Development and Validation of a Model to Predict Severe Hospital-Acquired Acute Kidney Injury in Non-Critically Ill Patients
Abstract
:1. Introduction
2. Methods
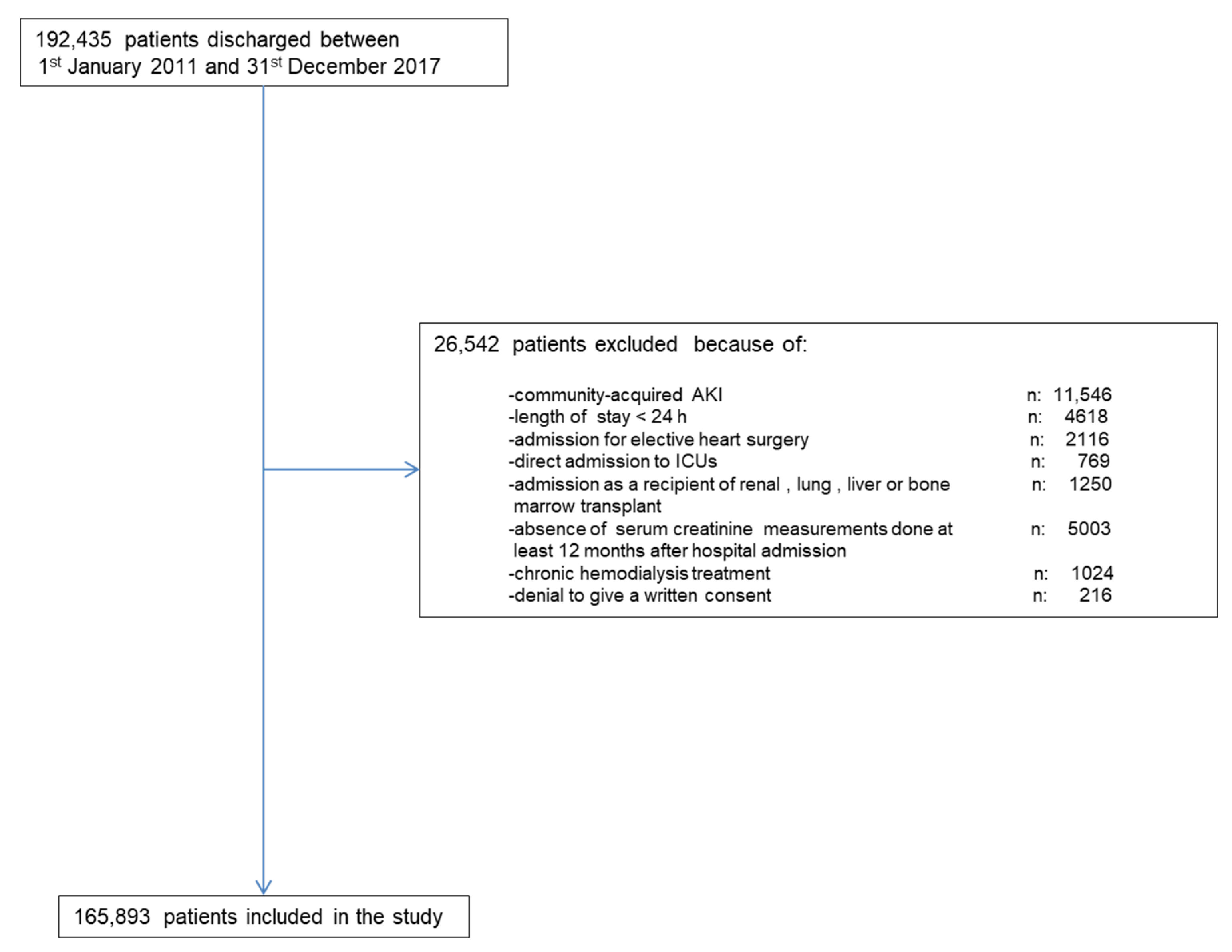
2.1. Study Set
2.2. Baseline Kidney Function
2.3. Definition of AKI Severe
2.4. AKI Detection
2.5. Clinical Evaluation at Hospital Admission and during Hospital Stay
2.6. Validation Set
2.7. Statistics
3. Results
3.1. Study Set
3.2. Validation SET
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kashani, K.; Rosner, M.H.; Haase, M.; Lewington, A.J.P.; O’Donoghue, D.J.; Wilson, F.P.; Nadim, M.K.; Silver, S.A.; Zarbock, A.; Ostermann, M.; et al. Quality Improvement Goals for Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2019, 14, 941–953. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.L.; Burdmann, E.A.; Cerdá, J.; Feehally, J.; Finkelstein, F.; García-García, G.; Godin, M.; Jha, V.; Lameire, N.H.; Levin, N.W.; et al. Recognition and Management of Acute Kidney Injury in the International Society of Nephrology 0by25 Global Snapshot: A Multinational Cross-Sectional Study. Lancet 2016, 387, 2017–2025. [Google Scholar] [CrossRef]
- Wonnacott, A.; Meran, S.; Amphlett, B.; Talabani, B.; Phillips, A. Epidemiology and Outcomes in Community-Acquired versus Hospital-Acquired AKI. Clin. J. Am. Soc. Nephrol. 2014, 9, 1007–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meier, P.; Bonfils, R.M.; Vogt, B.; Burnand, B.; Burnier, M. Referral Patterns and Outcomes in Noncritically Ill Patients with Hospital-Acquired Acute Kidney Injury. Clin. J. Am. Soc. Nephrol. 2011, 6, 2215–2225. [Google Scholar] [CrossRef] [Green Version]
- Yong, K.; Dogra, G.; Boudville, N.; Pinder, M.; Lim, W. Acute Kidney Injury: Controversies Revisited. Int. J. Nephrol. 2011, 2011, 762634. [Google Scholar] [CrossRef] [Green Version]
- Pruchnicki, M.C.; Dasta, J.F. Acute Renal Failure in Hospitalized Patients: Part I. Ann. Pharmacother. 2002, 36, 1261–1267. [Google Scholar] [CrossRef]
- Patschan, D.; Müller, G.A. Acute Kidney Injury. J. Inj. Violence Res. 2015, 7, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Forni, L.G.; Darmon, M.; Ostermann, M.; Oudemans-van Straaten, H.M.; Pettilä, V.; Prowle, J.R.; Schetz, M.; Joannidis, M. Renal Recovery after Acute Kidney Injury. Intensive Care Med. 2017, 43, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Bucaloiu, I.D.; Kirchner, H.L.; Norfolk, E.R.; Hartle, J.E.; Perkins, R.M. Increased Risk of Death and de Novo Chronic Kidney Disease Following Reversible Acute Kidney Injury. Kidney Int. 2012, 81, 477–485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collister, D.; Pannu, N.; Ye, F.; James, M.; Hemmelgarn, B.; Chui, B.; Manns, B.; Klarenbach, S. Alberta Kidney Disease Network. Health Care Costs Associated with AKI. Clin. J. Am. Soc. Nephrol. 2017, 12, 1733–1743. [Google Scholar] [CrossRef]
- Chertow, G.M.; Burdick, E.; Honour, M.; Bonventre, J.V.; Bates, D.W. Acute Kidney Injury, Mortality, Length of Stay, and Costs in Hospitalized Patients. JASN 2005, 16, 3365–3370. [Google Scholar] [CrossRef] [Green Version]
- Susantitaphong, P.; Cruz, D.N.; Cerda, J.; Abulfaraj, M.; Alqahtani, F.; Koulouridis, I.; Jaber, B.L. Acute Kidney Injury Advisory Group of the American Society of Nephrology. World Incidence of AKI: A Meta-Analysis. Clin. J. Am. Soc. Nephrol. 2013, 8, 1482–1493. [Google Scholar] [CrossRef] [Green Version]
- Ostermann, M. Acute Kidney Injury on Admission to the Intensive Care Unit: Where to Go from Here? Crit. Care 2008, 12, 189. [Google Scholar] [CrossRef] [Green Version]
- Mas-Font, S.; Ros-Martinez, J.; Pérez-Calvo, C.; Villa-Díaz, P.; Aldunate-Calvo, S.; Moreno-Clari, E. Prevention of acute kidney injury in intensive care units. Med. Intensiva 2017, 41, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Seller-Pérez, G.; Más-Font, S.; Pérez-Calvo, C.; Villa-Díaz, P.; Celaya-López, M.; Herrera-Gutiérrez, M.E. Acute kidney injury: Renal disease in the ICU. Med. Intensiva 2016, 40, 374–382. [Google Scholar] [CrossRef]
- Hoste, E.A.; Bagshaw, S.M.; Bellomo, R.; Cely, C.M.; Colman, R.; Cruz, D.N.; Edipidis, K.; Forni, L.G.; Gomersall, C.D.; Govil, D.; et al. Epidemiology of acute kidney injury in critically ill patients: The multinational AKI-EPI study. Intensive Care Med. 2015, 41, 1411–1423. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; McCulloch, C.E.; Fan, D.; Ordoñez, J.D.; Chertow, G.M.; Go, A.S. Community-Based Incidence of Acute Renal Failure. Kidney Int. 2007, 72, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrantes, F.; Feng, Y.; Ivanov, O.; Yalamanchili, H.B.; Patel, J.; Buenafe, X.; Cheng, V.; Dijeh, S.; Amoateng-Adjepong, Y.; Manthous, C.A. Acute Kidney Injury Predicts Outcomes of Non-Critically Ill Patients. Mayo Clin. Proc. 2009, 84, 410–416. [Google Scholar] [CrossRef]
- Harty, J. Prevention and Management of Acute Kidney Injury. Ulster Med. J. 2014, 83, 149–157. [Google Scholar] [PubMed]
- Cheng, P.; Waitman, L.R.; Hu, Y.; Liu, M. Predicting Inpatient Acute Kidney Injury over Different Time Horizons: How Early and Accurate? AMIA Annu. Symp. Proc. 2017, 2017, 565–574. [Google Scholar]
- Bedford, M.; Stevens, P.; Coulton, S.; Billings, J.; Farr, M.; Wheeler, T.; Kalli, M.; Mottishaw, T.; Farmer, C. Development of Risk Models for the Prediction of New or Worsening Acute Kidney Injury on or during Hospital Admission: A Cohort and Nested Study; Health Services and Delivery Research; NIHR Journals Library: Southampton, UK, 2016. [Google Scholar]
- James, M.T.; Hobson, C.E.; Darmon, M.; Mohan, S.; Hudson, D.; Goldstein, S.L.; Ronco, C.; Kellum, J.A.; Bagshaw, S.M. Acute Dialysis Quality Initiative (ADQI) Consensus Group. Applications for Detection of Acute Kidney Injury Using Electronic Medical Records and Clinical Information Systems: Workgroup Statements from the 15(Th) ADQI Consensus Conference. Can. J. Kidney Health Dis. 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyner, J.L.; Adhikari, R.; Edelson, D.P.; Churpek, M.M. Development of a Multicenter Ward-Based AKI Prediction Model. Clin. J. Am. Soc. Nephrol. 2016, 11, 1935–1943. [Google Scholar] [CrossRef]
- Hodgson, L.E.; Dimitrov, B.D.; Roderick, P.J.; Venn, R.; Forni, L.G. Predicting AKI in Emergency Admissions: An External Validation Study of the Acute Kidney Injury Prediction Score (APS). BMJ Open 2017, 7, e013511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Malley, K.J.; Cook, K.F.; Price, M.D.; Wildes, K.R.; Hurdle, J.F.; Ashton, C.M. Measuring Diagnoses: ICD Code Accuracy. Health Serv. Res. 2005, 40 Pt 2, 1620–1639. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, L.E.; Sarnowski, A.; Roderick, P.J.; Dimitrov, B.D.; Venn, R.M.; Forni, L.G. Systematic Review of Prognostic Prediction Models for Acute Kidney Injury (AKI) in General Hospital Populations. BMJ Open 2017, 7, e016591. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Hu, Y.; Yuan, B.; Zhang, X.; Chen, W.; Liu, K.; Liu, M. Which Risk Predictors Are More Likely to Indicate Severe AKI in Hospitalized Patients? Int. J. Med. Inform. 2020, 143, 104270. [Google Scholar] [CrossRef]
- Segarra, A.; Del Carpio, J.; Marco, M.P.; Jatem, E.; Gonzalez, J.; Chang, P.; Ramos, N.; de la Torre, J.; Prat, J.; Torres, M.J.; et al. Integrating Electronic Health Data Records to Develop and Validate a Predictive Model of Hospital-Acquired Acute Kidney Injury in Non-Critically Ill Patients. Clin. Kidney J. 2021, sfab094. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int. 2012, 2 (Suppl. 1), 1–138. [Google Scholar]
- Akaike, H. Likelihood of a Model and Information Criteria. J. Econom. 1981, 16, 3–14. [Google Scholar] [CrossRef]
- Cavanaugh, J.E. Unifying the Derivations for the Akaike and Corrected Akaike Information Criteria. Stat. Probab. Lett. 1997, 33, 201–208. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S. Confidence Interval Estimates of an Index of Quality Performance Based on Logistic Regression Models. Stat. Med. 1995, 14, 2161–2172. [Google Scholar] [CrossRef]
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.A.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and Elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [Green Version]
- Pencina, M.J.; D’Agostino, R.B.; D’Agostino, R.B.; Vasan, R.S. Evaluating the Added Predictive Ability of a New Marker: From Area under the ROC Curve to Reclassification and Beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef] [PubMed]
- Martin-Cleary, C.; Molinero-Casares, L.M.; Ortiz, A.; Arce-Obieta, J.M. Development and Internal Validation of a Prediction Model for Hospital-Acquired Acute Kidney Injury. Clin. Kidney J. 2021, 14, 309–316. [Google Scholar] [CrossRef] [Green Version]
- Tomašev, N.; Glorot, X.; Rae, J.W.; Zielinski, M.; Askham, H.; Saraiva, A.; Mottram, A.; Meyer, C.; Ravuri, S.; Protsyuk, I.; et al. A Clinically Applicable Approach to Continuous Prediction of Future Acute Kidney Injury. Nature 2019, 572, 116–119. [Google Scholar] [CrossRef]
- Bell, S.; James, M.T.; Farmer, C.K.T.; Tan, Z.; de Souza, N.; Witham, M.D. Development and External Validation of an Acute Kidney Injury Risk Score for Use in the General Population. Clin. Kidney J. 2020, 13, 402–412. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Vickers, A.J.; Cook, N.R.; Gerds, T.; Gonen, M.; Obuchowski, N.; Pencina, M.J.; Kattan, M.W. Assessing the Performance of Prediction Models: A Framework for Traditional and Novel Measures. Epidemiology 2010, 21, 128–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Austin, P.C.; Steyerberg, E.W. Graphical Assessment of Internal and External Calibration of Logistic Regression Models by Using Loess Smoothers. Stat. Med. 2014, 33, 517–535. [Google Scholar] [CrossRef]



| Variables | Total | Stage 3 AKI | Non-Stage 3 AKI | Sig |
|---|---|---|---|---|
| n | 165,893 | 995 (0.6) | 164,898 (99.4) | |
| Gender: Men. (n) % | 74,962 (45.2) | 517 (52.0) | 74,445 (45.1) | <0.001 |
| Age (years). mean (SD) | 54.9 (20.6) | 67.1 (21) | 53.9 (19.9) | <0.001 |
| Chronic comorbidities | ||||
| Diabetes. (n) % | 30,357 (18.3) | 450 (45.2) | 29,907 (18.1) | <0.001 |
| Hypertension. (n) % | 65,554 (39.5) | 707 (71.1) | 64,847 (39.3) | <0.001 |
| Ischemic Heart Disease. (n) % | 12,428 (7.5) | 169 (17.1) | 12,259 (7.4) | <0.001 |
| Ischemic Cerebrovascular disease. (n) % | 11,446 (6.9) | 78 (7.8) | 11,368 (6.9) | 0.136 |
| Ischemic Peripheral vascular disease. (n) % | 8706 (5.2) | 93 (9.3) | 8613 (5.2) | <0.001 |
| Chronic digestive disease. (n) % | 9627 (5.8) | 51 (5.1) | 9576 (5.8) | 0.198 |
| Chronic liver disease. (n) % | 5667 (3.4) | 105 (10.6) | 5562 (3.4) | <0.001 |
| Chronic congestive heart failure. (n) % | 14,344 (8.6) | 256 (25.7) | 14,088 (8.5) | <0.001 |
| Chronic obstructive pulmonary disease. (n) % | 23,272 (14.0) | 424 (42.6) | 22,848 (13.9) | <0.001 |
| Malignancy. (n) % | 23,504 (14.2) | 304 (30.6) | 23,200 (14.1) | <0.001 |
| Rheumatologic disease. (n) % | 6828 (4.1) | 41 (4.1) | 6787 (4.1) | 0.529 |
| Urologic disease. (n) % | 11,926 (7.2) | 148 (14.9) | 11,778 (7.1) | <0.001 |
| Chronic Kidney disease stages | <0.001 | |||
| 0 + I | 137,385 (82.8) | 583 (58.6) | 136,802 (83) | |
| II | 16,252 (9.8) | 109 (11.0) | 16,143 (9.8) | |
| III | 9265 (5.6) | 175 (17.6) | 9090 (5.5) | |
| IV | 2991 (1.8) | 128 (12.9) | 2863 (1.7) | |
| Clinical variables along hospital admission | ||||
| Urgent admission. (n) % | 108,577 (65.5) | 947 (95.2) | 107,630 (65.3) | <0.001 |
| Anaemia. (n) % | 23,291 (14.0) | 379 (38.1) | 22,912 (13.9) | <0.001 |
| Acute respiratory failure. (n) % | 7803 (4.7) | 308 (31.0) | 7495 (4.5) | <0.001 |
| Acute Hearth failure (n) % | 6204 (3.7) | 241 (24.2) | 5963 (3.6) | <0.001 |
| SIRS. (n) % | 2358 (1.4) | 235 (23.6) | 2123 (1.3) | <0.001 |
| Circulatory shock. (n) % | 2018 (1.2) | 280 (28.1) | 1738 (1.1) | <0.001 |
| Major surgery. (n) % | 61,583 (37.1) | 408 (41.0) | 61,675 (37.4) | <0.001 |
| Exposure to contrast media. (n) % | 14,698 (8.9) | 280 (28.1) | 14,418 (8.7) | <0.001 |
| Exposure to nephrotoxic drugs. (n) % | 85,863 (51.8) | 677 (68.0) | 85,186 (51.7) | <0.001 |
| Variable | B | S.E. | Wald | OR | 95% CI | p-Value |
|---|---|---|---|---|---|---|
| Age | 0.024 | 0.003 | 91.2 | 1.03 | 1.02–1.03 | 0.000 |
| Hypertension | 0.539 | 0.084 | 41.1 | 1.71 | 1.45–2.02 | 0.000 |
| Diabetes | 1.184 | 0.079 | 223.5 | 3.27 | 2.79–3.81 | 0.000 |
| Peripheral vascular disease | 0.597 | 0.135 | 19.7 | 1.82 | 1.39–2.37 | 0.000 |
| Anaemia | 0.664 | 0.075 | 78.0 | 1.94 | 1.67–2.25 | 0.000 |
| Chronic congestive hearth failure | 0.405 | 0.085 | 22.5 | 1.50 | 1.27–1.77 | 0.000 |
| Ischemic hearth disease | 0.653 | 0.107 | 37.6 | 1.92 | 1.56–2.37 | 0.000 |
| Chronic obstructive pulmonary disease | 0.469 | 0.096 | 23.9 | 1.60 | 1.32–1.93 | 0.000 |
| Chronic liver disease | 1.013 | 0.133 | 58.1 | 2.75 | 2.12–3.57 | 0.000 |
| Chronic urologic disease | 1.309 | 0.118 | 123.9 | 3.70 | 2.94–4.66 | 0.000 |
| CKD_stage | 469.9 | 0.000 | ||||
| CKD_stage(1) | 0.582 | 0.122 | 22.7 | 1.79 | 1.41–2.27 | 0.000 |
| CKD_stage(2) | 1.425 | 0.1 | 204.0 | 4.16 | 3.49–5.05 | 0.000 |
| CKD_stage(3) | 2.187 | 0.119 | 339.8 | 8.91 | 7.06–11.24 | 0.000 |
| SIRS | 0.698 | 0.128 | 29.6 | 2.01 | 1.56–2.59 | 0.000 |
| Shock | 2.055 | 0.122 | 286.1 | 7.81 | 6.15–9.9 | 0.000 |
| Acute Hearth Failure | 0.801 | 0.096 | 69.9 | 2.23 | 1.84–2.69 | 0.000 |
| Major_surgery | 1.213 | 0.083 | 211.8 | 3.36 | 2.85–3.96 | 0.000 |
| Acute respiratory failure | 1.283 | 0.106 | 147.4 | 3.61 | 2.93–4.44 | 0.000 |
| Nephrotoxic drugs | 0.345 | 0.078 | 19.8 | 1.41 | 1.21–1.64 | 0.000 |
| Exposure to contrast dyes | 0.931 | 0.085 | 119.5 | 2,53 | 2.15–2.99 | 0.000 |
| Urgent_admission | 1.899 | 0.161 | 139.0 | 6.68 | 4.87–9.15 | 0.000 |
| Constant | −11.211 | 0.237 | 2239.0 | 0.00 |
| Risk Deciles | Acute Kidney Injury = 0 | Acute Kidney INJURY = 1 | Total | ||
|---|---|---|---|---|---|
| Observed | Expected | Observed | Expected | ||
| <0.0001702 | 16,514 | 16,512.6 | 0 | 1.4 | 16,514 |
| 0.0001702–0.0003350 | 16,587 | 16,586.0 | 2 | 3.0 | 16,589 |
| 0.0003351–0.0004798 | 16,584 | 16,580.2 | 1 | 4.8 | 16,585 |
| 0.0004799–0.0007357 | 16,577 | 16,581.0 | 12 | 8.0 | 16,589 |
| 0.0007358–0.0011664 | 16,549 | 16,558.2 | 22 | 12.8 | 16,571 |
| 0.0011665–0.0016138 | 16,556 | 16,554.0 | 19 | 21.0 | 16,575 |
| 0.0016139–0.0027321 | 16,557 | 16,554.9 | 32 | 34.1 | 16,589 |
| 0.0027322–0.0044384 | 16,527 | 16,526.5 | 56 | 56.5 | 16,583 |
| 0.0044385–0.0098603 | 16,463 | 16,478.8 | 126 | 110.2 | 16,589 |
| >0.0098603 | 15,984 | 15,965.7 | 725 | 743.3 | 16,709 |
| Variables | Study Set | Validation Set | p-Value |
|---|---|---|---|
| n | 165,893 | 43,569 | |
| HA-AKI Stage 3 | 995 (0.60) | 271 (0.62) | 0.594 |
| Gender: Men. (n) % | 74,962 (45.2) | 19,606 (44.9) | 0.105 |
| Age (years). mean (SD) | 54.9 (20.6) | 55.7 (22.1) | 0.389 |
| Chronic comorbidities | |||
| Diabetes (n) % | 30,357 (18.3) | 7840 (17.9) | 0.048 |
| Hypertension (n) % | 65,554 (39.5) | 16,991 (38.9) | 0.059 |
| Ischemic Heart Disease (n) % | 12,428 (7.5) | 3033 (6.9) | <0.001 |
| Ischemic Cerebrovascular disease (n) % | 11,446 (6.9) | 2614 (6.0) | <0.001 |
| Ischemic Peripheral vascular disease (n) % | 8706 (5.2) | 2396 (5.5) | 0.037 |
| Chronic digestive disease (n) % | 9627 (5.8) | 2483 (5.7) | 0.407 |
| Chronic liver disease (n) % | 5667 (3.4) | 1307 (3.0) | <0.001 |
| Chronic congestive heart failure (n) % | 14,344 (8.6) | 3267 (7.5) | <0.001 |
| Chronic obstructive pulmonary disease (n) % | 23,272 (14) | 6535 (15.0) | <0.001 |
| Malignancy (n) % | 23,504 (14.2) | 6317 (14.5) | 0.081 |
| Rheumatologic disease (n) % | 6828 (4.1) | 1743 (4.0) | 0.285 |
| Urologic disease (n) % | 11,926 (7.2) | 3135 (7.1) | 0.971 |
| Chronic Kidney Disease stages | 0.2758 | ||
| 0 + I | 137,385 (82.8) | 36,162 (83.0) | |
| II | 16,252 (9.8) | 4182 (9.6) | |
| III | 9265 (5.6) | 2396 (5.5) | |
| IV | 2991 (1.8) | 829 (1.9) | |
| Clinical variables along hospital admission | |||
| Urgent admission (n) % | 108,577 (65.5) | 28,319 (65.0) | 0.077 |
| Anaemia (n) % | 23,291 (14.0) | 6186 (14.2) | 0.397 |
| Acute respiratory failure (n) % | 7803 (4.7) | 2178 (5.0) | 0.011 |
| Acute Hearth failure (n) % | 6204 (3.7) | 1655 (3.8) | 0.565 |
| SIRS (n) % | 2358 (1.4) | 653 (1.5) | 0.227 |
| Circulatory shock (n) % | 2018 (1.2) | 566 (1.3) | 0.167 |
| Major surgery (n) % | 61,583 (37.1) | 13,942 (32.0) | <0.001 |
| Exposure to contrast dyes (n) % | 14,698 (8.9) | 3.921 (9.0) | 0.36 |
| Exposure to nephrotoxic drugs (n) % | 85,863 (51.8) | 23,135 (53.1) | <0.001 |
| Acute Kidney Injury = 0 | Acute Kidney Injury = 1 | Total | |||
|---|---|---|---|---|---|
| Risk Deciles | Observed | Expected | Observed | Expected | |
| <0.0001486 | 4342 | 4343.4 | 2 | 0.58 | 4344 |
| 0.0001486–0.0002375 | 4347 | 4347.7 | 2 | 1.30 | 4349 |
| 0.0002376–0.0003818 | 4374 | 4371.8 | 0 | 2.12 | 4374 |
| 0.0003819–0.0006162 | 4353 | 4355.7 | 6 | 3.23 | 4359 |
| 0.0006163–0.0009573 | 4351 | 4352.3 | 6 | 4.70 | 4357 |
| 0.0009574–0.0015601 | 4345 | 4351.2 | 9 | 6.74 | 4358 |
| 0.0015602–0.0025301 | 4347 | 4345.1 | 8 | 9.86 | 4355 |
| 0.0025302–0.0044511 | 4349 | 4341.6 | 8 | 15.3 | 4357 |
| 0.0044512–0.0101964 | 4327 | 4329.1 | 31 | 28.8 | 4358 |
| >0.0101964 | 4159 | 4157.7 | 199 | 200.24 | 4358 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpio, J.D.; Marco, M.P.; Martin, M.L.; Ramos, N.; de la Torre, J.; Prat, J.; Torres, M.J.; Montoro, B.; Ibarz, M.; Pico, S.; et al. Development and Validation of a Model to Predict Severe Hospital-Acquired Acute Kidney Injury in Non-Critically Ill Patients. J. Clin. Med. 2021, 10, 3959. https://doi.org/10.3390/jcm10173959
Carpio JD, Marco MP, Martin ML, Ramos N, de la Torre J, Prat J, Torres MJ, Montoro B, Ibarz M, Pico S, et al. Development and Validation of a Model to Predict Severe Hospital-Acquired Acute Kidney Injury in Non-Critically Ill Patients. Journal of Clinical Medicine. 2021; 10(17):3959. https://doi.org/10.3390/jcm10173959
Chicago/Turabian StyleCarpio, Jacqueline Del, Maria Paz Marco, Maria Luisa Martin, Natalia Ramos, Judith de la Torre, Joana Prat, Maria J. Torres, Bruno Montoro, Mercedes Ibarz, Silvia Pico, and et al. 2021. "Development and Validation of a Model to Predict Severe Hospital-Acquired Acute Kidney Injury in Non-Critically Ill Patients" Journal of Clinical Medicine 10, no. 17: 3959. https://doi.org/10.3390/jcm10173959
APA StyleCarpio, J. D., Marco, M. P., Martin, M. L., Ramos, N., de la Torre, J., Prat, J., Torres, M. J., Montoro, B., Ibarz, M., Pico, S., Falcon, G., Canales, M., Huertas, E., Romero, I., Nieto, N., Gavaldà, R., & Segarra, A. (2021). Development and Validation of a Model to Predict Severe Hospital-Acquired Acute Kidney Injury in Non-Critically Ill Patients. Journal of Clinical Medicine, 10(17), 3959. https://doi.org/10.3390/jcm10173959






