; Vartianinen, E.; Sans, S.; Pasterkamp, G.; Hughes, M.; et al. Sex Differences and Similarities in Atrial Fibrillation Epidemiology, Risk Factors, and Mortality in Community Cohorts: Results from the BiomarCaRE Consortium (Biomarker for Cardiovascular Risk Assessment in Europe). Circulation 2017, 136, 1588–1597. [Google Scholar] [CrossRef] [Green Version]
- Denas, G.; Fedeli, U.; Gennaro, N.; Ferroni, E.; Corti, M.C.; Pengo, V. Death rates and causes in anticoagulated atrial fibrillation pa-tients: A population-based study. J. Cardiovasc. Med. 2020, 21, 415–419. [Google Scholar] [CrossRef]
- Morrone, D.; Kroep, S.; Ricci, F.; Renda, G.; Patti, G.; Kirchhof, P.; Chuang, L.-H.; Van Hout, B.; De Caterina, R. Mortality Prediction of the CHA2DS2-VASc Score, the HAS-BLED Score, and Their Combination in Anticoagulated Patients with Atrial Fibrillation. J. Clin. Med. 2020, 9, 3987. [Google Scholar] [CrossRef]
- Kim, D.; Yang, P.-S.; Lip, G.Y.; Joung, B. Atrial Fibrillation Increases the Risk of Early-Onset Dementia in the General Population: Data from a Population-Based Cohort. J. Clin. Med. 2020, 9, 3665. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.S.; Flattery, M.J.; Teljeur, C.; Ryan, M.; Smith, S.M. Effectiveness of systematic screening for the detection of atrial fibrillation. Cochrane Database Syst Rev. 2013, CD009586. [Google Scholar] [CrossRef] [Green Version]
- Freedman, B.; Camm, J.; Calkins, H.; Healey, J.S.; Rosenqvist, M.; Wang, J.; Albert, C.M.; Anderson, C.S.; Antoniou, S.; Benjamin, E.J.; et al. Screening for Atrial Fibrillation: A Report of the AF-SCREEN International Collaboration. Circulation 2017, 135, 1851–1867. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, R.B.; Haeusler, K.G.; Healey, J.S.; Freedman, B.; Boriani, G.; Brachmann, J.; Brandes, A.; Bustamante, A.; Casadei, B.; Crijns, H.J.G.M.; et al. Searching for Atrial Fibrillation Poststroke: A White Paper of the AF-SCREEN International Collaboration. Circulation 2019, 140, 1834–1850. [Google Scholar] [CrossRef]
- Rienstra, M.; Vermond, R.A.; Crijns, H.J.; Tijssen, J.G.; Van Gelder, I.C. Asymptomatic persistent atrial fibrillation and outcome: Results of the RACE study. Heart Rhythm. 2014, 11, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Rienstra, M.; Lubitz, S.A.; Mahida, S.; Sinner, M.F.; Ellinor, P.; Van Gelder, I.C.; Magnani, J.W.; Fontes, J.D.; Benjamin, E.J. Response to Letter Regarding Article, “Symptoms and Functional Status of Patients with Atrial Fibrillation: State of the Art and Future Research Opportunities”. Circulation 2012, 126, e350. [Google Scholar] [CrossRef] [Green Version]
- Flaker, G.C.; Belew, K.; Beckman, K.; Vidaillet, H.; Kron, J.; Safford, R.; Mickel, M.; Barrell, P. Asymptomatic atrial fibrillation: Demographic features and prognostic information from the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Am. Heart J. 2005, 149, 657–663. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, H.; Freedman, B.; Rosenqvist, M.; Virdone, S.; Al Mahmeed, W.; Ambrosio, G.; Camm, A.J.; Jacobson, B.; Jerjes-Sanchez, C.; Kayani, G.; et al. Clinical Outcomes in Asymptomatic and Symptomatic Atrial Fibrillation Presentations in GARFIELD-AF: Implications for AF Screening. Am. J. Med. 2021, 134, 893–901.e11. [Google Scholar] [CrossRef]
- Potpara, T.S.; Polovina, M.M.; Marinkovic, J.M.; Lip, G.Y. A comparison of clinical characteristics and long-term prognosis in asymptomatic and symptomatic patients with first-diagnosed atrial fibrillation: The Belgrade Atrial Fibrillation Study. Int. J. Cardiol. 2013, 168, 4744–4749. [Google Scholar] [CrossRef] [PubMed]
- Thind, M.; Holmes, D.N.; Badri, M.; Pieper, K.S.; Singh, A.; Blanco, R.G.; Steinberg, B.A.; Fonarow, G.C.; Gersh, B.J.; Mahaffey, K.W.; et al. Embolic and Other Adverse Outcomes in Symptomatic Versus Asymptomatic Patients with Atrial Fibrillation (from the ORBIT-AF Registry). Am. J. Cardiol. 2018, 122, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Senoo, K.; Suzuki, S.; Otsuka, T.; Sagara, K.; Matsuno, S.; Kano, H.; Uejima, T.; Oikawa, Y.; Yajima, J.; Nagashima, K.; et al. Progression to the persistent form in asymptomatic parox-ysmal atrial fibrillation. Circ. J. 2014, 78, 1121–1126. [Google Scholar] [CrossRef] [Green Version]
- Boriani, G.; Laroche, C.; Diemberger, I.; Fantecchi, E.; Popescu, M.I.; Rasmussen, L.H.; Sinagra, G.; Petrescu, L.; Tavazzi, L.; Maggioni, A.P.; et al. Asymptomatic Atrial Fibrillation: Clinical Correlates, Management, and Outcomes in the EORP-AF Pilot General Registry. Am. J. Med. 2014, 128, 509–518.e2. [Google Scholar] [CrossRef]
- Siontis, K.C.; Gersh, B.J.; Killian, J.M.; Noseworthy, P.A.; McCabe, P.; Weston, S.A.; Roger, V.L.; Chamberlain, A.M. Typical, atypical, and asymptomatic presen-tations of new-onset atrial fibrillation in the community: Characteristics and prognostic implications. Heart Rhythm Off. J. Heart Rhythm Soc. 2016, 13, 1418–1424. [Google Scholar] [CrossRef] [Green Version]
- Deeks, J.J.; D’Innes, J.; D’Amico, R.; Slowden, A.J.; Sakarovitch, C.; Song, F.; Petticrew, M.; Altman, D.G.; International Stroke Trial Collaborative Group; European Carotid Surgery Trial Collaborative Group. Evaluating non-randomized intervention studies. Health Technol. Assess. 2003, 7, 1–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 48. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, T.; Tachibana, H.; Sato, Y.; Ozawa, M.; Kunugida, F.; Nakamura, M. Efficacy of antiarrhythmic drug therapy in preventing recurrence of atrial fibrillation and long-term cardiovascular prognosis in patients with asymptomatic paroxysmal atrial fi-brillation. Int. Heart J. 2010, 51, 98–104. [Google Scholar] [CrossRef] [Green Version]
- Bakhai, A.; Darius, H.; De Caterina, R.; Smart, A.; Le Heuzey, J.-Y.; Schilling, R.J.; Zamorano, J.L.; Shah, M.; Bramlage, P.; Kirchhof, P. Characteristics and outcomes of atrial fibrillation patients with or without specific symptoms: Results from the PREFER in AF registry. Eur. Heart J.-Qual. Care Clin. Outcomes 2016, 2, 299–305. [Google Scholar] [CrossRef] [Green Version]
- Guerra, F.; Brambatti, M.; Nieuwlaat, R.; Marcucci, M.; Dudink, E.; Crijns, H.J.; Matassini, M.V.; Capucci, A. Symptomatic atrial fibrillation and risk of cardiovascular events: Data from the Euro Heart Survey. Europace 2017, 19, 1922–1929. [Google Scholar] [CrossRef]
- Xiong, Q.; Proietti, M.; Senoo, K.; Lip, G.Y. Asymptomatic versus symptomatic atrial fibrillation: A systematic review of age/gender differences and cardiovascular outcomes. Int. J. Cardiol. 2015, 191, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Banerjee, A.; Boriani, G.; Chiang, C.E.; Fargo, R.; Freedman, B.; Lane, D.A.; Ruff, C.T.; Turakhia, M.; Werring, D.; et al. Antithrombotic Therapy for Atrial Fibrillation: CHEST Guideline and Expert Panel Report. Chest 2018, 154, 1121–1201. [Google Scholar] [CrossRef] [Green Version]
- Boriani, G.; Vitolo, M.; Lane, A.D.; Potpara, T.S.; Lip, G.Y.H. Beyond the 2020 guidelines on atrial fibrillation of the European society of cardiology. Eur. J. Intern. Med. 2021, 86, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wachter, R.; Freedman, B. Subclinical Atrial Fibrillation and the Risk of Recurrent Ischemic Stroke. Thromb. Haemost. 2021, 121, 697–699. [Google Scholar] [CrossRef]
- Kitsiou, A.; Rogalewski, A.; Kalyani, M.; Deelawar, S.; Tribunyan, S.; Greeve, I.; Minnerup, J.; Israel, C.; Schäbitz, W.-R. Atrial Fibrillation in Patients with Embolic Stroke of Undetermined Source during 3 Years of Prolonged Monitoring with an Implantable Loop Recorder. Thromb. Haemost. 2021, 121, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Vitolo, M.; Imberti, J.F.; Potpara, T.S.; Lip, G.Y.H. What do we do about atrial high rate episodes? Eur. Heart J. Suppl. 2020, 22, O42–O52. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Laroche, C.; Diemberger, I.; Popescu, M.I.; Rasmussen, L.H.; Petrescu, L.; Crijns, H.J.G.M.; Tavazzi, L.; Maggioni, A.P.; Lip, G.Y.H. Glomerular filtration rate in patients with atrial fibrillation and 1-year outcomes. Sci. Rep. 2016, 6, 30271. [Google Scholar] [CrossRef]
- Boriani, G.; Vitolo, M.; Diemberger, I.; Proietti, M.; Valenti, A.C.; Malavasi, V.L.; Lip, G.Y.H. Optimizing indices of AF susceptibility and burden to evaluate AF severity, risk and outcomes. Cardiovasc. Res. 2021, cvab147. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H. The ABC pathway: An integrated approach to improve AF management. Nat. Rev. Cardiol. 2017, 14, 627–628. [Google Scholar] [CrossRef]
- Yoon, M.; Yang, P.-S.; Jang, E.; Yu, H.T.; Kim, T.-H.; Uhm, J.-S.; Kim, J.-Y.; Sung, J.-H.; Pak, H.-N.; Lee, M.-H.; et al. Improved Population-Based Clinical Outcomes of Patients with Atrial Fibrillation by Compliance with the Simple ABC (Atrial Fibrillation Better Care) Pathway for Integrated Care Management: A Nationwide Cohort Study. Thromb. Haemost. 2019, 119, 1695–1703. [Google Scholar] [CrossRef]
- Proietti, M.; Vitolo, M.; Lip, G.Y.H. Integrated care and outcomes in patients with atrial fibrillation and comorbidities. Eur. J. Clin. Investig. 2021, 51, e13498. [Google Scholar] [CrossRef] [PubMed]
- Proietti, M.; Lane, D.A. The Compelling Issue of Nonvitamin K Antagonist Oral Anticoagulant Adherence in Atrial Fibrillation Patients: A Systematic Need for New Strategies. Thromb. Haemost. 2020, 120, 369–371. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, C.; Hohnloser, S.H.; Jacob, J.; Walker, J.; Baldus, S.; Pfister, R. Non-Vitamin K Oral Anticoagulants in Comparison to Phenprocoumon in Geriatric and Non-Geriatric Patients with Non-Valvular Atrial Fibrillation. Thromb. Haemost. 2019, 119, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Basic, E.; Nabauer, M. Changes in Oral Anticoagulation Therapy over One Year in 51,000 Atrial Fibrillation Pa-tients at Risk for Stroke: A Practice-Derived Study. Thromb. Haemost. 2019, 119, 882–893. [Google Scholar] [PubMed]
- Vitolo, M.; Lane, A.D.; Boriani, G.; Lip, G.Y.H. The importance of adherence and persistence with oral anticoagulation treatment in patients with atrial fibrillation. Eur. Heart J.-Cardiovasc. Pharmacother. 2020, 7, f81–f83. [Google Scholar] [CrossRef]
- Mairesse, G.H.; Moran, P.; Van Gelder, I.C.; Elsner, C.; Rosenqvist, M.; Mant, J.; Banerjee, A.; Gorenek, B.; Brachmann, J.; Varma, N.; et al. Screening for atrial fibrillation: A European Heart Rhythm Association (EHRA) consensus document endorsed by the Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS), and Sociedad Latinoamericana de Estimulación Cardíaca y Electrofisiología (SOLAECE). Europace 2017, 19, 1589–1623. [Google Scholar] [CrossRef]
- Boriani, G.; Proietti, M. Screening for atrial fibrillation: Need for an integrated, structured approach. Eur. J. Intern. Med. 2019, 67, 33–35. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, J.; Shi, X.; Yao, Y.; Sun, Y.; Xia, Y.; Yu, B.; Liu, T.; Chen, Y.; Lyp, G.Y.H.; et al. Mobile health technology-supported atrial fibrillation screening and integrated care: A report from the mAFA-II trial Long-term Extension Cohort. Eur. J. Intern. Med. 2020, 82, 105–111. [Google Scholar] [CrossRef]
- Boriani, G.; Schnabel, R.B.; Healey, J.S.; Lopes, R.D.; Gurp, N.V.-V.; Lobban, T.; Camm, J.A.; Freedman, B. Consumer-led screening for atrial fibrillation using consumer-facing wearables, devices and apps: A survey of health care professionals by AF-SCREEN international collaboration. Eur. J. Intern. Med. 2020, 82, 97–104. [Google Scholar] [CrossRef]
- Curry, S.J.; Krist, A.H.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W., Jr.; Kemper, A.R.; Kubik, M.; et al. Screening for Atrial Fibrillation with Electro-cardiography: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 478–484. [Google Scholar]
- Jones, N.R.; Taylor, C.J.; Hobbs, F.D.R.; Bowman, L.; Casadei, B. Screening for atrial fibrillation: A call for evidence. Eur. Heart J. 2019, 41, 1075–1085. [Google Scholar] [CrossRef]
- Jones, N.R.; Crawford, W.; Yang, Y.; Hobbs, F.D.R.; Taylor, C.J.; Petrou, S. A Systematic Review of Economic Aspects of Service In-terventions to Increase Anticoagulation Use in Atrial Fibrillation. Thromb. Haemost. 2021. [Google Scholar] [CrossRef]
- Corley, S.D.; Epstein, A.E.; DiMarco, J.P.; Domanski, M.J.; Geller, N.; Greene, H.L.; Josephson, R.A.; Kellen, J.C.; Klein, R.C.; Krahn, A.D.; et al. Relationships between sinus rhythm, treatment, and survival in the Atrial Fibrillation Follow-Up Investigation of Rhythm Management (AFFIRM) Study. Circulation 2004, 109, 1509–1513. [Google Scholar] [PubMed]
- Engdahl, J.; Holmén, A.; Rosenqvist, M.; Strömberg, U. A prospective 5-year follow-up after population-based systematic screening for atrial fibrillation. Europace 2018, 20, f306–f311. [Google Scholar] [CrossRef] [PubMed]
- Wallenhorst, C.; Martinez, C.; Freedman, B. Risk of Ischemic Stroke in Asymptomatic Atrial Fibrillation Incidentally Detected in Primary Care Compared with Other Clinical Presentations. Thromb. Haemost. 2021. [Google Scholar] [CrossRef]
- Li, Y.-G.; Pastori, D.; Farcomeni, A.; Yang, P.-S.; Jang, E.; Joung, B.; Wang, Y.-T.; Guo, Y.-T.; Lip, G.Y.H. A Simple Clinical Risk Score (C2HEST) for Predicting Incident Atrial Fibrillation in Asian Subjects. Chest 2018, 155, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.H.; Skjøth, F.; Nielsen, P.B.; Larsen, T.B. Evaluation of the C. Am. J. Cardiol. 2020, 125, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Boriani, G.; Palmisano, P.; Malavasi, V.; Fantecchi, E.; Vitolo, M.; Bonini, N.; Imberti, J.; Valenti, A.; Schnabel, R.; Freedman, B. Clinical Factors Associated with Atrial Fibrillation Detection on Single-Time Point Screening Using a Hand-Held Single-Lead ECG Device. J. Clin. Med. 2021, 10, 729. [Google Scholar] [CrossRef]
- Ding, E.Y.; Marcus, G.M.; McManus, D.D. Emerging Technologies for Identifying Atrial Fibrillation. Circ. Res. 2020, 127, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Olier, I.; Ortega-Martorell, S.; Pieroni, M.; Lip, G.Y.H. How machine learning is impacting research in atrial fibrillation: Implications for risk prediction and future management. Cardiovasc. Res. 2021, 117, 1700–1717. [Google Scholar] [CrossRef] [PubMed]
| Study (Year) | Study Design | Asymptomatic AF | Symptomatic AF | FU (Years) | Outcomes | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Age (Mean/Median) | Male n (%) | DM n (%) | HF n (%) | CAD n (%) | N | Age (Mean/Median) | Male n (%) | DM n (%) | HF n (%) | CAD n (%) | ||||
| Flaker GC. [19] (2005) | RCT | 481 | 70 ± 8.3 | 370 (76.9) | 100 (21) | 64 (13) | 137 (28) | 3576 | 69.7 ± 9 | 2095 (58.5) | 713 (20) | 873 (24) | 1413 (40) | 3.5 (mean) | All cause death, stroke |
| Komatsu [29] T. (2010) | Retrospective | 45 | 67 ± 9.9 | 35 (77.7) | 14 (31) | NA | NA | 289 | 66.6 ± 11.8 | 194 (67.1) | 28 (10) | NA | NA | 5 ± 0.9 | CV death, TE event |
| Potpara TS. [21] (2013) | Prospective | 146 | 53.1 ± 13 | 122 (83.5) | 17 (11.6) | 12 (8.2) | 7 (4.8) | 954 | 52.6 ± 12.1 | 589 (61.7) | 59 (6.2) | 71 (7.4) | 46 (4.8) | 9.9 ± 6.1 | All cause death, CV death, TE events, stroke |
| Rienstra M. [17] (2014) | RCT | 157 | 67 ± 9 | 113 (71.9) | 14 (9) | 37 (34) | 25 (16) | 365 | 69 ± 9 | 217 (67.7) | 39 (11) | 224 (61) | 118 (32) | 2.3 ± 0.6 | CV death, TE events |
| Senoo K. [23] (2014) | Retrospective | 468 | 61.2 ± 12.6 | 373 (79.7) | 48 (10.3) | NA | 36 (7.7) | 708 | 61.5 ± 13.5 | 502 (71) | 85 (12) | NA | 48 (6.8) | 3.3 ± 2.5 | All cause death, CV death, stroke |
| Boriani G. [24] (2015) | Prospective | 1237 | 72 (64–78) | 804 (65) | 276 (22.3) | 544 (44.3) | 496 (40.1) | 1882 | 68 (61–76) | 1056 (56.1) | 365 (19.4) | 933 (49.6) | 636 (33.8) | 1 ± 0.1 | All cause death, TE events |
| Bakhai A. [30] (2016) | Prospective | 501 | 71.5 ± 9.3 | 387 (77.2) | 101 (20.2) | 44 (8.8) | 80 (16) | 5695 | 71.8 ± 10.4 | 3347 (58.8) | 1267 (22.3) | 1290 (22.7) | 1237 (24.4) | 1 | Stroke |
| Guerra F. [31] (2017) | Prospective | AS 252AA 903 | AS 67.6 ± 11.4 AA 68.1 ± 11.7 | AS 157 (62.3) AA 600 (66.4) | AS 50 (19.9) AA 184 (20.4) | AS 57 (22.7) AA 203 (22.6) | AS 7 (2.8) AA 150 (16.7) | SS 896 SA 1556 | SS 65.9 ± 11.6 SA 64.4 ± 13.6 | SS 497 (55.5) SA 872 (56) | SS 164 (18.4) SA 244 (15.7) | SS 357 (40.2) SA 418 (27) | SS 134 (15.1) SA 181 (11.7) | 1 | TE events, stroke |
| Thind M. [22] (2018) | Prospective | 3582 | 76 (68–82) | 2313 (64.6) | 1061 (29.6) | 1011 (28.2) | 1292 (36.1) | 5737 | 74 (66–81) | 3062 (53.4) | 1686 (29.4) | 1958 (34.1) | 2041 (35.6) | 2.6 | All cause death |
| Gibbs H. [20] (2021) | Prospective | 13,235 | 72 (65–79) | 8501 (64.2) | 3037 (23) | 1786 (13.5) | 1219 (9.2) | 38797 | 70 (62–78) | 20,541 (52.9) | 8509 (21.9) | 9953 (25.7) | 4317 (11.2) | 2 | All cause death, TE events |
| Study (Year) | Asymptomatic AF | Symptomatic AF | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Thromboembolic Risk | Antithrombotic Treatment n (%) | N | Thromboembolic Risk | Antithrombotic Treatment n (%) | |||||
| Flaker GC. [19] (2005) | 481 | NA | Aspirin 100 (21) Warfarin 438 (91) | 3576 | NA | Aspirin 980 (27) Warfarin 2993 (84) | ||||
| Komatsu T. [29] (2010) | 45 | CHADS2 (mean): 1.63 ± 1.27 | Aspirin 19 (42) Warfarin 11 (24) None 15 (34) | 289 | CHADS2 (mean): 1.14 ± 1.18 | Aspirin 80 (28) Warfarin 66 (23) None 143 (49) | ||||
| Potpara TS. [21] (2013) | 146 | CHADS2 ≥ 2, 21 (14.4) CHA2DS2-VASC ≥ 2, 48 (32.9) | Aspirin 70 (47.9) OAC 59 (40.4) None 17 (11.6) | 954 | CHADS2 ≥ 2, 96 (10.1) CHA2DS2-VASC ≥ 2, 348 (36.5) | Aspirin 463 (48.6) OAC 203 (21.3) None 287 (30.1) | ||||
| Rienstra M. [17] (2014) | 157 | CHADS2 (mean): 1.2 ± 1.1 | NA | 365 | CHADS2 (mean): 1.7 ± 1.1 | NA | ||||
| Senoo K. [23] (2014) | 468 | CHADS2 ≥ 2, 98 (20.9) | Antiplatelets 177 (40.9) Warfarin 150 (32.1) Dabigatran 8 (1.7) | 708 | CHADS2 ≥ 2, 172 (24.3) | Antiplatelets 291 (39.2) Warfarin 234 (33.1) Dabigatran 66 (9.3) | ||||
| Boriani G. [24] (2015) | 1237 | CHADS2 (mean): 2 ± 1.31 CHA2DS2-VASC (mean): 3.41 ± 1.78 | Antiplatelets 400 (32.3)OAC 1027 (83) None 45 (3.6) | 1882 | CHADS2 (mean): 1.87 ± 1.25 CHA2DS2-VASC (mean): 3.14 ± 1.79 | Antiplatelets 668 (35.5) OAC 1528 (81.2) None 105 (5.6) | ||||
| Bakhai A. [30] (2016) | 501 | CHA2DS2-VASC (mean): 2.9 ± 1.7 | Antiplatelets 55 (11) VKA 359 (71.7) NOAC 26 (5.2) None 28 (5.6) | 5695 | CHA2DS2-VASC (mean): 3.4 ± 1.8 | Antiplatelets 634 (11.1) VKA 3781 (66.4) NOAC 359 (6.3) None 336 (5.9) | ||||
| Guerra F. [31] (2017) | AS 252AA 903 | AS CHADS2 (mean): 1.5 ± 1CHA2DS2-VASC (mean): 2.9 ± 1.8 | AA CHADS2 (mean): 1.5 ± 1CHA2DS2-VASC (mean): 2.9 ± 1.7 | AS Antiplatelets 73 (29.4) OAC 144 (58.8) | AA Antiplatelets 258 (28.9) OAC 529 (59.8) | SS 896SA 1556 | SS CHADS2(mean): 1.7 ± 1 CHA2DS2-VASC (mean): 3.2 ± 2 | SA CHADS2(mean): 1.4 ± 1 CHA2DS2-VASC (mean): 2.7 ± 1.8 | SS Antiplatelets 285 (32.2) OAC 637(72.4) | SA Antiplatelets 508 (32.9) OAC 964 (63.8) |
| Thind M. [22] (2018) | 3582 | CHADS2: 2 (1–3) CHA2DS2-VASC: 4 (3–5) | Antiplatelets 1662 (46.4) OAC 2762 (77.1) | 5737 | CHADS2: 2 (1–3) CHA2DS2-VASC: 4 (3–5) | Antiplatelets 2767 (48.2) OAC 4292 (74.8) | ||||
| Gibbs H. [20] (2021) | 13,235 | CHA2DS2-VASC: 3 (2–4) | Antiplatelets 2350 (17.9) NOAC ± antiplatelets 3906 (29.8) VKA ± antiplatelets 5187 (39.6) None 1659 (12.7) | 38,797 | CHA2DS2-VASC: 3 (2–4) | Antiplatelets 8411 (22) NOAC ± antiplatelets 10,217 (26.7) VKA ± antiplatelets 14,996 (39.3)None 4581 (12) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgreccia, D.; Manicardi, M.; Malavasi, V.L.; Vitolo, M.; Valenti, A.C.; Proietti, M.; Lip, G.Y.H.; Boriani, G. Comparing Outcomes in Asymptomatic and Symptomatic Atrial Fibrillation: A Systematic Review and Meta-Analysis of 81,462 Patients. J. Clin. Med. 2021, 10, 3979. https://doi.org/10.3390/jcm10173979
Sgreccia D, Manicardi M, Malavasi VL, Vitolo M, Valenti AC, Proietti M, Lip GYH, Boriani G. Comparing Outcomes in Asymptomatic and Symptomatic Atrial Fibrillation: A Systematic Review and Meta-Analysis of 81,462 Patients. Journal of Clinical Medicine. 2021; 10(17):3979. https://doi.org/10.3390/jcm10173979
Chicago/Turabian StyleSgreccia, Daria, Marcella Manicardi, Vincenzo Livio Malavasi, Marco Vitolo, Anna Chiara Valenti, Marco Proietti, Gregory Y. H. Lip, and Giuseppe Boriani. 2021. "Comparing Outcomes in Asymptomatic and Symptomatic Atrial Fibrillation: A Systematic Review and Meta-Analysis of 81,462 Patients" Journal of Clinical Medicine 10, no. 17: 3979. https://doi.org/10.3390/jcm10173979
APA StyleSgreccia, D., Manicardi, M., Malavasi, V. L., Vitolo, M., Valenti, A. C., Proietti, M., Lip, G. Y. H., & Boriani, G. (2021). Comparing Outcomes in Asymptomatic and Symptomatic Atrial Fibrillation: A Systematic Review and Meta-Analysis of 81,462 Patients. Journal of Clinical Medicine, 10(17), 3979. https://doi.org/10.3390/jcm10173979









