Treatment of Adolescents with Concurrent Substance Use Disorder and Attention-Deficit/Hyperactivity Disorder: A Systematic Review
Abstract
:1. Introduction
2. Methods
- (a)
- Studies were original studies published between 2000 and 2021 (11 April 2021) in peer-reviewed, English-language journals.
- (b)
- Studies evaluated the effectiveness of treatment of ADHD in adolescents (12–20 years old) with or without concurrent SUD.
- (c)
- Study treatments involved a pharmacological, psychosocial, or complementary (e.g., dietary) intervention targeted at ADHD.
- (d)
- (e)
- Studies were randomized controlled trials (RCTs), controlled clinical trials, randomized cross-over studies, or relevant meta-analyses.
- (f)
- Outcome measures included validated rating scales for ADHD, and—in studies involving patients with comorbid SUD—a quantitative measure of consumption of substances (e.g., days/frequency of use/abstinence).
- (g)
- Studies had to include a minimum of 10 (ADHD plus SUD) or a minimum of 20 (ADHD without SUD) adolescent patients per treatment condition.
- (h)
- Studies in mixed samples of children and adolescents, or adolescents and adults had to meet inclusion criterion.
- (i)
- Studies in mixed samples of children and adolescents, or adolescents and adults had to have separate outcomes analyzed and reported for the adolescent subgroup.
3. Results
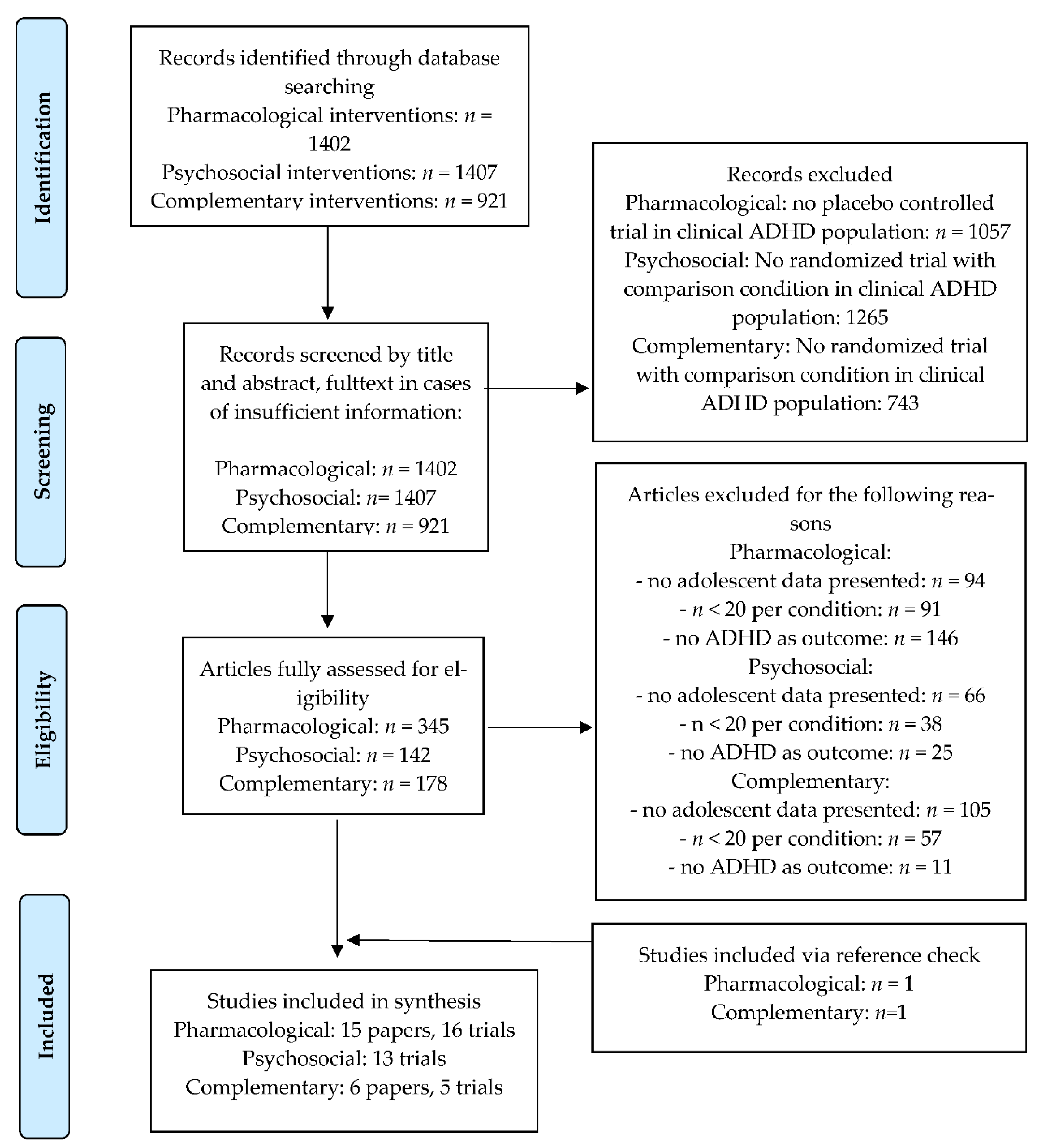
3.1. Literature Search
3.2. Pharmacological Interventions
3.2.1. Adolescents with Concurrent ADHD and SUD
3.2.2. Adolescents with ADHD but without SUD Comorbidity
3.3. Childhood ADHD and Later SUD
3.3.1. Childhood ADHD and the Risk of Later SUD
3.3.2. Stimulant Treatment of Childhood ADHD and the Risk of Later SUD
3.4. Psychosocial Interventions
3.4.1. Adolescents with Concurrent ADHD and SUD
3.4.2. Adolescents with ADHD but without SUD Comorbidity
3.5. Complementary Interventions
3.5.1. Adolescents with Concurrent ADHD and SUD
3.5.2. Adolescents with ADHD but without SUD Comorbidity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lee, S.S.; Humphreys, K.L.; Flory, K.; Liu, R.; Glass, K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: A meta-analytic review. Clin. Psychol. Rev. 2011, 31, 328–341. [Google Scholar] [CrossRef] [Green Version]
- Groenman, A.P.; Janssen, T.W.P.; Oosterlaan, J. Childhood Psychiatric Disorders as Risk Factor for Subsequent Substance Abuse: A Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 556–569. [Google Scholar] [CrossRef]
- Armstrong, T.D.; Costello, E.J. Community Studies on Adolescent Substance Use, Abuse, or Dependence and Psychiatric Comorbidity. J. Consult. Clin. Psychol. 2002, 70, 1224–1239. [Google Scholar] [CrossRef]
- Van Emmerik-van Oortmerssen, K.; van de Glind, G.; van den Brink, W.; Smit, F.; Crunelle, C.L.; Swets, M.; Schoevers, R.A. Prevalence of attention-deficit-hyperactivity disorder in substance use disorder patients: A meta-analysis and meta-regression analysis. Drug Alcohol Depend. 2012, 122, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Kramer, T.L.; Robbins, J.M.; Phillips, S.D.; Miller, T.; Burns, B.J. Detection and outcomes of substance use disorders in adolescents seeking mental health treatment. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 1318–1326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couwenbergh, C.; van den Brink, W.; Zwart, K.; Vreugdenhil, C.; van Wijngaarden-Cremers, P.; van der Gaag, R.J. Comorbid psychopathology in adolescents and young adults treated for substance use disorders: A review. Eur. Child Adolesc. Psychiatry 2006, 15, 319–328. [Google Scholar] [CrossRef]
- Derks, E.M.; Vink, J.M.; Willemsen, G.; van den Brink, W.; Boomsma, D.I. Genetic and environmental influences on the relationship between adult ADHD symptoms and self-reported problem drinking in 6024 Dutch twins. Psychol. Med. 2014, 44, 2673–2683. [Google Scholar] [CrossRef] [PubMed]
- Vink, J.M.; Schellekens, A. Relating addiction and psychiatric disorders. Science 2018, 361, 1323–1324. [Google Scholar]
- Abdellaoui, A.; Smit, D.J.A.; van den Brink, W.; Denys, D.; Verweij, K.J.H. Genomic Relationships across Psychiatric Disorders Including Substance Use Disorders. Drug Alcohol Depend. 2021, 220, 108535. [Google Scholar] [CrossRef]
- Volkow, N.D.; Wang, G.J.; Newcorn, J.; Telang, F.; Solanto, M.V.; Fowler, F.S.; Swanson, L.M. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch. Gen. Psychiatry 2007, 64, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Adisetiyo, V.; Gray, K.M. Neuroimaging the Neural Correlates of Increased Risk for Substance Use Disorders in Attention-Deficit/Hyperactivity Disorder-A Systematic Review. Am. J. Addict. 2017, 26, 99–111. [Google Scholar] [CrossRef]
- Molina, B.S.; Pelham, W.E., Jr. Attention-deficit/hyperactivity disorder and risk of substance use disorder: Developmental considerations, potential pathways, and opportunities for research. Annu. Rev. Clin. Psychol. 2014, 10, 607–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taurines, R.; Schmitt, J.; Renner, T.; Conner, A.C.; Warnke, A.; Romanos, M. Developmental comorbidity in attention-deficit/hyperactivity disorder. Atten. Deficit Hyperact. Disord. 2010, 2, 267–289. [Google Scholar] [CrossRef]
- Ormel, J.; Raven, D.; van Oort, F.; Hartman, C.A.; Reijneveld, S.A.; Veenstra, R.; Vollebergh, W.A.M.; Buitelaar, J.; Verhulst, F.C.; Oldehinkel, A.J. Mental health in Dutch adolescents: A TRAILS report on prevalence, severity, age of onset, continuity and co-morbidity of DSM disorders. Psychol. Med. 2015, 45, 345–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ottosen, C.; Larsen, J.T.; Faraone, S.V.; Chen, Q.; Hartman, C.; Larsson, H.; Petersen, L.; Dalsgaard, S. Sex differences in comorbidity patterns of attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2019, 58, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.M.; Groen, Y.; Fuermaier, A.B.M.; Tucha, O. The female side of pharmacotherapy for ADHD-A systematic literature review. PLoS ONE 2020, 15, e0239257. [Google Scholar] [CrossRef]
- McHugh, R.K.; Votaw, V.R.; Sugarman, D.E.; Greenfield, S.F. Sex and gender differences in substance use disorders. Clin. Psychol. Rev. 2018, 66, 12–23. [Google Scholar] [CrossRef]
- Landelijke Stuurgroep Multidisciplinaire Richtlijnontwikkeling in de GGZ. Multidisciplinaire Richtlijn ADHD bij Kinderen en Jeugdigen; Trimbos Institute: Utrecht, The Netherlands, 2005. [Google Scholar]
- National Health and Medical Research Council. Draft Australian Guidelines on Attention Deficit Hyperactivity Disorder. (ADHD). 2009. Available online: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/ch54_draft_guidelines.pdf (accessed on 25 June 2021).
- Scottish Intercollegiate Guidelines Network (SIGN). Edinburgh: The Network; Management of Attention Deficit and Hyperkinetic Disorders in Children and Young People: A National Clinical Guideline (Internet). October 2009. Available online: http://www.sign.ac.uk/ (accessed on 25 June 2021).
- Association of the Scientific Medical Societies in Germany. 2018. Available online: https://en.wikipedia.org/wiki/Association_of_the_Scientific_Medical_Societies_in_Germany (accessed on 25 June 2021).
- Canadian ADHD Resource Alliance (CADDRA). Canadian ADHD Practice Guidelines, 4th ed.; CADDRA: Toronto, ON, Canada, 2018. [Google Scholar]
- National Institute for Health and Care Excellence (NICE). Attention Deficit Hyperactivity Disorder: Diagnosis and Management. NG87. March 2018. Available online: www.nice.org.uk/guidance/ng87 (accessed on 25 June 2021).
- Wolraich, M.L.; Hagan, J.F.; Allan, C.; Chan, E.; Davidson, D.; Earls, M.; Evans, S.W.; Flinn, S.K.; Froelich, T.; Frost, J.; et al. Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 2019, 144, e20192528. [Google Scholar] [CrossRef] [Green Version]
- Spencer, T.J.; Faraone, S.V.; Michelson, D.; Adler, L.A.; Reimherr, F.W.; Glatt, S.J.; Biederman, J. Atomoxetine and adult attention-deficit/hyperactivity disorder: The effects of comorbidity. J. Clin. Psychiatry 2006, 67, 415–420. [Google Scholar] [CrossRef]
- Wilens, T.E.; Kratochvil, C.; Newcorn, J.H.; Gao, H. Do children and adolescents with ADHD respond differently to atomoxetine? J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 149–157. [Google Scholar] [CrossRef]
- Findling, R.L.; Turnbow, J.; Burnside, J.; Melmed, R.; Civil, R.; Li, Y. A randomized, double blind, multicenter, parallel-group, placebo-controlled, dose-optimization study of the methylphenidate transdermal system for the treatment of ADHD in adolescents. CNS Spectr. 2010, 15, 419–430. [Google Scholar] [CrossRef]
- McAweeney, M.; Rogers, N.L.; Huddleston, C.; Moore, D.; Gentile, J.P. Symptom prevalence of ADHD in a community residential substance abuse treatment program. J. Atten. Disord. 2010, 13, 601–608. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2000. [Google Scholar]
- World Health Organization; Division of Mental Health. Classification of Diseases (ICD). 2018 version. 1994. Available online: https://icd.who.int/browse11/l-m/en (accessed on 25 June 2021).
- Sterne, J.A.; Savovíc, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Higgins, J.P.T. RoB2: A revised tool for assessing risk of bias in randomised trials. Br. Med J. 2019, 366, 4898. [Google Scholar] [CrossRef] [Green Version]
- Harbour, R.; Miller, J. A new system for grading recommendations in evidence based guidelines. Br. Med J. 2001, 323, 334–336. [Google Scholar] [CrossRef] [Green Version]
- Pelham, W.E.; Smith, B.H.; Evans, S.W.; Bukstein, O.; Gnagy, E.M.; Greiner, A.R.; Sibley, M.H. The effectiveness of short-and long-acting stimulant medications for adolescents with ADHD in a naturalistic school setting. J. Atten. Disord. 2013, 21, 40–45. [Google Scholar] [CrossRef]
- Newcorn, J.H.; Nagy, P.; Childress, A.C.; Frick, G.; Yan, B.; Pliszka, S. Randomized, double-blind, placebo-controlled acute comparator trials of lisdexamfetamine and extended-release methylphenidate in adolescents with attention-deficit/hyperactivity disorder. CNS Drugs 2017, 31, 999–1014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunill, R.; Castells, X.; Tobias, A.; Capella, D. Pharmacological treatment of attention deficit hyperactivity disorder with co-morbid drug dependence. J. Psychopharmacol. 2014, 29, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Szobot, C.M.; Rohde, L.A.; Katz, B.; Ruaro, P.; Schaefer, T.; Walcher, M.; Bukstein, O.; Pechansky, F. A randomized crossover clinical study showing that methylphenidate-SODAS improves attention-deficit/hyperactivity disorder symptoms in adolescents with substance use disorder. Braz. J. Med. Biol. Res. 2008, 41, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Riggs, P.D.; Winhusen, T.; Davies, R.D.; Leimberger, J.D.; Mikulich-Gilbertson, S.; Klein, C.; Macdonald, M. Randomized controlled trial of osmotic-release methylphenidate with cognitive-behavioral therapy in adolescents with attention-deficit/hyperactivity disorder and substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 903–914. [Google Scholar] [CrossRef] [Green Version]
- Riggs, P.D.; Hall, S.K.; Mikulich-Gilbertson, S.K.; Lohman, M.; Kayser, A. A randomized controlled trial of pemoline for attention-deficit/hyperactivity disorder in substance-abusing adolescents. J. Am. Acad. Child Adolesc. Psychiatry 2004, 43, 420–429. [Google Scholar] [CrossRef]
- Thurstone, C.; Riggs, P.D.; Salomonsen-Sautel, S.; Mikulich-Gilbertson, S.K. Randomized, controlled trial of atomoxetine for attention-deficit/hyperactivity disorder in adolescents with substance use disorder. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 573–582. [Google Scholar] [PubMed] [Green Version]
- Wilens, T.E.; McBurnett, K.; Bukstein, O.; McGough, J.; Greenhill, L.; Lerner, M.; Sten, M.A. Multisite controlled study of OROS methylphenidate in the treatment of adolescents with attention-deficit/hyperactivity disorder. Arch. Pediatrics Adolesc. Med. 2006, 160, 82–90. [Google Scholar] [CrossRef] [Green Version]
- Spencer, T.J.; Wilens, T.E.; Biederman, J.; Weisler, R.H.; Read, S.C.; Pratt, R. Efficacy and safety of mixed amphetamine salts extended release (Adderall XR) in the management of attention-deficit/hyperactivity disorder in adolescent patients: A 4-week, randomized, double-blind, placebo-contrlled, parallel-group study. Clin. Ther. 2006, 28, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Findling, R.L.; Childress, A.C.; Cutler, A.J.; Gasior, M.; Hamdani, M.; Ferreira-Cornwell, M.C. Efficacy and safety of lisdexamfetamine dimesylate in adolescents with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Bostic, J.Q.; Biederman, J.; Spencer, T.J.; Wilens, T.E.; Prince, J.B.; Monuteaux, M.C.; Sienna, M.; Polisner, D.A.; Hatch, M. Pemoline Treatment of Adolescents with Attention Deficit Hyperactivity Disorder: A Short-Term Controlled Trial. J. Child Adolesc. Psychopharmacol. 2000, 10, 205–216. [Google Scholar] [CrossRef]
- Bangs, M.E.; Emslie, G.J.; Spencer, T.J.; Ramsey, J.L.; Carlson, C.; Bartky, E.J.; Busner, J.; Duesenberg, D.A.; Harshawat, P.; Kaplan, S.L.; et al. Efficacy and safety of atomoxetine in adolescents with attention-deficit/hyperactivity disorder and major depression. J. Child Adolesc. Psychopharmacol. 2007, 17, 407–420. [Google Scholar]
- Biederman, J.; Melmed, R.D.; Patel, A.; McBurnett, K.; Konow, J.; Lyne, A.; Scherer, N. A Randomized, Double-Blind, Placebo-Controlled Study of Guanfacine Extended Release in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder. Pediatrics 2008, 121, e73–e84. [Google Scholar] [CrossRef]
- Sallee, F.R.; McGough, J.; Wigal, T.; Donahue, J.; Lyne, A.; Biederman, J. Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: A placebo-controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 155–165. [Google Scholar] [CrossRef]
- Wilens, T.E.; Robertson, B.; Sikirica, V.; Harper, L.; Young, J.L.; Bloomfield, R.; Lyne, A. A randomized, placebo-controlled trial of guanfacine extended release in adolescents with attention-deficit/hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 916–925. [Google Scholar] [CrossRef]
- Cerrillo-Urbina, A.J.; García-Hermoso, A.; Pardo-Guijarro, M.J.; Sánchez-López, M.; Santos Gómez, J.L.; Martínez-Vizcaíno, V. The effects of lang-acting stimulant and nonstimulant medications in children and adolescents with attention-deficit/hyperactivity disorder: A meta-analysis of randomized controlled trials. J. Child Adolesc. Psychopharmacol. 2018, 28, 494–507. [Google Scholar] [CrossRef] [PubMed]
- McGough, J.; McCrachen, J.; Swanson, J.; Riddle, M.; Kollins, S.; Greenhill, L.; Abikoff, H.; Davies, M.; Chuang, S.; Wigal, T.; et al. Pharmacogenetics of methylphenidate response in preschoolers with ADHD. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 1314–1322. [Google Scholar] [CrossRef]
- Charach, A.; Yeung, E.; Climans, T.; Lillie, E. Childhood attention-deficit/hyperactivity disorders and future substance use disorders: Comparative meta-analyses. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 9–21. [Google Scholar] [CrossRef]
- Erskine, H.E.; Norman, R.E.; Ferrari, A.J.; Chan, G.C.; Copeland, W.E.; Whiteford, H.A.; Scott, J.G. Long-Term Outcomes of Attention-Deficit/Hyperactivity Disorder and Conduct Disorder: A Systematic Review and Meta-Analysis. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Groenman, A.; Oosterlaan, J.; Rommelse, N.; Franke, B.; Roeyers, H.; Oades, R.D.; Sergeant, J.A.; Buitelaar, J.K.; Faraone, S.V. Substance use disorders in adolescents with attention deficit hyperactivity disorder: A 4-year follow-up study. Addiction 2013, 108, 1503–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, B.S.G.; Howard, A.L.; Swanson, J.M.; Stehli, A.; Mitchell, J.T.; Kennedy, T.M.; Epstein, J.N.; Arnold, L.E.; Hechtman, L.; Vitiello, B.; et al. Substance use through adolescence into early adulthood after childhood-diagnosed ADHD: Findings from the MTA longitudinal study. J. Child Psychol. Psychiatry 2018, 56, 692–702. [Google Scholar] [CrossRef]
- Wilens, T.E.; Faraone, S.V.; Biederman, J.; Gunawardene, S. Does stimulant therapy of attention-deficit/hyperactivity disorder beget later substance abuse? A meta-analytic review of the literature. Pediatrics 2003, 111, 179–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphreys, K.L.; Eng, T.; Lee, S.S. Stimulant medication and substance use outcomes: A meta-analysis. JAMA Pychiatry 2013, 70, 740–749. [Google Scholar]
- Molina, B.S.G.; Hinshaw, S.P.; Arnold, L.E.; Swanson, J.M.; Pelham, W.E.; Hechtman, L.; Lu, B. Adolescent substance use in the multimodal treatment study of attention-deficit/hyperactivity disorder (ADHD) (MTA) as a function of childhood ADHD, Random assignment to childhood treatments, and subsequent medication (2013). J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 250–263. [Google Scholar] [CrossRef] [Green Version]
- Groenman, A.P.; Oosterlaan, J.; Rommelse, N.N.J.; Franke, B.; Greven, C.U.; Hoekstra, P.J.; Hartman, C.A.; Luman, M.; Roeyers, H.; Oades, R.D.; et al. Stimulant treatment for attention-deficit hyperactivity disorder and risk of developing substance use disorder. Br. J. Psychiatry 2013, 203, 112–119. [Google Scholar] [CrossRef] [Green Version]
- Dalsgaard, S.; Mortensen, P.B.; Frydenberg, M.; Thomsen, P.H. ADHD, stimulant treatment in childhood and subsequent substance abuse in adulthood-a naturalistic long-term follow-up study. Addict. Behav. 2014, 39, 325–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Groenman, A.P.; Schweren, L.J.S.; Weeda, W.; Luman, M.; Noordermeer, S.D.S.; Heslenfeld, D.J.; Franke, B.; Faraone, S.V.; Rommelse, N.; Hartman, C.A.; et al. Stimulant treatment profiles predicting co-occurring substance use disorders in individuals with attention-deficit/hyperactivity disorder. Eur. Child Adolesc. Psychiatry 2019, 28, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Chang, Z.; Lichtenstein, P.; Halldner, L.; D’Onofrio, B.; Serlachius, E.; Fazel, S.; Långström, N.; Larsson, H. Stimulant ADHD medication and risk for substance abuse. J. Child Psychol. Psychiatry 2014, 55, 878–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinn, P.D.; Chang, Z.; Hur, K.; Gibbons, R.D.; Lahey, B.B.; Rickert, M.E. ADHD medication and substance-related problems. Am. J. Psychiatry 2017, 174, 179. [Google Scholar] [CrossRef] [Green Version]
- Evans, S.W.; Serpell, Z.N.; Schultz, B.K.; Pastor, D.A. Cumulative benefits of secondary school-based treatment of students with attention deficit hyperactivity disorder. Sch. Psychol. Rev. 2007, 36, 256–273. [Google Scholar] [CrossRef]
- Evans, S.W.; Langberg, J.M.; Schultz, B.K.; Vaughn, A.; Altaye, M.; Marshall, S.A.; Zoromski, A.K. Evaluation of a school-based treatment program for young adolescents with ADHD. J. Consult. Clin. Psychol. 2016, 84, 15. [Google Scholar] [CrossRef] [PubMed]
- Langberg, J.M.; Epstein, J.N.; Becker, S.P.; Girio-Herrera, E.; Vaughn, A.J. Evaluation of the homework, organization, and planning skills (HOPS) intervention for middle school students with attention deficit hyperactivity disorder as implemented by school mental health providers. Sch. Psychol. Rev. 2012, 41, 342–364. [Google Scholar] [CrossRef]
- Schramm, S.A.; Hennig, T.; Linderkamp, F. Training problem solving and organizational skills in adolescents with attention-deficit/hyperactivity disorder: A randomized controlled trial. J. Cogn. Educ. Psychol. 2016, 15, 391–411. [Google Scholar] [CrossRef]
- Steeger, C.M.; Gondoli, D.M.; Gibson, B.S.; Morrissey, R.A. Combined cognitive and parent training interventions for adolescents with ADHD and their mothers: A randomized controlled trial. Child Neuropsychol. 2016, 22, 394–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkley, R.A.; Edwards, G.; Laneri, M.; Fletcher, K.; Metevia, L. The Efficacy of Problem-Solving Communication Training Alone, Behavior Management Training Alone, and Their Combination for Parent–Adolescent Conflict in Teenagers with ADHD and ODD. J. Consult. Clin. Psychol. 2001, 69, 926–941. [Google Scholar] [CrossRef]
- Boyer, B.E.; Geurts, H.M.; Prins, P.J.; Van der Oord, S. Two Novel CBTs for Adolescents with ADHD: The Value of Planning Skills. Eur. Child Adolesc. Psychiatry 2015, 24, 1075–1090. [Google Scholar] [CrossRef]
- Meyer, J.; Ramklint, M.; Hallerbäck, M.U.; Lööf, M.; Isaksson, J. Evaluation of a structured skills training group for adolescents with attention-deficit/hyperactivity disorder: A randomised controlled trial. Eur. Child Adolesc. Psychiatry 2021, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sibley, M.H.; Graziano, P.A.; Kuriyan, A.B.; Coxe, S.; Pelham, W.E.; Rodriguez, L.; Ward, A. Parent–teen behavior therapy+ motivational interviewing for adolescents with ADHD. J. Consult. Clin. Psychol. 2016, 84, 699. [Google Scholar] [CrossRef] [PubMed]
- Sibley, M.H.; Rodriguez, L.; Coxe, S.; Page, T.; Espinal, K. Parent–Teen Group versus Dyadic Treatment for Adolescent ADHD: What Works for Whom? J. Clin. Child Adolesc. Psychol. 2019, 49, 476–492. [Google Scholar] [CrossRef] [PubMed]
- Sibley, M.H.; Graziano, P.A.; Coxe, S.; Bickman, L.; Martin, P. Effectiveness of Motivational Interviewing–Enhanced Behavior Therapy for Adolescents with Attention-Deficit/Hyperactivity Disorder: A Randomized Community-Based Trial. J. Am. Acad. Child Adolesc. Psychiatry 2020, 60, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Sprich, S.E.; Safren, S.A.; Finkelstein, D.; Remmert, J.E.; Hammerness, P. A randomized controlled trial of cognitive behavioral therapy for ADHD in medication-treated adolescents. J. Child Psychol. Psychiatry 2016, 57, 1218–1226. [Google Scholar] [CrossRef] [Green Version]
- Vidal, R.; Castells, J.; Richarte, V.; Palomar, G.; García, M.; Nicolau, R.; Ramos-Quiroga, J.A. Group therapy for adolescents with attention-deficit/hyperactivity disorder: A randomized controlled trial. J. Am. Acad. Child Adolesc. Psychiatry 2015, 54, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, G.M.; Mohamed, S. Effect of Regular Aerobic Exercises on Behavioral, Cognitive and Psychological Response in Patients with Attention Deficit-Hyperactivity Disorder. Life Sci. J. 2011, 8, 366–371. [Google Scholar]
- Bink, M.; Bongers, I.L.; Popma, A.; Janssen, T.W.; van Nieuwenhuizen, C. 1-Year Follow-up of Neurofeedback Treatment in Adolescents with Attention-Deficit Hyperactivity Disorder: Randomised Controlled Trial. BJPsych Open 2016, 2, 107–115. [Google Scholar] [CrossRef] [Green Version]
- Gray, S.A.; Chaban, P.; Martinussen, R.; Goldberg, R.; Gotlieb, H.; Kronitz, R.; Tannock, R. Effects of a computerized working memory training program on working memory, attention, and academics in adolescents with severe LD and comorbid ADHD: A randomized controlled trial. J. Child Psychol. Psychiatry 2012, 53, 1277–1284. [Google Scholar] [CrossRef]
- Matsudaira, T.; Gow, R.V.; Kelly, J.; Murphy, C.; Potts, L.; Sumich, A.; Taylor, E. Biochemical and psychological effects of omega-3/6 supplements in male adolescents with attention-deficit/hyperactivity disorder: A randomized, placebo-controlled, clinical trial. J. Child Adolesc. Psychopharmacol. 2015, 25, 775–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konstenius, M.; Jayaram-Lindstrom, N.; Guterstam, J.; Beck, O.; Philips, B.; Franck, J. Methylphenidate for attention deficit hyperactivity disorder and drug relapse in criminal offenders with substance dependence: A 24-week randomized placebo-controlled trial. Addiction 2014, 109, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Levin, F.R.; Mariani, J.J.; Specker, S.; Mooney, M.; Mahony, A.; Brooks, D.J.; Grabowski, J. Extended-release mixed amphetamine salts vs placebo for comorbid adult attention-deficit/hyperactivity disorder and cocaine use disorder. A randomized clinical trial. JAMA Psychiatry 2015, 72, 593–602. [Google Scholar] [CrossRef]
- Cornforth, C.; Sonuga-Barke, E.; Coghill, D. Stimulant drug effects on attention deficit/hyperactivity disorder: A review of the effects of age and sex of patients. Curr. Pharm. Des. 2010, 16, 2424–2433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buitelaar, J.K.; Van der Gaag, R.J.; Swaab-Barneveld, H.; Kuiper, M. Prediction of clinical response to methylphenidate in children with attention-deficit hyperactivity disorder. J. Am. Acad. Child Adolesc. Psychiatry 1995, 34, 1025–1032. [Google Scholar] [CrossRef]
- Jensen, P.S.; Hinshaw, S.P.; Kraemer, H.C.; Lenora, N.; Newcorn, J.H.; Abikoff, H.B.; March, J.S. ADHD comorbidity findings from the MTA study: Comparing comorbid subgroups. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Hinshaw, S.P. Moderators and mediators of treatment outcome for youth with ADHD understanding for whom and how interventions work. J. Pediatric Psychol. 2007, 32, 664–675. [Google Scholar] [CrossRef]
- Tamm, L.; Trello-Rishel, K.; Riggs, P.; Nakonezny, P.; Acosta, M.; Bailey, G.; Winhusen, T. Predictors of treatment response in adolescents with comorbid substance use disorder and attention-deficit/hyperactivity disorder. J. Subst. Abuse Treat. 2013, 44, 224–230. [Google Scholar] [CrossRef] [Green Version]
- Schubiner, H.; Saules, K.K.; Arfken, C.L.; Johanson, C.; Schuster, C.R.; Lockhart, N.; Donlin, E.A.; Pihlgren, E. Double-blind placebo-controlled trial of methylphenidate in the treatment of adult ADHD patients with comorbid cocaine dependence. Exp. Clin. Psychopharmacol. 2002, 10, 286–294. [Google Scholar] [CrossRef]
- Levin, F.R.; Upadhyaya, H.P. Diagnosing ADHD in adults with substance use disorder: DSM-IV criteria and differential diagnosis. J. Clin. Psychiatry 2007, 68, e18. [Google Scholar] [CrossRef]
- Crunelle, C.L.; Veltman, D.J.; van Emmerik-van Oortmerssen, K.; Booij, J.; van den Brink, W. Impulsivity in adult ADHD patients with and without cocaine dependence. Drug Alcohol Depend. 2013, 129, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Van Emmerik-van Oortmerssen, K.; Vedel, E.; Kramer, F.J.; Blankers, M.; Dekker, J.J.M.; van den Brink, W.; Schoevers, R.A. Integrated cognitive behavioral therapy for ADHD in adult substance use disorder patients: Results of a randomized clinical trial. Drug Alcohol Depend. 2019, 197, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Özgen, H.; Spijkerman, R.; Noack, M.; Holtmann, M.; Schellekens, A.S.A.; van de Glind, G.; Banaschewski, T.; Barta, C.; Begeman, A.; Casas, M.; et al. International consensus statement for the screening, diagnosis, and treatment of adolescents with concurrent attention-deficit/hyperactivity disorder and substance use disorder. Eur. Addict. Res. 2020, 26, 223–232. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | Population | SUD Diagnosis | Study Design and Treatment | Concurrent Treatment | Treatment Completers | Primary Outcome Measurement: ADHD | Primary Outcome Measurement: SUD | Primary Outcome | Level of Evidence (SIGN) |
|---|---|---|---|---|---|---|---|---|---|
| Adolescents with ADHD and SUD | |||||||||
| Methylphenidate | |||||||||
| Szobot, 2008 [36] | 16 adolescents aged 15–21 years (mean 17.4 years; 100% male) with ADHD and cannabis an/or cocaine use disorder (DSM-IV) | Dependence: cannabis (93.8%) or cocaine (43.8%) | 6-week, randomized, SB, PC, crossover study: 2 × 3 weeks: MPH-SODAS (0.3–1.2 mg/kg/day) or placebo | No concurrent Tx for ADHD or SUD | 87.5% in both treatment conditions combined | Mean change over time on mother-reported SNAP-IV and investigator-rated CGI by treatment condition | Mean change over time in adolescent-reported days of substance use past 1 week by treatment condition | Significant improvement in ADHD symptoms and global functioning (both: p ≤ 0.001) in MPH-SODAS condition vs. placebo; no treatment effect on substance use | 2 − |
| Riggs, 2011 [37] | 303 adolescents aged 13–18 years (mean 16.5 years; 78.9% male) with ADHD and SUD (DSM-IV) | Dependence: cannabis (66.7%), alcohol (29.7%), cocaine (7.3%), hallucinogen (5%), sedative (2.6%), amphetamine (1.3%), or other (1.2%) Abuse: alcohol (26.4%), cannabis (25.4%), opiate (12.2%), hallucinogen (7.6%), sedative (7.3%), amphetamine (3%), cocaine (2.6%), or other (4%) | 16 weeks, RCT, DB, PC; parallel groups: OROS-MPH (max. 72 mg/day, titrated fixed dose) or placebo | 16-week individual CBT/MET for SUD | OROS-MPH: 78.1%; placebo: 71.7% | Mean change over time on adolescent-reported ADHD-RS-IV by treatment group | Mean change over time in adolescent-reported days of substance use past 4 weeks (TLFB) by treatment group | No significant difference between both groups in reduction of ADHD symptoms (d = 0.22; ns) and substance use (d = 0.05; ns) | 1 + |
| Pemoline | |||||||||
| Riggs, 2004 [38] | 69 adolescents aged 13–19 years (mean 15.8 years; 84.1% male) with ADHD, SUD, and CD (DSM-IV) | Dependence: alcohol (47.8%) or cannabis (73.9%) | 12 weeks, RCT, DB, PC; parallel groups: pemoline (75–112.5 mg/day) or placebo | No concurrent Tx for ADHD, SUD or CD | Pemoline: 54.3%; placebo: 50.0% | % responders on clinician-rated CGI (ADHD-symptoms “much improved” or “very much improved”) at study endpoint and mean change over time on parent-rated CHI by treatment group | Mean change over time in adolescent-reported days of substance use past 30 days (TLFB) and total number of negative urine drug screens by treatment group | Significantly more responders in pemoline group vs. placebo (d = 0.5; p = 0.05); no treatment effect on ADHD symptoms on CHI (d = 0.34; ns) and substance use (d = 0.05; ns) | 1 − |
| Atomoxetine | |||||||||
| Thurstone, 2010 [39] | 70 adolescents aged 13–19 years (mean 16.1 years; 78.6% male) with ADHD and SUD (DSM-IV) | Alcohol (28.6%), cannabis (95.7%), cocaine (2.9%), amphetamine (1.4%), or hallucinogen (1.4%) | 12 weeks, RCT, DB, PC; parallel groups: atomoxetine (<70 kg: 1.1–1.5 mg/kg/day; ≥70 kg: max. 100 mg/day) or placebo | 12-week individual CBT/MET for SUD | Atomoxetine: 91.4%; placebo: 94.3% | Mean change over time on an adolescent-reported DSM-IV checklist by treatment group | Mean change over time in adolescent-reported days of substance use past 4 weeks (TLFB) by treatment group | No significant difference between both groups in reduction of ADHD symptoms (d = 0.10; ns) and substance use (d = 0.35; ns) | 1 + |
| Adolescents with ADHD, without SUD | |||||||||
| Methylphenidate | |||||||||
| Wilens 2006 [40] | 177 adolescents aged 13–18 years (mean 14.6 years; 80.2% male) with ADHD (DSM-IV) without a history of nonresponse to MPH, who responded favorably to OROS-MPH in an open-label titration phase | 2 weeks, RCT, DB, PC; parallel groups: OROS-MPH (18–72 mg/day, titrated fixed dose) or placebo | Subjects in behavioral treatment at study enrollment could continue their treatment | OROS-MPH: 81.6%; placebo: 68.9% | Mean change over time on adolescent-reported ADHD-RS-IV by treatment group | NA | Significant improvement in ADHD symptoms in OROS-MPH group vs. placebo (d = 0.56; p = 0.001) | 1 − | |
| Findling, 2010a [27] | 217 adolescents aged 13–17 years (mean 14.6 years; 74.4% male) with ADHD (DSM-IV-TR) | 7 weeks, RCT, DB, PC; parallel groups: MTS (10, 15, 20, or 30 mg/day, titrated fixed dose) or placebo | No concurrent Tx | MTS: 65.5%; placebo: 40.3% | Mean change over time on adolescent-reported ADHD-RS-IV by treatment group | NA | Significant improvement in ADHD symptoms in MTS group vs. placebo (d = 1.33; p < 0.001) | 1 − | |
| Pelham, 2013 [33] | 30 adolescents aged 12–17 years (mean 14.1 years; 90% male) with ADHD (DSM-III) | 12-week, randomized, DB, PC, conditions cross-over study: IR-MPH (max. 75 mg/day, titrated dose) or pemoline (max. 112.5 mg/day, titrated dose) or placebo in a naturalistic school setting | Not reported | Not reported | Mean change over time on teacher-reported inattention/overactivity subscale of IOWA-CRS by treatment condition | NA | Significant improvement in ADHD symptoms in IR-MPH condition vs. placebo (d = 0.53; p < 0.05), but not in pemoline condition vs. placebo (d = 0.21; ns) | 2 − | |
| Newcorn 2017a [34] | 464 adolescents aged 13–17 years (mean 14.7 years; 66.4% male) with ADHD (DSM-IV-TR) | 8 weeks, RCT, DB, PC; parallel groups: OROS-MPH (12–72 mg/day, titrated flexible dose) or LDX (30–70 mg/day, titrated flexible dose) or placebo | Not reported | OROS-MPH: 84.9%; LDX: 83.3%; placebo: 73.1% | Mean change over time on parent-reported ADHD-RS-IV by treatment group | NA | Significant improvement in ADHD symptoms in OROS-MPH (d = 0.97; p < 0.0001) and LDX (d = 1.16; p < 0.0001) vs. placebo. No significant difference between OROS-MPH and LDX (p = 0.07) | 1 − | |
| Newcorn 2017b [34] | 549 adolescents aged 13–17 years (mean 14.7 years; 66.0% male) with ADHD (DSM-IV-TR) | 6 weeks, RCT, DB, PC; parallel groups: OROS-MPH (72 mg/day, titrated fixed dose) or LDX (70 mg/day, titrated fixed dose) or placebo | Not reported | OROS-MPH: 84.5%; LDX: 82.6%; placebo: 88.2% | Mean change over time on parent-reported ADHD-RS-IV by treatment group | NA | Significant improvement in ADHD symptoms in OROS-MPH (d = 0.50; p < 0.0001) and LDX (d = 0.82; p < 0.0001) vs. placebo. Significant improvement in LDX vs. OROS-MPH (d = 0.33; p = 0.0013) | 1 + | |
| (Lis)dexamphetamine/mixed amphetamine salts | |||||||||
| Spencer, 2006 [41] | 287 adolescents aged 13–17 years (mean 14.2 years; 65.5% male) with ADHD (DSM-IV-TR) | 4 weeks, RCT, DB, PC; parallel groups: MAS-XR (10, 20, 30, 40 mg/day, forced dose titration) or placebo | Not reported | 92.8% in all treatment groups combined | Mean change over time on adolescent-reported ADHD-RS-IV by treatment group | NA | Significant improvement in ADHD symptoms in MAS-XR group vs. placebo (d = 0.80; p ≤ 0.001) | 1 + | |
| Findling, 2011 [42] | 314 adolescents aged 13–17 years (mean 14.6 years; 70.3% male) with ADHD (DSM-IV-TR) | 4 weeks, RCT, DB, PC; parallel groups: LDX (30, 50, 70 mg/day, forced dose titration) or placebo | Subjects in behavioral treatment at study enrollment could continue their treatment | LDX: 83.4%; placebo: 87.3% | Mean change over time on adolescent-reported ADHD-RS-IV by treatment group | NA | Significant improvement in ADHD-symptoms in LDX groups vs. placebo (30 mg: d = 0.80; 50 mg: d = 1.23; 70 mg: d = 1.09; all: p ≤ 0.0056) | 1 + | |
| Pemoline | |||||||||
| Bostic, 2000 [43] | 21 adolescents aged 12–17 years (mean 14.1 years; 85.7% male) with ADHD (DSM-IV) | 10-week, randomized, DB, PC, crossover study: 2 × 4-week: pemoline (1–3 mg/kg) or placebo | Not reported | 71.4% in both treatment groups combined | Mean change over time on adolescent-reported ADHD-RS-IV by treatment condition | NA | Significant improvement in ADHD symptoms in pemoline condition vs. placebo (d = 2.05; p = 0.001) | 2 − | |
| Atomoxetine | |||||||||
| Bangs, 2007 [44] | 142 adolescents aged 12–18 years (mean 14.5 years; 73.2% male) with ADHD and major depression (DSM-IV) | 9 weeks, RCT, DB, PC; parallel groups: atomoxetine (1.2–1.8 mg/kg/day) or placebo | No concurrent Tx | Atomoxetine: 81.9%; placebo: 87.1% | Mean change over time on parent-reported ADHD-RS-IV and CDRS-R by treatment group | NA | Significant improvement in ADHD symptoms in atomoxetine group vs. placebo (d = 0.99; p <0.001); no significant difference in reduction of depressive symptoms (d = 0.20; ns) | 1 − | |
| Guanfacine | |||||||||
| Biederman, 2008 [45] | Subgroup of 80 adolescents (gender for subset not reported) aged 13–17 years with ADHD (DSM-IV) | 8 weeks, RCT, DB, PC; parallel groups: GXR (2, 3, and 4 mg/day, fixed dose escalation) or placebo | Not reported | Not reported for the adolescent subgroup | Mean change over time on parent-reported ADHD-RS-IV by treatment group | NA | No significant difference between GXR groups and placebo in reduction of ADHD symptoms (p > 0.05) | 1 − | |
| Sallee, 2009 [46] | Subgroup of 80 adolescents (gender for subset not reported) aged 13–17 years with ADHD (DSM-IV-TR) | 9 weeks, RCT, DB, PC; parallel groups: GXR (1, 2, 3, and 4 mg/day, fixed dose escalation) or placebo | Not reported | Not reported for the adolescent subgroup | Mean change over time on parent-reported ADHD-RS-IV by treatment group | NA | No significant difference between GXR groups and placebo in reduction of ADHD symptoms (p = 0.20) | 1 − | |
| Wilens 2015 [47] | 314 adolescents aged 13–18 years (mean 14.5 years; 64.7% male) with ADHD (DSM-IV-TR) | 13 weeks, RCT, DB, PC; parallel groups: GXR (4 to 7 mg/day dependent on weight and optimal dose titration) or placebo | Subjects in behavioral treatment at study enrollment could continue their treatment | GXR: 74.5%; placebo: 70.1% | Mean change over time on adolescent-reported ADHD-RS-IV by treatment group | NA | Significant improvement in ADHD symptoms in GXR group vs. placebo (d = 0.52; p < 0.001) | 1 − | |
| Study | Bias Arising from/Due to: | |||||
|---|---|---|---|---|---|---|
| Rnd | Int | Mis | Mea | Sel | Overall | |
| Adolescents with ADHD + SUD | ||||||
| Riggs, 2011 [37] |  |  |  |  |  |  |
| Szobot, 2008 [36] |  |  |  |  |  |  |
| Riggs, 2004 [38] |  |  |  |  |  |  |
| Thurstone, 2010 [39] |  |  |  |  |  |  |
| Adolescents with ADHD | ||||||
| Pelham, 2013 [33] |  |  |  |  |  |  |
| Findling, 2010 [27] |  |  |  |  |  |  |
| Wilens, 2006 [40] |  |  |  |  |  |  |
| Findling, 2011 [42] |  |  |  |  |  |  |
| Spencer, 2006 [41] |  |  |  |  |  |  |
| Bostic, 2000 [43] |  |  |  |  |  |  |
| Bangs, 2007 [44] |  |  |  |  |  |  |
| Biederman, 2008 [45] |  |  |  |  |  |  |
| Sallee, 2009 [46] |  |  |  |  |  |  |
| Wilens, 2015 [47] |  |  |  |  |  |  |
| Newcorn, 2017a [34] |  |  |  |  |  |  |
| Newcorn, 2017b [34] |  |  |  |  |  |  |


 low risk/high risk/some concerns. Rnd—randomization proces; Int—intended interventions; Mis—missing outcome data; Mea—measurement of the outcome; Sel—selection of the reported result.
low risk/high risk/some concerns. Rnd—randomization proces; Int—intended interventions; Mis—missing outcome data; Mea—measurement of the outcome; Sel—selection of the reported result.| AUTHOR, YEAR | Population | Study Design | Intervention | Treatment Completers | Outcome Measures | ADHD Outcomes | Functional Outcomes | Quality Rating |
|---|---|---|---|---|---|---|---|---|
| Clinic interventions: adolescent-focused | ||||||||
| Boyer, 2015 [68] | 159 adolescents with ADHD (DSM-IV-TR) aged 12–17 years (mean 14.4; 74% male) 78% medicated (only MPH. stable). No significant differences in medication use at baseline, post-test, and 3-month follow-up, and no differences in number of adolescents receiving additional psychosocial treatment between end of treatment and follow-up. | 2-arm RCT, 8-week treatment duration, stratified randomization on gender, medication (y/n), DB, 8 sessions of Plan My Life (PML) or 8 sessions Solution-Focused Treatment (SFT). | PML, n = 83, 8 adolescent sessions (frequency NR) CBT-based planning skills training, 2 parent sessions. SFT, n = 76, SFT = 8 adolescent sessions of self-formulated problem solving. Both PML and SFT also contained psychoeducation, MI, and personal treatment goal setting/monitoring and rewards. | ITT analyses End of treatment: PML: 79 (95%), SFT: 67 (88%), no significant differences in number of completers between conditions. Follow-up: PML: 77 (93%), SFT: 59 (71%), higher drop-out in SFT at follow-up. | ADHD-symptoms with Disruptive Behavior Disorder (DBD) rating scale (Pelham, et al., 1992) parent report. Planning and executive functions (BRIEF, Gioia, 2000). Neuropsychological measures (computer tasks). | No significant interactions between time and group were found on reported ADHD. | No differences between conditions at the end of treatment and 2-month follow-up. | 1 − |
| Sprich, 2016 [73] | 46 adolescents with ADHD (DSM-IV) aged 14–18 years (mean 15.1; 78% male), all on stable medication before randomization but changes allowed (n = 15), or all on stable medication but use varied. Weekly medication monitoring. 78% reported medication use at baseline and 84% at post-treatment. Group differences and control for medication or other therapy not reported. | 2-arm cross-over RCT, SB, 4-month treatment duration, stratified randomization based on sex and ADHD severity, 12 sessions of CBT (approximately 17 weeks) vs. Wait-List (WL) control, assessments at the beginning and end of treatment and 4-month follow up (but unclear comparisons at follow-up). | CBT, n = 24, 7 modules over 12 individual sessions with a therapist (psychoeducation, organization/planning, distractibility, and adaptive thinking procrastination). Parents were included for 10 min at the end of each session (psychoeducation and goal-setting). In addition, two optional parent-only sessions (parenting style and contingency management). 94% of study completers participated in all 12 sessions. For the treatment completers, the average completion time was 17.3 weeks. WL, n = 22, no treatment for four months. | ITT analyses End of treatment (4 months after baseline): CBT: 21% (88%), WL: 22 (100%). | Independent evaluator administered parent and adolescent DSM-IV ADHD rating scales (Barkley 1990). Clinical Global Impression (CGI, NIMH, 1985). Categorical responder status (Steele, 2006). | Controlled effect sizes (dcorr) comparing CBT vs. WL on parent-rated and adolescent-rated ADHD total-score symptom score at the end of treatment were 0.5 and 0.43, respectively. Article reports group differences tested in one analysis based on cross-over design, but comparisons are unclear. | 1 − | |
| Vidal, 2015 [74] | 119 adolescents with ADHD (DSM-IV) aged 15–21 years (mean 17.2; 68% male), 100% medicated, stabilized doses for at least 2 months. n = 12 discontinued medication between pre-test and follow-up. No differences between conditions. Medication use and absence of other interventions was monitored weekly. | 2-arm RCT, SB, treatment duration NR, group CBT (12 sessions) vs. Wait-List (WL) control group, assessments at baseline and at the end of treatment, assessments of control and intervention group coincided. | CBT, n = 59, participants received 12 manualized group sessions provided by a trained clinical psychologist (n = 2) based on CBT and MI, including psychoeducation (1 session), impulsivity/motivation (5 sessions), and planning strategy/attention (6 sessions). Parents were not involved. WL, n = 60, only monitoring of adherence and continuation of medication, no CBT or other psychological treatment. All participants were monitored weekly for medication adherence and the absence of other treatments. | ITT analyses End of treatment: CBT: 45 (76%), WL: 44 (73%). | ADHD by Adolescent and Parent reports (ADHD-RS, DSM-IV: Dupaul, 1998), CGI (NIMH, 1985), Weiss Functional Impairment Rating Scale, CADDRA 2000). Depression (BDI, Beck, 1941), Anxiety (STAI, Spielberger, 1986). | Controlled effect sizes (dcorr) comparing CBT vs. WL on parent-rated and adolescent-rated ADHD total-symptom scores at the end of treatment were 1.06 and 0.99, respectively. | Participants in the CBT group showed significant improvement in parent-reported functional impairment compared with participants in the WL condition. No significant differences on self-reported functional impairment, anxiety, and depression. | 1 − |
| Meyer, 2021 [69] | 184 adolescents with ADHD (DSM-5) aged 15–18 years received Structured Skills Training Group (SSTG): mean age 16.5 years (SD = 0.88), 34% males; control group: mean age 16.7 (SD = 0.94), 38% male. 77% received ADHD medication; 36% received additional medication. Current ADHD medication assessed at baseline, post-treatment, and follow-up by parent report. Pharmacological treatment needed to be stable for study inclusion and participants were requested not to take part in any other psychological treatment during the study. 18–21% underwent major changes in medication during study. No significant differences. | 2-arm RCT, SB, treatment duration NR, SSTG group-based dialectical behavior therapy (DBT) originally developed for adults with ADHD vs. psychoeducation control group (Control). Baseline assessment 2 weeks before treatment, with post-treatment assessment at 2 weeks and 6 months after treatment. | SSTG, n = 93, participants received 14 weekly manualized group sessions of 2h each provided by a trained clinical psychologist (n = 2), including psychoeducation, strategies managing ADHD, DBT elements, and homework assignments. CONTROL, n = 91, manual-based psychoeducation group program (SKILLS), 3 2-h sessions including information about relevant ADHD-related issues (symptomatology, strengths and challenges, sleep and diet, stress management, etc.) and a book with tools facilitating schoolwork. Average treatment fidelity was only measured for SSTG, and was considered acceptable to good. | ITT analyses SSTG: Baseline: 85 (91%), Post-treatment: 74 (80%), Follow-up: 71 (76%), Included in analyses: 85 (91%). CONTROL: Baseline: 79 (87%), Post-treatment: 61 (67%), Follow-up: 57 (63%), Included in analyses: 79 (87%). | Primary outcomes: Adult ADHD self-report scale for adolescents (ASRS-A) Sonnby et al., 2015). Self- and parent-rated functional impairment by Child Sheehan Disability Scale (CSDS; Whiteside, 2009). Impact of ADHD symptoms (IAS) on well-being; scale constructed for this study. Global Quality of Life scale (GQL, Ivarsson, 2010). Five Facet Mindfulness Questionnaire (FFMQ, Ba2r, 2008). Secondary measures included questionnaires on behavioral and emotional problems, stress, anxiety, and sleep problems. | No between group-differences in improved ADHD-symptoms. Only significant within-group effects: Moderate effects for parent-reported ADHD symptoms in the SSTG group (d = 0.59 (T1–T2), d = 0.62 (T1–T3)). | No between group-differences; small within-group differences (d = 0.26–0.45). | 1 − |
| Clinic interventions: parent/Family-focused | ||||||||
| Barkley, 2001 [67] | 97 adolescents with ADHD (DSM-IV) aged 12–18 years (mean: not reported; 90% male) Criteria: stable medication, no other social therapies. Medication, reported for all assessments, 56% medicated at baseline. No significant differences in medication use at baseline, end of treatment, and follow-up. | 2-arm RCT, 9 week treatment duration, sequential randomization to Problem solving Communication Training (PCT) or PCT + Behavior Parent Training (PCT + BPT), assessments at baseline and at the end of treatment. | PCT, n = 58, 18 twice-weekly sessions of problem solving, communication training, and cognitive restructuring). PCT + PBT, n = 39, 9 twice-weekly sessions PCT followed by 9 twice-weekly sessions PBT (positive parenting, point system/contingency management). | No ITT analyses End of treatment: PCT: 36 (62%), PCT + PBT: 32 (82%). Follow-up: PCT: 33 (57%), PCT + PBT: 29 (74%). Higher drop-out in PSCT than in BMT/PSCT. | ADHD/ODD DSM-IV Raing Scale (DuPaul et al., 1998), parent, adolescent and teacher report Conflict Behavior Scale (CBQ, Prinz, Foster, Kent, and O’Leary, 1979) and other conflict measures. | No significant interactions between time and group were found on reported ADHD. | No differences between conditions at the end of treatment and at 2-month follow-up. | 1 − |
| Sibley 2016 [70] | 128 adolescents with ADHD (DSM-IV-TR) aged 11–15 years (mean = 12.8; 65% male, mostly Hispanic), additional medication and other treatment use was allowed. 34% used medication (no significant differences between conditions at post-test and follow-up, no differences in participants who changed dose or started new medication), 6.4% received individual therapy, no group differences in use of other interventions (academic tutoring, educational accommodation, and individual therapy) | 2-arm RCT 10 week treatment duration, stratified randomization on medication status and oversampling of Supporting Teen’s Academic Needs Daily (STAND) compared with treatment as usual (TAU), assessments pre/post intervention (timing unclear) and at 6 months follow-up. | STAND, n = 67, received 10 family therapy sessions, 50 min with parents and teens by MI- and STAND trained clinicians. Treatment includes 4 selections out of 7 modular MI and CBT-based sessions to train academic/organization/problem solving skills. In addition, parents were invited to 4 group sessions, but these were not well attended (22–52% per session). TAU: n = 61, families were encouraged to seek services in the community. | ITT analyses End of treatment: STAND: 60 (90%), TAU: 55 (90%). Follow-up: STAND: 55 (82%), TAU: 51 (84%). Missing data per condition NR. 95% post-test data available from at least two sources, at follow-up this was 87%. No differences between completers (at least one source) and non-completers. Pre-treatment differences on IQ and ADHD-subtypes (included as covariates). | ADHD (DBD, Pelham, 1992). OTP and Grade Point Average (GPA) defined as primary outcome measures. Parent and teacher ratings of OTP (AAPC, blinded ref). Official school grades (electronic gradebook, Quarterly GPA) Parent-rated Parent-Teen conflict (CBQ-20, Prinz 1979) Parenting stress (CSQ, Brannan, 1997) Parent OTP involvement (PAMS, blinded ref) Recorded homework Bookbag organization (Evans, 2009). | Controlled effect sizes (dcorr) comparing STAND vs. TAU on parent-rated ADHD total-score symptom score were 0.72 at the end of treatment and 0.59 at follow-up. No differences on teacher-rated ADHD. | Stronger improvements at post-test and FU on parent-rated OTP, parenting problems and homework for STAND than TAU. No differences on GPA, teacher-rated outcomes, and adolescent-rated parent−teen conflict. | 1 − |
| Sibley 2019 [71] | 123 adolescents with ADHD (DSM-5) aged 11–17 years (mean 13.6; 80% male), clinic-based treatment but recruitment via schools, and additional medication and other treatment use was allowed. 42% used medication at baseline (no significant differences across assessments), and medication use was controlled and monitored during data-collection and controlled for in analyses. | 2-arm RCT, treatment duration 10–12 weeks, randomization in 20 waves to Dyadic Supporting Teen’s Academic Needs Daily (STAND) or Group STAND, assessments at baseline and at the end of treatment and at 6-month follow-up. | Dyadic STAND, n = 63 received 10 weekly parent−teen dyadic therapy sessions, 60 min by MI- and STAND trained clinicians (see Sibley 2016). Group STAND: n = 60 received 8 weekly group sessions, consisting of 75 min of separate group sessions for parents and adolescents, and 15 min of final blended parent−teen group session (total 90 min) by trained clinicians. Therapy dose and fidelity scores did not differ between interventions. However, MI integrity was higher in Dyadic than Group STAND, but Group STAND showed higher levels of MITI giving information than Dyadic STAND, and parents in Group STAND reported a greater self-efficacy and normalization of their difficulties than parents in the Group STAND. | ITT analyses End of treatment: Dyadic STAND: 59 (94%), TAU: 58 (97%). Follow-up: STAND: 50 (79%), TAU: 55 (92%). Differences not tested. | ADHD (DSM-5 ADHD-rating scale, Sibley and Kuriyan, 2016). OTP and Grade Point Average (GPA) defined as primary outcome measures. Parent and teacher ratings of OTP (AAPC, blinded ref). Official school grades (electronic gradebook, quarterly GPA). Parent-rated Parent−Teen conflict (CBQ-20, Prinz 1979). Parenting stress (CSQ, Brannan, 1997). Parent OTP involvement (PAMS, blinded ref). Recorded homework Bookbag organization (Evans, 2009). | No significant differences in treatment outcomes between conditions. Parents with elevated ADHD-scores and at least moderate depression symptoms and high conflict dyads benefitted more from the Dyadic than group-based STAND. | No between-group differences on the other functional outcomes reported. | 1 − |
| Sibley 2020 [72] | 287 adolescents with ADHD (DSM-5) aged 11–17 years (mean 14.0; 71% male), clinic-based treatment at community clinics, with additional medication allowed but monitored and controlled for in the analyses. Of the participants in the intervention group, 31.2% used medication at baseline compared with 23.6% in the control group (usual care). From baseline to post-treatment: increased medication use in the intervention group compared with usual care. | 2-arm RCT, treatment duration varied (only mean session reported per condition), stratified randomization procedure within agency to either Teen’s Academic Needs Daily (STAND) or Usual Care (UC) Baseline assessment, post-test at 16 weeks post baseline and follow-up at 12 weeks after the post-treatment assessment. | STAND: n = 138, 10 weekly parent−teen dyadic therapy sessions, 60 min by STAND trained clinicians (see Sibley 2016). Received number of sessions: 13.99 (SD = 13.80). UC: n = 140, received mean 17.38 (SD = 15.26) weekly therapy sessions of usual care. Coding of 78 available UC audio tapes using STAND fidelity checklists indicated high treatment differentiation (53.8% of items were not present on any UC recordings). | ITT analyses End of treatment: STAND: 114 (83%), UC: 111 (79%). Follow-up: STAND: 112 (81%), TAU: 106 (76%). Differences were not significant. | Primary outcomes: Parent and teacher reports ADHD-symptoms (Conners-3, 2008); parent and teacher DSM-5 ADHD checklists (Sibley and Kuriyan, 2016). Secondary outcomes: Academic impairment: OTP and Grade Point Average (GPA) Parent and teacher ratings of Adolescent Academic Problems Checklist (AAPC, blinded ref). Family impairment: Parent and adolescent rated Conflict Behavior Questionnaire-20 (CBQ-20). Disciplinary incidents: Counts of all disciplinary incidents (e.g., detention, in-school suspension) during academic quarter immediately preceding each assessment. | No significant differences in improved treatment outcomes between conditions. | No between-group differences on other functional outcomes reported. | 1 − |
| School interventions: adolescent-focused | ||||||||
| Evans 2007 [62] | 79 adolescents with ADHD (DSM-IV-TR) aged 10–14 years (mean 11.9; 77% male). Prevalence of medication use examined in analyses, but prevalence and group differences NR. | 2-arm RCT, treatment duration NR, cluster-randomization per school (n = 5, 2:2, 1 extra school added to the control group), 15 sessions of training and consultation model of the Challenging Horizons Program (CHP-C) or “treatment advice” (Control), assessments pre- and post-treatment (after 6 months) and at 12-, 18-, 24-, and 30-months of follow-up. | CHP-C, n = 42, 15 sessions targeting academic and social skills, individual guidance by mentors. Monthly medication monitoring (if symptoms above threshold, option for additional medication or psychosocial treatment, 92% opted for additional psychosocial interventions). Control, n = 37, parents received summaries of baseline intake, a list of local treatment providers, and could pursue treatment of their choice. | ITT-analyses NR, but Hierarchical Linear Modeling have been ITT. Reported Year 3 completion rates do not match N final evaluation. Year 1: CHP-C: 40 (95%), Control: 32 (88%). Year 2: CHP-C: 37 (88%), Control: 25 (66%). Year 3: CHP-C: 32 (76%), Control: 22 (61%). | ADHD, parent-report (DBD, Pelham, 1992). Behavior Assessment System for Children (BASC; Reynolds and Kamphaus, 1993). Impairment Rating Scale (IRS, Fabiano, 2006). Grades Social Skills Rating System (SSRS, Gresham and Elliot, 1990). | Results of the analyses suggest small cumulative benefits of CHP-C on inattention. | Small cumulative benefits of CHP-C on social functioning. | 1 − |
| Evans 2016 [63] | 326 adolescents with ADHD (DSM-IV-TR), grades 6–8 (mean: NR; 70% male). 43–52% medicated at baseline, no group differences. | 3-arm RCT at nine schools, treatment duration 1 year, stratified randomization for site and medication at baseline. 1 (academic) year of the afterschool Challenging Horizons Program (CHP-AS) or CHP-M or “treatment advice” (control). | CHP-AS, n = 112, twice per week group-based afterschool program targeting social impairment, education/study skills group. Individual guidance/monitoring by a primary counselor (PC). CHP-M, n = 110, weekly individual mentor meetings monitoring and delivery of subset of the CHP-AS interventions. CC, n = 104, summary report of intake evaluation on request and list of local treatment providers. | ITT analyses End of treatment: CHP-AS: 104 (91%), CHP-M: 108 (98%), CC: 104 (100%), Follow-up: CHP-AS: 104 (95%) CHP-M: 108 (%) CC: Higher treatment drop-out in CHP-AS (22% of 112 or 105, not clear) compared with CHP-M (3% of 110/108 not clear). Treatment-drop-out in CC not reported. | ADHD, parent-report (DBD, Pelham, 1992). Organization, time-management, planning skills (COSS: Abikoff, 2000). Homework problems (CPS, Evans, 2012). Overall academic functioning (HPC, Anesko, 1987). Interpersonal functioning (IRS, Fabiano, 2006). | Only the interaction between time and group for parent-rated inattention symptoms was significant. Compared with Control, CHP-AS only showed stronger benefits for parent-rated inattention symptoms (no differences on parent-rated hyperactivity and teacher ADHD-ratings). Compared with CHP-M, CHP-AS showed stronger improvements in parent-rated inattention at follow-up assessment only. | Compared with Control, CHP-AS showed stronger benefits on organization/planning skills, homework, and academic skills. No differences for social and teacher-rated academic functioning. Compared with CHP-M, CHP-AS showed stronger improvements on task planning and academic functioning at the follow-up assessment only. | 1 − |
| Langberg 2012 [64] | 47 adolescents with ADHD (DSM-IV) aged 11–14 years (mean: not reported; 55% male). 66% medicated. | 2-arm RCT, treatment duration 11 weeks, Homework Organization and Planning Skills (HOPS) intervention with Waiting list-control group (WL), assessments at baseline and end of treatment 3-month follow-up in intervention group. | HOPS, n = 23, 16 individual school-day bi-weekly/weekly 20 min sessions over 11-week period on school materials organization, homework recording and management, and planning/time management including skill tracking with a point/reward system. Two 1 h parent meetings with students. WL: waiting list control group | ITT, but no info about attrition or treatment drop-out. Seems all participants completed the interventions and assessments, but could also be as selection? HOPS was provided between 11–19 weeks (M = 13.8, Med = 14). | No primary/secondary outcomes defined. Homework (parent-rated HPC, Ramirez, 1987). Organizational skills (COSS, parent, teacher, and student report, Abikoff, 2008). Parent-rated ADHD (DSM-IV-based scale VADPRS, Vanderbildt) Parent Skills Implementation Questionnaire. | No differences between HOPS intervention and WL control on ADHD (interaction, p = 0.059). | HOPS intervention resulted in a stronger improvement of organizational skills than for the WL control. | 1 − |
| Schramm 2016 [65] | n = 113 adolescents with ADHD (DSM-IV-TR) aged 12–17 years (mean = 14.0; 85% male). No selection criteria for medication use and other interventions. 50.4% medicated, lower medication use in 38.9% in waiting list control group compared with the training intervention group (60%) and active control group (51.4%). | 3-arm RCT, treatment duration approximately 3–6 months, stratified for gender, 6-month CBT-based adolescent-directed problem solving and organizational skills training (INT) or 6-month progressive muscle relaxation (active control (AC)) or 3-month waiting-list control group (WL), assessments at baseline and at the end of treatment. | INT: n = 40, participants received CBT-based individual training sessions provided by trained special education or psychology students (max. 20, 60 min) combined with a behavioral component based on contingency management by parents and teachers. AC, n = 36, participants received twice-weekly group (4–5 pp) sessions of PMR (12–15 sessions, 60 min). WL, n = 37 | ITT-based last observation carried forward. End of treatment: INT: 39 (98%), AC: 34 (94%), WL: 36 (97%). Overall, 6.4% missing data (higher in teacher reports). | ADHD DSM-IV symptom Checklists (SBB-HKS, Dopfner, 2007). Parent and teacher reports. Hyperactivity-subscale SDQ (Goodman, 1997). Academic Enablers (AVL, Lauth, 2004). Meta-cognitive skills (WSW, reading strategies, Schlagmuller, 2007). Int/Ext problems, alertness, flexibility, inhibition, self-rated outcomes on ADHD, etc. | Compared with WL, INT resulted in stronger reductions of parent and teacher-rated ADHD, teacher-rated learning behavior, internalizing problems, and self-rated learning problems compared with WL. No differences between INT and PMR. | Compared with WL, INT resulted in stronger reductions of teacher-rated learning behavior, internalizing problems, and self-rated learning problems. No differences between INT and PMR. | 1 − |
| School interventions: Parent/Family-focused | ||||||||
| Steeger 2016 [66] | n = 104 (randomized from n = 108) adolescents with ADHD (DSM-IV) aged 11–15 years (mean 12.5; 69% male). 84% medicated Criteria: stable medication, 84% medicated at baseline, and no significant differences between groups. | 4-arm RCT: 2 × 2 mixed group factorial design, treatment duration of 5 weeks, randomization to Cogmed Working Memory Training (CWMT) and group Behavioral Parent Training (BPT) or to CWMT and Control-BPT (CNT-BPT) or to Control-CWMT (CNT-CMWT) and BPT, or to CNT-CMWT and CNT-BPT, assessments at baseline and at the end of treatment. | CWMT + BPT, n = 26, CWMT: 25-day high-dose adaptive computerized WM training (Cogmed). BPT: 5-week group BPT program based on COPE aimed at mother−adolescent interactions, adolescent compliance, and maternal control, reducing conflict and adolescent ODD. CNT-CWMT + BPT, n = 26, 25 day low-dose non-adaptive computerized WM training (Cogmed) + BPT: see above. CWMT-CNT-BPT: n = 26, 5 CWMT: see above. CNT-BPT: active control program of didactic lectures on adolescent development, homework of weekly readings self-help guide. No facilitation of practice/ feedback. CNT-CWMT + CNT-BPT, n = 26, CNT-CWT: see above. CNT = BPT: see above. | No ITT analyses but analyses on completers-only (n = 96), excluding participants with IQ < 70 (n = 3) and participants with mothers with <75% BPT attendance (n = 2), final sample n = 91. End of treatment: CWMT + BPT, n = 22 (85%), CWMT + CNT-BPT, n = 23 (88%), CNT-CWMT + BPT, n = 25 (96%), CWMT + CNT-BPT, n = 26 (100%). | Mother and teacher ratings of ADHD Rating Scale-IV (ADHD-RS, DuPaul 1998). Executive functioning (BRIEF, Gioia, 2000). Mother-reported: Parenting behavior (APQ, Frick 1991). Mother–adolescent conflict (CBQ, Robin, 2002). Oppositional behaviors (CBCL, Achenbach, 2001). | No significant differences between conditions on ADHD-symptoms and parenting variables. | No significant differences between conditions on parenting variables. Interaction effect on global functioning showed better outcomes of participants in the control-CWMT + BPT group. | 1 − |
| Bias Arising from/Due to: | Rnd | Int | Mis | Mea | Sel | Overall | |
|---|---|---|---|---|---|---|---|
| Psychosocial Interventions | |||||||
| 1 | Barkley, 2001 [67] |  |  |  |  |  |  |
| 2 | Boyer, 2015 [68] |  |  |  |  |  |  |
| 3 | Evans, 2007 [62] |  |  |  |  |  |  |
| 4 | Evans, 2016 [63] |  |  |  |  |  |  |
| 5 | Langberg, 2012 [64] |  |  |  |  |  |  |
| 6 | Meyer, 2021 [69] |  |  |  |  |  |  |
| 7 | Schramm, 2016 [65] |  |  |  |  |  |  |
| 8 | Sibley, 2016 [70] |  |  |  |  |  |  |
| 9 | Sibley, 2019 [71] |  |  |  |  |  |  |
| 10 | Sibley, 2020 [72] |  |  |  |  |  |  |
| 11 | Sprich, 2016 [73] |  |  |  |  |  |  |
| 12 | Steeger, 2016 [66] * |  |  |  |  |  |  |
| 13 | Vidal, 2015 [74] |  |  |  |  |  |  |
| Complementary Interventions | |||||||
| 14 | Ahmed, 2011 [75] |  |  |  |  |  |  |
| 15 | Bink, 2016 [76] |  |  |  |  |  |  |
| 16 | Gray, 2012 [77] |  |  |  |  |  |  |
| 17 | Matsudaira, 2015 [78] |  |  |  |  |  |  |


 : green = low risk of bias, orange = some concerns and red = high risk of bias. Rnd—randomization process; Int—intended interventions; Mis—missing outcome data; Mea—measurement of the outcome; Sel—selection of the reported result. * Since Steeger, 2016, examined both a psychosocial and a complementary intervention, this trial was included in the review of both psychosocial and complementary interventions.
: green = low risk of bias, orange = some concerns and red = high risk of bias. Rnd—randomization process; Int—intended interventions; Mis—missing outcome data; Mea—measurement of the outcome; Sel—selection of the reported result. * Since Steeger, 2016, examined both a psychosocial and a complementary intervention, this trial was included in the review of both psychosocial and complementary interventions.| Author, Year | Population | Study Design | Intervention | Treatment Completers | Outcome Measures | ADHD Outcomes | Functional Outcomes | Quality Rating |
|---|---|---|---|---|---|---|---|---|
| Gray, 2012 [77] | 60 adolescents aged 12–17 years (mean 14.3; 87% male) with ADHD (DSM-version not reported) and learning disabilities, 98% medicated. | 8-week SB, RCT unbalanced randomization (3:2 assignment to the working memory (WM) training), 5-week working memory training program (WM), or 5-week mathematics training program (MT) (unbalanced randomization). Post-test assessment 3 weeks after the end of treatment. | WM, n = 36, 45-min training sessions of Cogmed at school, 4–5 days a week for 5 weeks. MT, n = 24, 45-min training sessions of mathematics training program (Academy of Math; (Torlakovic, 2011), 4–5 days a week for 5 weeks. | ITT analyses WM: 32 (89%), MT: 20 (83%). | ADHD-symptoms were assessed with the Strengths and Weakness of ADHD-symptoms and Normal-behavior scale (SWAN, Swanson et al., 2001) and the IOWA Conners scale (Pelham et al., 1989). Academic Progress (WRAT-4PM; Roid and Ledbetter, 2006). WM and attention measures, i.e., CANTAB. | No group differences on ADHD and other measures. Only differences on WM criterion measures. | 1 − | |
| Steeger, 2016 [66] | n = 104 (randomized from n = 108), adolescents with ADHD (DSM-IV) aged 11–15 years (mean 12.5; 69% male), 84% medicated. | 7-week RCT with four conditions: 5-week Cogmed working memory training (CWMT) combined with behavioral parent training (BPT), 5-week CWMT with a control parent intervention (CNT-BPT), 5-week control version of CWMT (CNT-CWMT) combined with BPT, or 5-week CNT-CWMT combined with CNT-CBT. | CWMT + BPT, n = 26, CWMT: 25-day high-dose adaptive computerized WM training (Cogmed). BPT: 5-week treatment group BPT program based on COPE aimed at positive mother−adolescent interactions, adolescent compliance, and maternal control, reducing conflict and adolescent ODD. CNT-CWMT + BPT, n = 26, 25-day low-dose non-adaptive computerized WM training (Cogmed) + BPT: see above. CWMT-CNT-BPT: 5 CWMT: see above. CNT-BPT: Control parent intervention of didactic lectures on adolescent development and homework of weekly readings from self-help guide. No facilitation of practice or feedback. CNT-CWMT + CNT-BPT, n = 26, CNT-CWT: see above. CNT = BPT: see above. | No ITT-analyses, but analyses on completers-only excluding participants with IQ < 70 and participants with mothers with <75% BPT attendance, final sample, n = 91. n = 108 included, n = 104 randomized, and n = 8 dropped out. Drop-out = 8% of 104 pp, higher drop-out in CWMT than in CWMT-CNTR. | ADHD Rating Scale-IV (ADHD-RS, DuPaul, 1998) mother and teacher report. Executive functioning (BRIEF, Gioia, 2000). Mother-reported: Parenting behavior (APQ, Frick, 1991). Mother–adolescent conflict (CBQ, Robin, 2002). Oppositional behaviors (CBCL, Achenbach, 2001). | No significant differences between conditions on ADHD-symptoms and parenting variables. | No significant differences between conditions on parenting variables. Interaction effect on global functioning showing better outcomes of participant in the control-CWMT + BPT group. | 1 − |
| Bink, 2016 [76] | 90 adolescents with ADHD (DSM-IV-TR) aged 12–24 years (mean 16.0; 100% male), 49–52% medicated, no between group differences in medication use at baseline and follow-up. | 1-year un-blinded, RCT unbalanced randomization (2:1) stratified randomization for age groups of 12–15, 16–20, and 21–24 years. 25-week neurofeedback training (NF) + treatment as usual (TAU) or 25-week TAU. | NF + TAU, n= 59, 25 weeks of 2–3 weekly 30 min training sessions of a theta/SMR training (Lubar 2003) + At least 5 weeks TAU consisting of regular cognitive–behavioral therapy, systemic therapy, and/or supportive counselling for the adolescent and/or his parent(s). TAU: n = 31, at least 25 weeks of TAU (see above). | ITT analyses on n = 87 (n = 56 NF + TAU, n = 31 TAU) End of treatment NF + TAU: 45 (76%) TAU: 26 (85%) 1-year follow-up 41 (73%) TAU: 19 (61%) | MINI ADHD-subscale (Sheehan et al., 1998). ADHD Rating Scale-IV (ADHD-RS, DuPaul, 1998). Youth Self-Report (YSR, Achenbach, 1991). Neuropsychological measures (computer tasks). | No significant differences between NF + TAU and TAU on all outcome measures. | 1 − | |
| Matsudaira, 2015 [78] | 75 adolescents with ADHD (DSM-IV) aged 12–16 years (mean 13.7; 100% male), 19.7% psychostimulant medication. No differences between conditions. | 12 weeks, DB, RCT placebo-controlled, stratified randomization by day/boarding school and age group (12–14 years and 15–17 years). Long chain-polyunsaturated fatty acid (LC-PUFA) with placebo. | LC-PUFA, n = 38, 12 weeks of daily dose of six LC-PUFA capsules of omega-3 fatty acids (EPA 558 mg and DHA 174 mg), omega-6 fatty acid У-linoleic acid 60 mg, and vitamin E 9.6 mg (in the natural form, α-tocopherol). Placebo, n = 38, 12 weeks of daily dose of placebo (medium chain triglycerides). | “ITT” analyses on n = 69 (LC-PUFA, n = 33, Placebo, n = 36) Per protocol on n = 50 End of treatment: LC-PUFA: 23 (61%) Placebo: 27 (71%) | ADHD measured by Conners’ Teacher Rating Scales (CTRS-L), which assessed each of 59 items of child behavior on a four-point scale (Conners et al., 1998). | No differences in ADHD ratings between LC-PUFA and placebo at 12-weeks of follow-up. | 1 − | |
| Ahmed, 2011 [75] | 84 adolescents with ADHD (DSM-IV-TR) aged 11–16 years (mean = 13.8; 64% male), medicated: % not reported. | 10-week RCT, no details on randomization. 10-week aerobic moderate intensity exercise program (MA exercise) or no intervention. Blinding not reported. | MA Exercise, n = 42, 10 weeks, 3 days a week 40–50 min aerobic sessions and home program parental instruction of 30 min outdoor walking in weekends. No exercise, n = 42, 10 weeks. | No information on completers/drop-out rates. No between group differences on physical characteristics (weight) and outcome measures. | Attention problems, motor skills, task orientation, emotional and oppositional behavior, and academic and classroom behavior: modified Conner’s Rating Scale (Conners, et al., 1998). | Stronger improvement in attention problems in participants who received the MA exercise program compared with the control group. | 1 − |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özgen, H.; Spijkerman, R.; Noack, M.; Holtmann, M.; Schellekens, A.; Dalsgaard, S.; van den Brink, W.; Hendriks, V. Treatment of Adolescents with Concurrent Substance Use Disorder and Attention-Deficit/Hyperactivity Disorder: A Systematic Review. J. Clin. Med. 2021, 10, 3908. https://doi.org/10.3390/jcm10173908
Özgen H, Spijkerman R, Noack M, Holtmann M, Schellekens A, Dalsgaard S, van den Brink W, Hendriks V. Treatment of Adolescents with Concurrent Substance Use Disorder and Attention-Deficit/Hyperactivity Disorder: A Systematic Review. Journal of Clinical Medicine. 2021; 10(17):3908. https://doi.org/10.3390/jcm10173908
Chicago/Turabian StyleÖzgen, Heval, Renske Spijkerman, Moritz Noack, Martin Holtmann, Arnt Schellekens, Søren Dalsgaard, Wim van den Brink, and Vincent Hendriks. 2021. "Treatment of Adolescents with Concurrent Substance Use Disorder and Attention-Deficit/Hyperactivity Disorder: A Systematic Review" Journal of Clinical Medicine 10, no. 17: 3908. https://doi.org/10.3390/jcm10173908
APA StyleÖzgen, H., Spijkerman, R., Noack, M., Holtmann, M., Schellekens, A., Dalsgaard, S., van den Brink, W., & Hendriks, V. (2021). Treatment of Adolescents with Concurrent Substance Use Disorder and Attention-Deficit/Hyperactivity Disorder: A Systematic Review. Journal of Clinical Medicine, 10(17), 3908. https://doi.org/10.3390/jcm10173908






