How to Measure Intraocular Pressure: An Updated Review of Various Tonometers
Abstract
1. Introduction
2. Indentation Tonometry
3. Applanation Tonometry
4. Non-Contact Tonometry (Air-Puff Tonometry)
5. Pneumotonometry
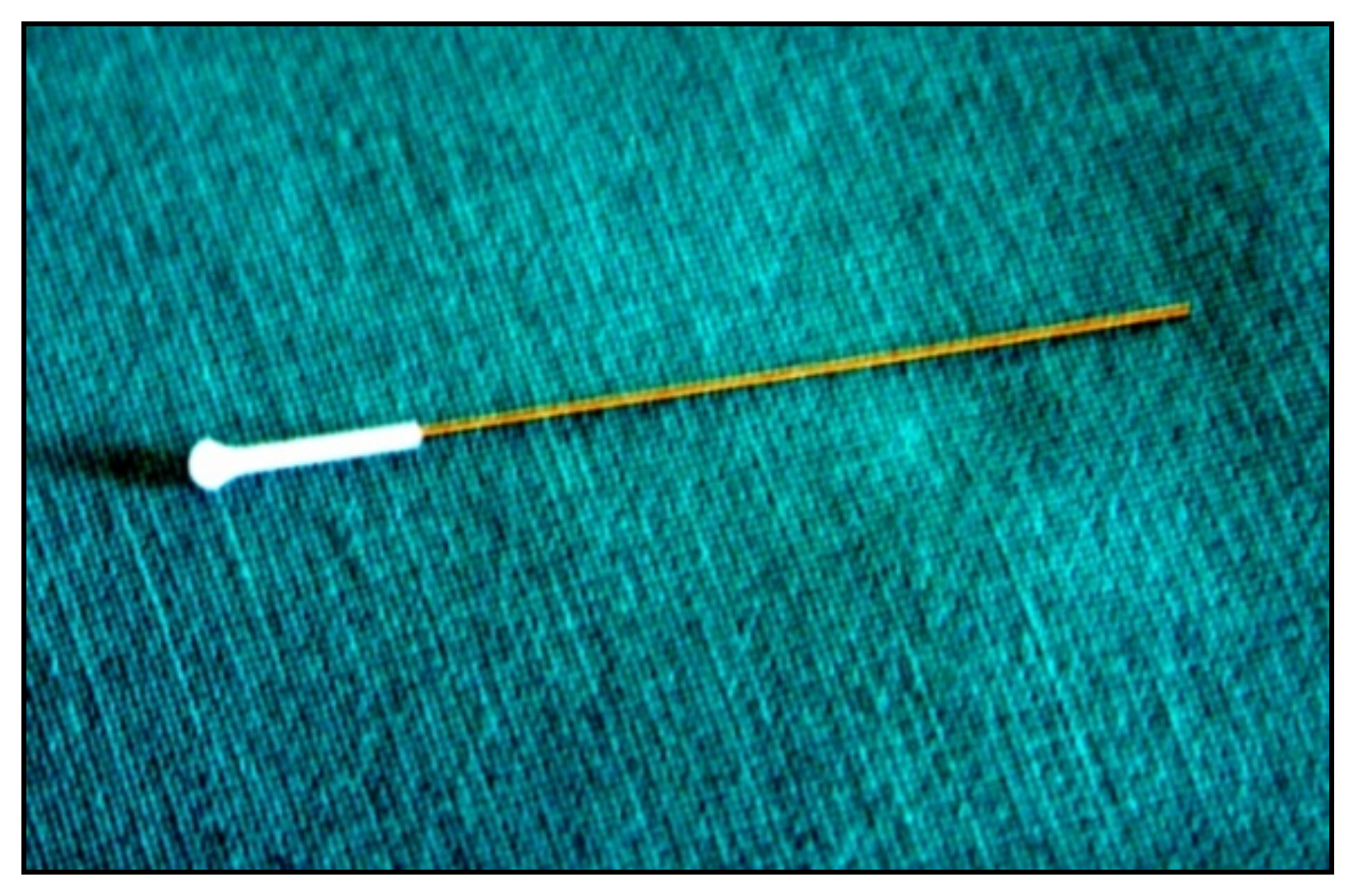
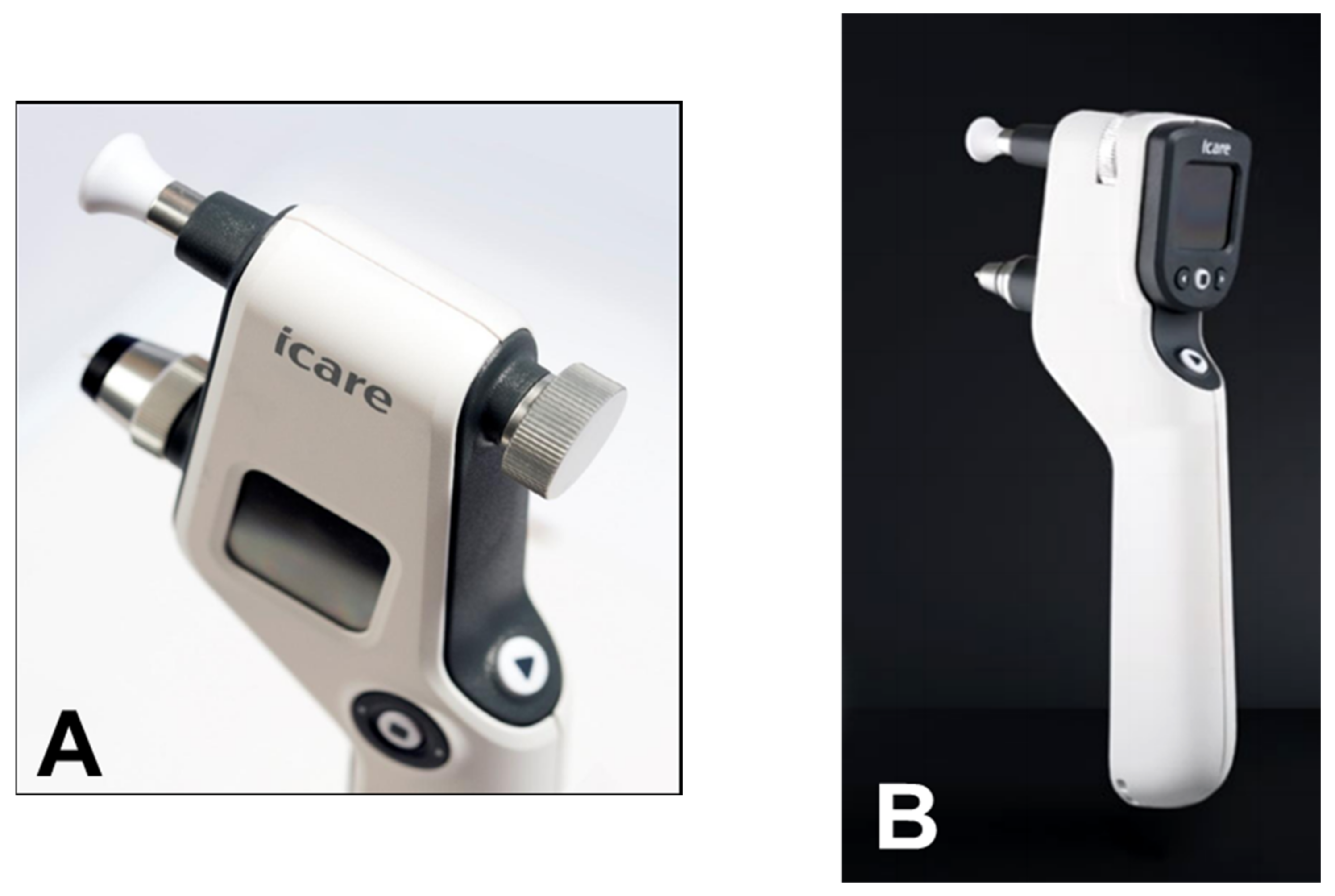
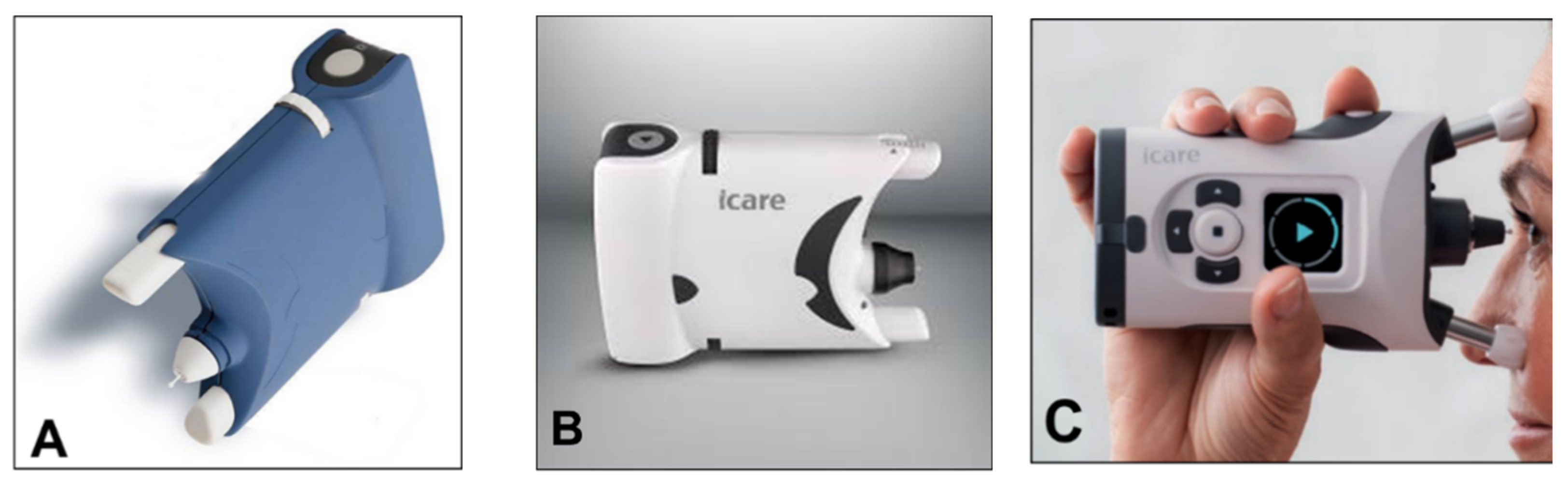
6. Rebound Tonometry
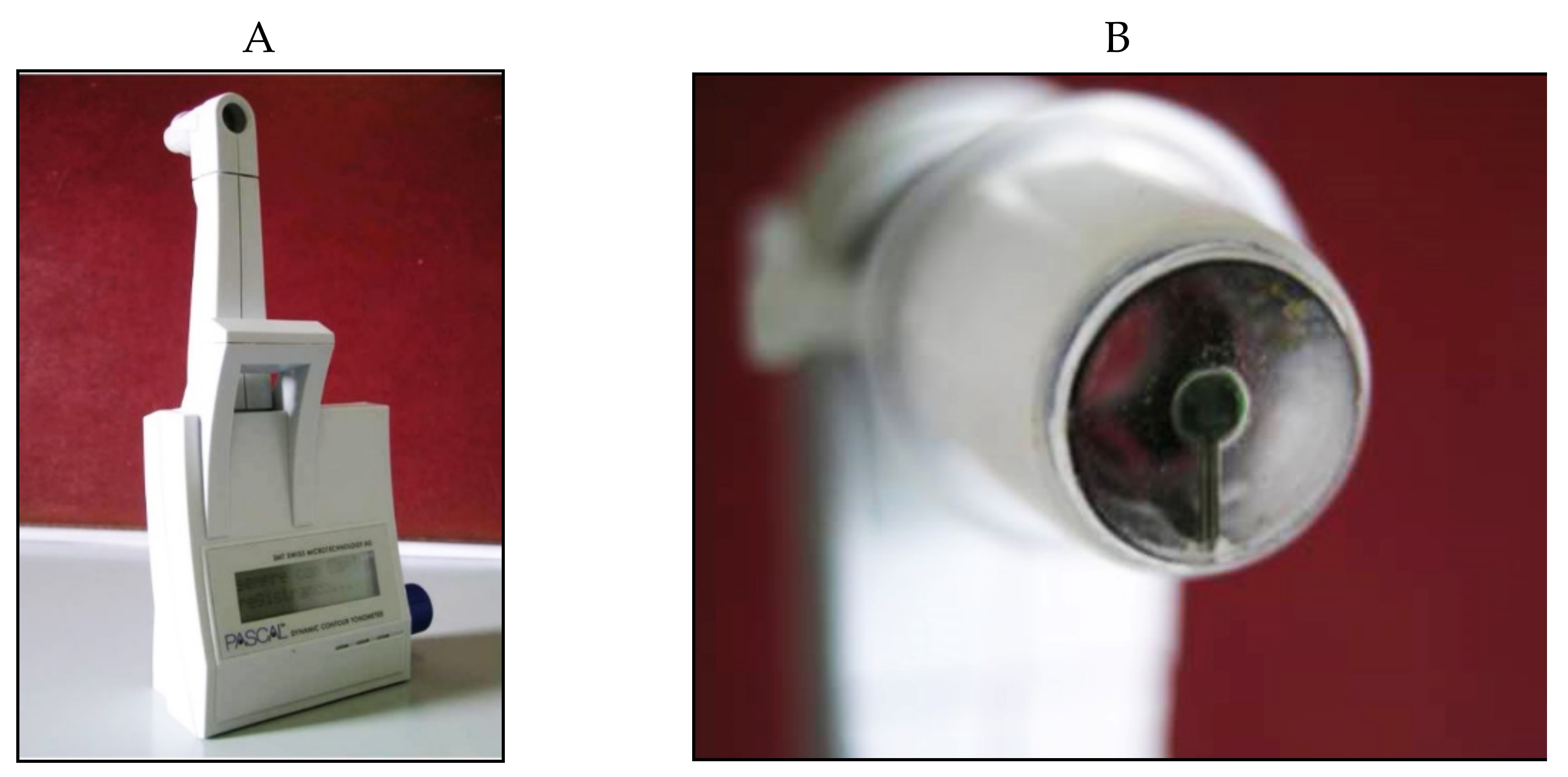
7. Dynamic Contour Tonometry
8. Applanation Resonance Tonometry
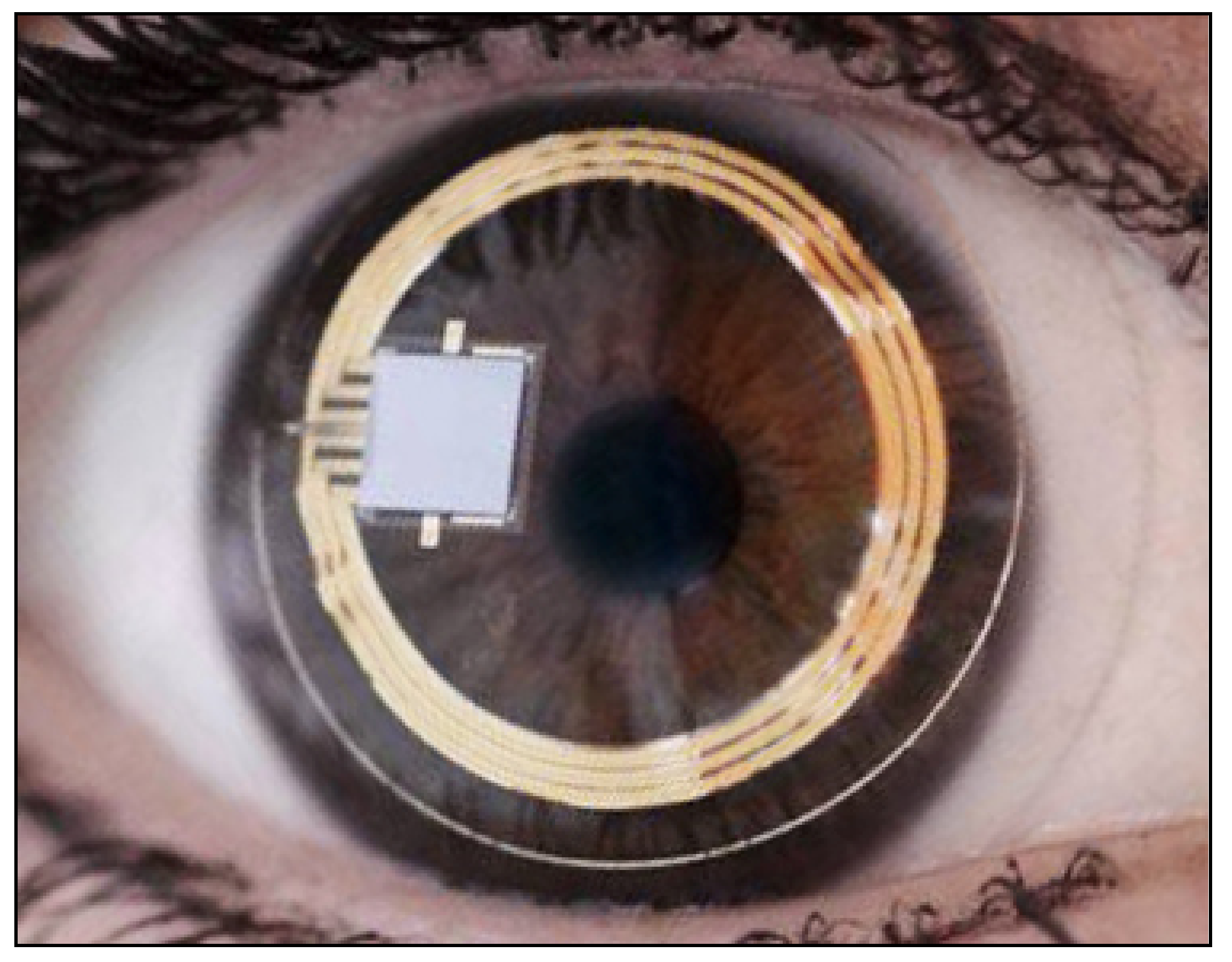
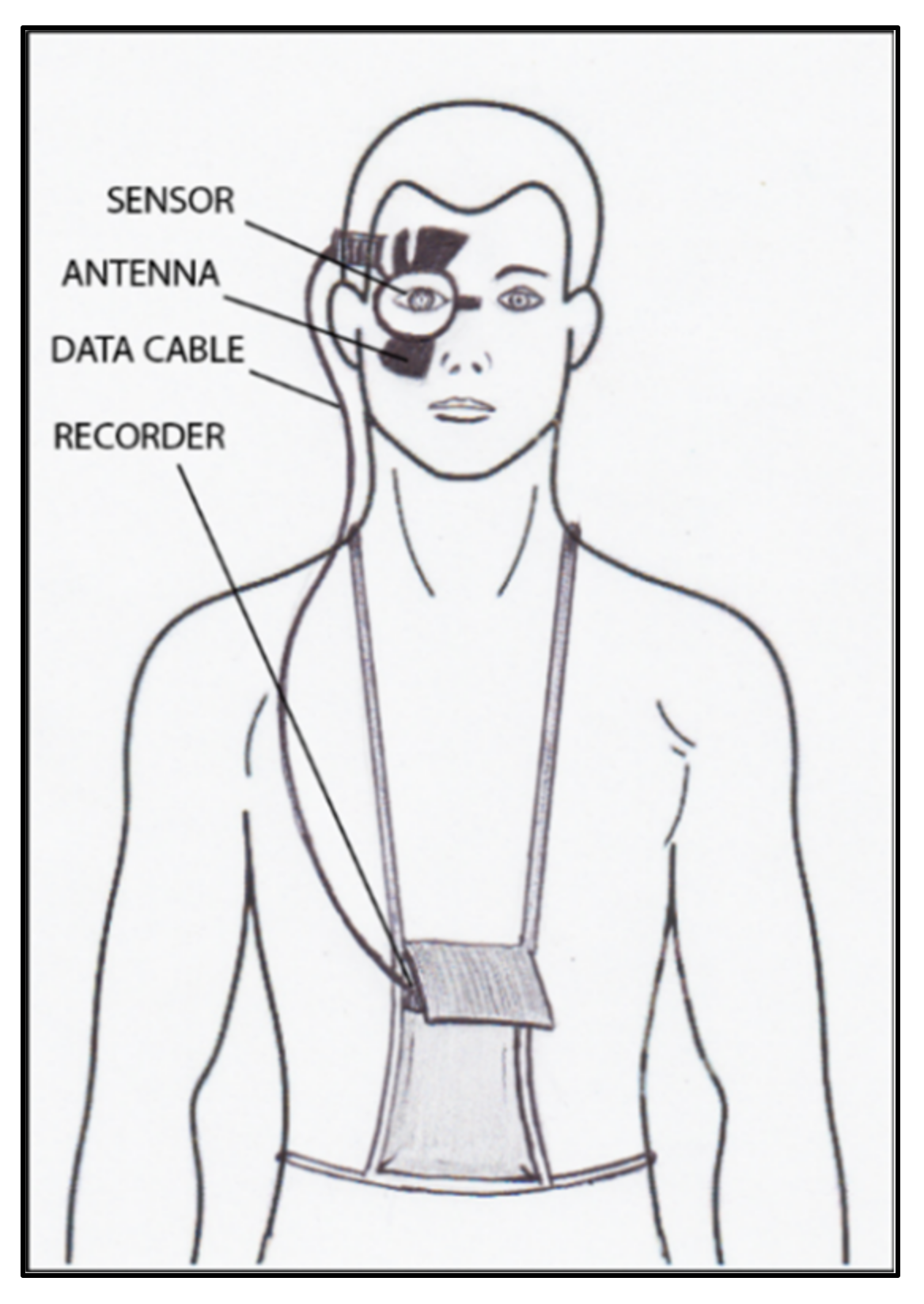
9. Continuous IOP Monitoring
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Kniestedt, C.; Punjabi, O.; Lin, S.; Stamper, R.L. Tonometry through the Ages. Surv. Ophthalmol. 2008, 53, 568–591. [Google Scholar] [CrossRef]
- Stamper, R.L. A History of Intraocular Pressure and Its Measurement. Optom. Vis. Sci. 2011, 88, E16–E28. [Google Scholar] [CrossRef] [PubMed]
- Brusini, P. Intraocular Pressure and Its Measurement. In Atlas of Glaucoma, 3rd ed.; Choplin, N.T., Traverso, C.E., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 29–36. [Google Scholar]
- Albert, D.M.; Keeler, R. The Pressure: Before and after Schiøtz. Ophthalmol. Glaucoma 2020, 3, 409–413. [Google Scholar] [CrossRef]
- Kyari, F.; Nolan, W.; Gilbert, C. Ophthalmologists’ practice patterns and challenges in achieving optimal management for glaucoma in Nigeria: Results from a nationwide survey. BMJ Open. 2016, 6, e012230. [Google Scholar] [CrossRef]
- Nagarajan, S.; Velayutham, V.; Ezhumalai, G. Comparative evaluation of applanation and indentation tonometers in a community ophthalmology setting in Southern India. Saudi. J. Ophthalmol. 2016, 30, 83–87. [Google Scholar] [CrossRef]
- Lasseck, J.; Jehle, T.; Feltgen, N.; Lagrèze, W.A. Comparison of intraocular tonometry using three different non-invasive tonometers in children. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008, 246, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Ohana, O.; Varssano, D.; Shemesh, G. Comparison of intraocular pressure measurements using Goldmann tonometer, I-care pro, Tonopen XL, and Schiotz tonometer in patients after Descemet stripping endothelial keratoplasty. Indian J. Ophthalmol. 2017, 65, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Goldmann, H.; Schmidt, T. Über Applanationstonometrie. Acta. Ophthalmol. 1957, 134, 221–242. [Google Scholar] [CrossRef]
- Ehlers, N.; Bramsen, T.; Sperling, S. Applanation tonometry and central corneal thickness. Acta. Ophthalmol. 2009, 53, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, M.M.; Stein, R.A.; Hassanein, K. The Effect of Corneal Thickness on Applanation Tonometry. Am. J. Ophthalmol. 1993, 115, 592–596. [Google Scholar] [CrossRef]
- Herndon, L.W.; Choudhri, S.A.; Cox, T.; Damji, K.F.; Shields, M.B.; Allingham, R.R. Central Corneal Thickness in Normal, Glaucomatous, and Ocular Hypertensive Eyes. Arch. Ophthalmol. 1997, 115, 1137–1141. [Google Scholar] [CrossRef]
- Wolfs, R.C.; Klaver, C.; Vingerling, J.R.; Grobbee, D.E.; Hofman, A.; de Jong, P.T. Distribution of Central Corneal Thickness and Its Association With Intraocular Pressure: The Rotterdam Study. Am. J. Ophthalmol. 1997, 123, 767–772. [Google Scholar] [CrossRef]
- Doughty, M.J.; Zaman, M.L. Human Corneal Thickness and Its Impact on Intraocular Pressure Measures: A Review and Meta-analysis Approach. Surv. Ophthalmol. 2000, 44, 367–408. [Google Scholar] [CrossRef]
- Park, S.J.; Ang, G.S.; Nicholas, S.; Wells, A.P. The Effect of Thin, Thick, and Normal Corneas on Goldmann Intraocular Pressure Measurements and Correction Formulae in Individual Eyes. Ophthalmology 2012, 119, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.D.; Gordon, M.O.; Gao, F.; Beiser, J.A.; Miller, J.P.; Kass, M. Adjusting Intraocular Pressure for Central Corneal Thickness Does Not Improve Prediction Models for Primary Open-Angle Glaucoma. Ophthalmology 2012, 119, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Gunvant, P.; O’Leary, D.J.; Baskaran, M.; Broadway, D.C.; Watkins, R.J.; Vijaya, L. Evaluation of Tonometric Correction Factors. J. Glaucoma 2005, 14, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Brandt, J.D.; Beiser, J.A.; Kass, M.; Gordon, M.O. Central corneal thickness in the ocular hypertension treatment study (OHTS). Ophthalmology 2001, 108, 1779–1788. [Google Scholar] [CrossRef]
- Gazzard, G.; Jayaram, H.; Roldan, A.M.; Friedman, D.S. When gold standards change: Time to move on from Goldmann tonometry? Br. J. Ophthalmol. 2021, 105, 1–2. [Google Scholar] [CrossRef]
- Kotecha, A.; White, E.; Schlottmann, P.G.; Garway-Heath, D. Intraocular Pressure Measurement Precision with the Goldmann Applanation, Dynamic Contour, and Ocular Response Analyzer Tonometers. Ophthalmology 2010, 117, 730–737. [Google Scholar] [CrossRef]
- Whitacre, M.M.; Stein, R. Sources of error with use of Goldmann-type tonometers. Surv. Ophthalmol. 1993, 38, 1–30. [Google Scholar] [CrossRef]
- Choudhari, N.S.; Rao, H.L.; Ramavath, S.; Rekha, G.; Rao, A.; Senthil, S.; Garudadri, C.S. How Often the Goldmann Applanation Tonometer Should be Checked for Calibration Error? J. Glaucoma 2016, 25, 908–913. [Google Scholar] [CrossRef] [PubMed]
- Frenkel, R.E.P.; Hong, Y.J.; Shin, D.H. Comparison of the Tono-Pen to the Goldmann Applanation Tonometer. Arch. Ophthalmol. 1988, 106, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Iester, M.; Mermoud, A.; Achache, F.; Roy, S. New TonoPen XL: Comparison with the Goldmann tonometer. Eye 2001, 15, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, M.J.; Varikuti, V.N.V.; Weiner, A. Real-world comparison between the Tonopen and Goldmann applanation tonometry in a university glaucoma clinic. Int. Ophthalmol. 2021, 41, 1815–1825. [Google Scholar] [CrossRef]
- Salvetat, M.L.; Zeppieri, M.; Tosoni, C.; Brusini, P. Comparisons between Pascal dynamic contour tonometry, the TonoPen, and Goldmann applanation tonometry in patients with glaucoma. Acta. Ophthalmol. Scand. 2006, 85, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Tonnu, P.-A.; Ho, T.; Sharma, K.; White, E.; Bunce, C.; Garway-Heath, D. A comparison of four methods of tonometry: Method agreement and interobserver variability. Br. J. Ophthalmol. 2005, 89, 847–850. [Google Scholar] [CrossRef]
- Tonnu, P.-A.; Ho, T.; Newson, T.; El Sheikh, A.; Sharma, K.; White, E.; Bunce, C.; Garway-Heath, D. The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometry, non-contact tonometry, the Tono-Pen XL, and Goldmann applanation tonometry. Br. J. Ophthalmol. 2005, 89, 851–854. [Google Scholar] [CrossRef]
- Kontadakis, G.A.; Pennos, A.; Pentari, I.; Kymionis, G.D.; Pallikaris, I.G.; Ginis, H. Accuracy of dynamic contour tonometry, Goldmann applanation tonometry, and Tono-Pen XL in edematous corneas. Ther. Adv. Ophthalmol. 2020, 12. [Google Scholar] [CrossRef]
- Cronemberger, S.; Veloso, A.W. Comparison of Tono-Pen Avia and Handheld Applanation Tonometry in Primary Congenital Glaucoma. J. Glaucoma 2021, 30, e227–e230. [Google Scholar] [CrossRef]
- Deuter, C.M.E.; Schlote, T.; Hahn, G.A.; Bende, T.; Derse, M. Messung des Augeninnendrucks mit dem Tono-Pen im Vergleich zum Applanationstonometer nach Goldmann—eine klinische Studie an 100 Augen. Klin. Mon. Für Augenheilkd. 2002, 219, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Gopesh, T.; Camp, A.; Unanian, M.; Friend, J.; Weinreb, R.N. Rapid and Accurate Pressure Sensing Device for Direct Measurement of Intraocular Pressure. Transl. Vis. Sci. Technol. 2020, 9, 28. [Google Scholar] [CrossRef]
- Grolman, B. A new tonometer system. Optom. Vis. Sci. 1972, 49, 646–660. [Google Scholar] [CrossRef]
- Hansen, M.K. Clinical comparison of the XPERT non-contact tonometer and the conventional Goldmann applanation tonometer. Acta. Ophtahlmol. Scand. 1995, 73, 176–180. [Google Scholar] [CrossRef]
- Vincent, S.; Vincent, R.A.; Shields, D.; Lee, G.A. Comparison of intraocular pressure measurement between rebound, non-contact and Goldmann applanation tonometry in treated glaucoma patients. Clin. Exp. Ophthalmol. 2011, 40, e163–e170. [Google Scholar] [CrossRef] [PubMed]
- Demirci, G.; Erdur, S.K.; Tanriverdi, C.; Gulkilik, G.; Ozsutçu, M. Comparison of rebound tonometry and non-contact airpuff tonometry to Goldmann applanation tonometry. Ther. Adv. Ophthalmol. 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Stock, R.A.; Ströher, C.; Sampaio, R.R.; Mergener, R.A.; Bonamigo, E.L. A Comparative Study between the Goldmann Applanation Tonometer and the Non-Contact Air-Puff Tonometer (Huvitz HNT 7000) in Normal Eyes. Clin. Ophthalmol. 2021, 15, 445–451. [Google Scholar] [CrossRef]
- Moseley, M.J.; Evans, N.M.; Fielder, A.R. Comparison of a new non-contact tonometer with Goldmann applanation. Eye 1989, 3, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Kyei, S.; Assiamah, F.; Kwarteng, M.A.; Gboglu, C.P. The Association of Central Corneal Thickness and Intraocular Pressure Measures by Non-Contact Tonometry and Goldmann Applanation Tonometry among Glaucoma Patients. Ethiop. J. Health Sci. 2020, 30, 999–1004. [Google Scholar] [CrossRef]
- Rebours, A.K.; Kouassi, F.; Soumahoro, M.; Ellalie, C.K.C.; Siméon, K.A.N.; Agbohoun, R. Comparaison de la tonomérie de Goldmann à la tonométrie à air pulsé à propos de 159 patients à Abidjan. J. Français D’Ophtalmol. 2021, 44, 41–47. [Google Scholar] [CrossRef]
- Atkinson, P.L.; Wishart, P.K.; James, J.N.; Vernon, S.A.; Reid, F. Deterioration in the accuracy of the Pulsair non-contact tonometer with use: Need for regular calibration. Eye 1992, 6, 530–534. [Google Scholar] [CrossRef]
- Britt, J.M. Microaerosol Formation in Noncontact ’Air-Puff’ Tonometry. Arch. Ophthalmol. 1991, 109, 225–228. [Google Scholar] [CrossRef]
- Lai, T.H.T.; Tang, E.W.; Chau, S.K.Y.; Fung, K.S.C.; Li, K.K.W. Stepping up infection control measures in ophthalmology during the novel coronavirus outbreak: An experience from Hong Kong. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 1049–1055. [Google Scholar] [CrossRef]
- Almazyad, E.M.; Ameen, S.; Khan, M.A.; Malik, R. Guidelines and recommendations for tonometry use during the COVID-19 era. Middle East Afr. J. Ophthalmol. 2020, 27, 73–78. [Google Scholar] [CrossRef]
- Mostafa, I.; Bianchi, E.; Brown, L.; Tatham, A.J. What is the best way to measure intraocular pressure (IOP) in a virtual clinic? Eye 2021, 35, 448–454. [Google Scholar] [CrossRef]
- Luce, D.A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract. Refract. Surg. 2005, 31, 156–162. [Google Scholar] [CrossRef]
- Kynigopoulos, M.; Schlote, T.; Kotecha, A.; Tzamalis, A.; Pajic, B.; Haefliger, I. Repeatability of Intraocular Pressure and Corneal Biomechanical Properties Measurements by the Ocular Response Analyser. Klin. Mon. Für Augenheilkd. 2008, 225, 357–360. [Google Scholar] [CrossRef]
- Martinez-De-La-Casa, J.M.; García-Feijóo, J.; Fernández-Vidal, A.; Mendez-Hernandez, C.; Garcia-Sanchez, J. Ocular Response Analyzer versus Goldmann Applanation Tonometry for Intraocular Pressure Measurements. Investig. Opthalmol. Vis. Sci. 2006, 47, 4410–4414. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Weinreb, R.N. Evaluation of the Influence of Corneal Biomechanical Properties on Intraocular Pressure Measurements Using the Ocular Response Analyzer. J. Glaucoma 2006, 15, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Touboul, D.; Roberts, C.; Kérautret, J.; Garra, C.; Maurice-Tison, S.; Saubusse, E.; Colin, J. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J. Cataract. Refract. Surg. 2008, 34, 616–622. [Google Scholar] [CrossRef]
- Bayoumi, N.H.L.; Bessa, A.S.; El Massry, A.A.K. Ocular Response Analyzer and Goldmann Applanation Tonometry. J. Glaucoma 2010, 19, 627–631. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, Z.; Li, L.; Sun, R.; Zhang, H. Comparison of intraocular pressure measured by ocular response analyzer and Goldmann applanation tonometer after corneal refractive surgery: A systematic review and meta-analysis. BMC Ophthalmol. 2020, 20, 23–29. [Google Scholar] [CrossRef]
- Lascaratos, G.; Garway-Heath, D.F.; Russell, R.A.; Crabb, D.P.; Zhu, H.; Hirn, C.; Kotecha, A.; Suzuki, K.; UKGTS Investigators. Intraocular pressure (IOP) measured with the ocular response ana-lyser is a better predictor of glaucoma progression than Goldmann IOP in the United Kingdom Glaucoma Treatment study (UKGTS). Investig. Ophthalmol. Vis. Sci. 2014, 55, 128–138. [Google Scholar]
- Susanna, B.N.; Ogata, N.G.; Daga, F.B.; Susanna, C.N.; Diniz-Filho, A.; Medeiros, F.A. Association between Rates of Visual Field Progression and Intraocular Pressure Measurements Obtained by Different Tonometers. Ophthalmology 2019, 126, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Congdon, N.G.; Broman, A.T.; Bandeen-Roche, K.; Grover, D.; Quigley, H.A. Central Corneal Thickness and Corneal Hysteresis Associated With Glaucoma Damage. Am. J. Ophthalmol. 2006, 141, 868–875. [Google Scholar] [CrossRef]
- Medeiros, F.A.; Meira-Freitas, D.; Lisboa, R.; Kuang, T.-M.; Zangwill, L.M.; Weinreb, R.N. Corneal Hysteresis as a Risk Factor for Glaucoma Progression: A Prospective Longitudinal Study. Ophthalmology 2013, 120, 1533–1540. [Google Scholar] [CrossRef]
- Susanna, B.N.; Ogata, N.G.; Jammal, A.A.; Susanna, C.N.; Berchuck, S.I.; Medeiros, F.A. Corneal Biomechanics and Visual Field Progression in Eyes with Seemingly Well-Controlled Intraocular Pressure. Ophthalmology 2019, 126, 1640–1646. [Google Scholar] [CrossRef] [PubMed]
- Fujino, Y.; Murata, H.; Matsuura, M.; Nakakura, S.; Shoji, N.; Nakao, Y.; Kiuchi, Y.; Asaoka, R. The Relationship between Corneal Hysteresis and Progression of Glaucoma After Trabeculectomy. J. Glaucoma 2020, 29, 912–917. [Google Scholar] [CrossRef]
- Kirgiz, A.; Erdur, S.K.; Atalay, K.; Gurez, C. The Role of Ocular Response Analyzer in Differentiation of Forme Fruste Keratoconus from Corneal Astigmatism. Eye Contact Lens. Sci. Clin. Pr. 2019, 45, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Randleman, J.B. Post-laser in-situ keratomileusis ectasia: Current understanding and future directions. Curr. Opin. Ophthalmol. 2006, 17, 406–412. [Google Scholar] [CrossRef]
- Reznicek, L.; Muth, D.; Kampik, A.; Neubauer, A.S.; Hirneiss, C. Evaluation of a novel Scheimpflug-based non-contact tonometer in healthy subjects and patients with ocular hypertension and glaucoma. Br. J. Ophthalmol. 2013, 97, 1410–1414. [Google Scholar] [CrossRef]
- Salvetat, M.L.; Zeppieri, M.; Tosoni, C.; Felletti, M.; Grasso, L.; Brusini, P. Corneal Deformation Parameters Provided by the Corvis-ST Pachy-Tonometer in Healthy Subjects and Glaucoma Patients. J. Glaucoma 2015, 24, 568–574. [Google Scholar] [CrossRef]
- Lopes, B.T.; Roberts, C.J.; Elsheikh, A.; Vinciguerra, R.; Vinciguerra, P.; Reisdorf, S.; Berger, S.; Koprowski, R.; Ambrósio, R. Repeatability and Reproducibility of Intraocular Pressure and Dynamic Corneal Response Parameters Assessed by the Corvis ST. J. Ophthalmol. 2017, 2017, 8515742. [Google Scholar] [CrossRef]
- Serbecic, N.; Beutelspacher, S.; Markovic, L.; Roy, A.S.; Shetty, R. Repeatability and reproducibility of corneal biomechanical parameters derived from Corvis ST. Eur. J. Ophthalmol. 2020, 30, 1287–1294. [Google Scholar] [CrossRef]
- Luebke, J.; Bryniok, L.; Neuburger, M.; Jordan, J.F.; Boehringer, D.; Reinhard, T.; Wecker, T.; Anton, A. Intraocular pressure measurement with Corvis ST in comparison with applanation tonometry and Tomey non-contact tonometry. Int. Ophthalmol. 2019, 39, 2517–2521. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Kiuchi, Y.; Okumichi, H. Evaluation of biomechanically corrected intraocular pressure using Corvis ST and comparison of the Corvis ST, noncontact tonometer, and Goldmann applanation tonometer in patients with glaucoma. PLoS ONE 2020, 15, e0238395. [Google Scholar] [CrossRef]
- Bao, F.; Huang, W.; Zhu, R.; Lu, N.; Wang, Y.; Li, H.; Wu, S.; Lin, H.; Wang, J.; Zheng, X.; et al. Effectiveness of the Goldmann Applanation Tonometer, the Dynamic Contour Tonometer, the Ocular Response Analyzer and the Corvis ST in Measuring Intraocular Pressure following FS-LASIK. Curr. Eye Res. 2019, 45, 144–152. [Google Scholar] [CrossRef]
- Yang, K.; Xu, L.; Fan, Q.; Gu, Y.; Song, P.; Zhang, B.; Zhao, D.; Pang, C.; Ren, S. Evaluation of new Corvis ST parameters in normal, Post-LASIK, Post-LASIK keratectasia and keratoconus eyes. Sci. Rep. 2020, 10, 5676. [Google Scholar] [CrossRef] [PubMed]
- Durham, D.G.; Bigliano, R.P.; Masino, J.A. Pneumatic applanation tonometer. Trans. Am. Acad. Ophthalmol. Otolaryngol. Am. Acad. Ophthalmol. Otolaryngol. 1965, 69, 1029–1047. [Google Scholar]
- West, C.E.; Capella, J.A.; Kaufman, H.E. Measurement of Intraocular Pressure with a Pneumatic Applanation Tonometer. Am. J. Ophthalmol. 1972, 74, 505–509. [Google Scholar] [CrossRef]
- Guildford, J.; O’day, D.M. Applanation Pneumotonometry in Screening for Glaucoma. South. Med. J. 1985, 78, 1081–1083. [Google Scholar] [CrossRef] [PubMed]
- Abbasoglu Özlem, E.; Bowman, R.; Cavanagh, H.; McCulley, J.P. Reliability of intraocular pressure measurements after myopic excimer photorefractive keratectomy. Ophthalmology 1998, 105, 2193–2196. [Google Scholar] [CrossRef]
- Zadok, D.; Tran, D.B.; Twa, M.; Carpenter, M.; Schanzlin, D.J. Pneumotonometry versus Goldmann tonometry after laser in situ keratomileusis for myopia. J. Cataract. Refract. Surg. 1999, 25, 1344–1348. [Google Scholar] [CrossRef]
- Langham, M.E. Discussion on pneumatic applanation tonometer. Tr. Am. Acad. Ophth. Otolaryng. 1965, 69, 1042. [Google Scholar]
- Langham, M.E.; McCarthy, E. A Rapid Pneumatic Applanation Tonometer. Arch. Ophthalmol. 1968, 79, 389–399. [Google Scholar] [CrossRef]
- Silver, D.M.; Farrell, R.A. Validity of pulsatile ocular blood flow measurements. Surv. Ophthalmol. 1994, 38, S72–S80. [Google Scholar] [CrossRef]
- Esgin, H.; Alimgil, M.; Erda, S. Clinical comparison of the ocular blood flow tonograph and the Goldmann applanation tonometer. Eur. J. Ophthalmol. 1998, 8, 162–166. [Google Scholar] [CrossRef]
- Gunvant, P.; Baskaran, M.; Vijaya, L.; Joseph, I.S.; Watkins, R.J.; Nallapothula, M.; Broadway, D.C.; O’Leary, D.J. Effect of corneal parameters on measurements using the pulsatile ocular blood flow tonograph and Goldmann applanation tonometer. Br. J. Ophthalmol. 2004, 88, 518–522. [Google Scholar] [CrossRef]
- Bhan, A.; Bhargava, J.; Vernon, S.A.; Armstrong, S.; Bhan, K.; Tong, L.; Sung, V. Repeatability of ocular blood flow pneumotonometry. Ophthalmology 2003, 110, 1551–1554. [Google Scholar] [CrossRef]
- Spraul, C.W.; Lang, G.E.; Ronzani, M.; Högel, J.; Lang, G.K. Reproducibility of measurements with a new slit lamp-mounted ocular blood flow tonograph. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998, 236, 274–279. [Google Scholar] [CrossRef]
- Kontiola, A.I. A new induction-based impact method for measuring intraocular pressure. Acta. Ophthalmol. Scand. 2000, 78, 142–145. [Google Scholar] [CrossRef]
- Brusini, P.; Salvetat, M.L.; Zeppieri, M.; Tosoni, C.; Parisi, L. Comparison of ICare Tonometer with Goldmann Applanation Tonometer in Glaucoma Patients. J. Glaucoma 2006, 15, 213–217. [Google Scholar] [CrossRef]
- Kageyama, M.; Hirooka, K.; Baba, T.; Shiraga, F. Comparison of ICare Rebound Tonometer with Noncontact Tonometer in Healthy Children. J. Glaucoma 2011, 20, 63–66. [Google Scholar] [CrossRef]
- Salvetat, M.L.; Zeppieri, M.; Miani, F.; Tosoni, C.; Parisi, L.; Brusini, P. Comparison of iCare tonometer and Goldmann applanation tonometry in normal corneas and in eyes with automated lamellar and penetrating keratoplasty. Eye 2011, 25, 642–650. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Realini, T.; McMillan, B.; Gross, R.L.; Devience, E.; Balasubramani, G.K. Assessing the Reliability of Intraocular Pressure Measurements Using Rebound Tonometry. J. Glaucoma 2021, 30, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Rosentreter, A.; Athanasopoulos, A.; Schild, A.M.; Lappas, A.; Cursiefen, C.; Dietlein, T.S. Rebound, Applanation, and Dynamic Contour Tonometry in Pathologic Corneas. Cornea 2013, 32, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Badakere, S.V.; Chary, R.; Choudhari, N.S.; Rao, H.L.; Garudadri, C.; Senthil, S. Agreement of Intraocular Pressure Measurement of Icare ic200 with Goldmann Applanation Tonometer in Adult Eyes with Normal Cornea. Ophthalmol. Glaucoma 2021, 4, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Ve, R.S.; Jose, J.; Pai, H.V.; Biswas, S.; Parimi, V.; Poojary, P.; Nagarajan, T. Agreement and repeatability of Icare ic100 tonometer. Indian J. Ophthalmol. 2020, 68, 2122–2125. [Google Scholar] [CrossRef]
- Halkiadakis, I.; Stratos, A.; Stergiopoulos, G.; Patsea, E.; Skouriotis, S.; Mitropoulos, P.; Papaconstantinou, D.; Georgopoulos, G. Evaluation of the Icare-ONE rebound tonometer as a self-measuring intraocular pressure device in normal subjects. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012, 250, 1207–1211. [Google Scholar] [CrossRef]
- Rosenfeld, E.; Barequet, D.; Mimouni, M.; Fischer, N.; Kurtz, S. Role of home monitoring with iCare ONE rebound tonometer in glaucoma patients management. Int. J. Ophthalmol. 2021, 14, 405–408. [Google Scholar] [CrossRef]
- Cvenkel, B.; Velkovska, M.A.; Jordanova, V.D. Self-measurement with Icare HOME tonometer, patients’ feasibility and acceptability. Eur. J. Ophthalmol. 2019, 30, 258–263. [Google Scholar] [CrossRef]
- Quérat, L.; Chen, E. Clinical Use of iC are Home® tonometer. Acta. Ophthalmol. 2020, 98, e131–e132. [Google Scholar] [CrossRef]
- Gao, F.; Liu, X.; Zhao, Q.; Pan, Y. Comparison of the iCare rebound tonometer and the Goldmann applanation tonometer. Exp. Ther. Med. 2017, 13, 1912–1916. [Google Scholar] [CrossRef]
- Kiddee, W.; Tanjana, A. Variations of Intraocular Pressure Measured by Goldmann Applanation Tonometer, Tono-Pen, iCare Rebound Tonometer, and Pascal Dynamic Contour Tonometer in Patients With Corneal Edema After Phacoemulsification. J. Glaucoma 2021, 30, 317–324. [Google Scholar] [CrossRef]
- Valero, B.; Fénolland, J.-R.; Rosenberg, R.; Sendon, D.; Mesnard, C.; Sigaux, M.; Giraud, J.-M.; Renard, J.-P. Fiabilité et reproductibilité des mesures de la pression intraoculaire par le tonomètre Icare ® Home (modèle TA022) et comparaison avec les mesures au tonomètre à aplanation de Goldmann chez des patients glaucomateux. J. Français D’Ophtalmol. 2017, 40, 865–875. [Google Scholar] [CrossRef]
- Nakakura, S.; Mori, E.; Fujio, Y.; Fujisawa, Y.; Matsuya, K.; Kobayashi, Y.; Tabuchi, H.; Asaoka, R.; Kiuchi, Y. Comparison of the Intraocular Pressure Measured Using the New Rebound Tonometer Icare ic100 and Icare TA01i or Goldmann Applanation Tonometer. J. Glaucoma 2019, 28, 172–177. [Google Scholar] [CrossRef]
- Kato, Y.; Nakakura, S.; Matsuo, N.; Yoshitomi, K.; Handa, M.; Tabuchi, H.; Kiuchi, Y. Agreement among Goldmann applanation tonometer, iCare, and Icare PRO rebound tonometers; non-contact tonometer; and Tonopen XL in healthy elderly subjects. Int. Ophthalmol. 2017, 38, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Scuderi, G.; Cascone, N.C.; Regine, F.; Perdicchi, A.; Cerulli, A.; Recupero, S.M. Validity and Limits of the Rebound Tonometer (ICare®): Clinical Study. Eur. J. Ophthalmol. 2011, 21, 251–257. [Google Scholar] [CrossRef]
- Nakakura, S.; Asaoka, R.; Terao, E.; Nagata, Y.; Fukuma, Y.; Oogi, S.; Shiraishi, M.; Kiuchi, Y. Evaluation of rebound tonometer iCare IC200 as compared with IcarePRO and Goldmann applanation tonometer in patients with glaucoma. Eye Vis. 2021, 8, 25. [Google Scholar] [CrossRef]
- Liu, J.; De Francesco, T.; Schlenker, M.; Ahmed, I.I. Icare Home Tonometer: A Review of Characteristics and Clinical Utility. Clin. Ophthalmol. 2020, 14, 4031–4045. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, L.; Xu, J.; Chen, X.; Gu, Y.; Ren, Y.; Wang, K. Comparability of three intraocular pressure measurement: iCare pro rebound, non-contact and Goldmann applanation tonometry in different IOP group. BMC Ophthalmol. 2019, 19, 225. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.L.; Lim, L.T.; Ooi, C.Y.; Rahman, M. Comparison of Icare Rebound Tonometer and Perkins Applanation Tonometer in Community Eye Screening. Asia-Pac. J. Ophthalmol. 2019, 8, 229–232. [Google Scholar] [CrossRef]
- Subramaniam, A.G.; Allen, P.; Toh, T. Comparison of the Icare ic100 rebound tonometer and the Goldmann applanation tonometer in 1000 eyes. Ophthalmic Res. 2020, 64, 321–326. [Google Scholar] [CrossRef]
- Muttuvelu, D.V.; Baggesen, K.; Ehlers, N. Precision and accuracy of the ICare tonometer—Peripheral and central IOP measurements by rebound tonometry. Acta. Ophthalmol. 2010, 90, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, C.; Bachmann, L.M.; Thiel, M.A. Intraocular Pressure Measurements Using Dynamic Contour Tonometry after Laser In Situ Keratomileusis. Investig. Opthalmol. Vis. Sci. 2003, 44, 3790–3794. [Google Scholar] [CrossRef] [PubMed]
- Kanngiesser, H.E.; Kniestedt, C.; Robert, Y.C.A. Dynamic Contour Tonometry. J. Glaucoma 2005, 14, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Kamppeter, B.A.; Jonas, J.B. Dynamic Contour Tonometry for Intraocular Pressure Measurement. Am. J. Ophthalmol. 2005, 140, 318–320. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, P.; Morbio, R.; Marraffa, M.; Marchini, G. Comparison of Goldmann applanation tonometry and dynamic contour tonometry in healthy and glaucomatous eyes. Eye 2008, 23, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Fogagnolo, P.; Figus, M.; Frezzotti, P.; Iester, M.; Oddone, F.; Zeppieri, M.; Ferreras, A.; Brusini, P.; Rossetti, L.; Orzalesi, N. Test-retest variability of intraocular pressure and ocular pulse amplitude for dynamic contour tonometry: A multicentre study. Br. J. Ophthalmol. 2009, 94, 419–423. [Google Scholar] [CrossRef] [PubMed]
- Katsimpris, J.M.; Theoulakis, P.E.; Vasilopoulos, K.; Skourtis, G.; Papadopoulos, G.E.; Petropoulos, I.K. Correlation between Central Corneal Thickness and Intraocular Pressure Measured by Goldmann Applanation Tonometry or Pascal Dynamic Contour Tonometry. Klin. Mon. Für Augenheilkd. 2015, 232, 414–418. [Google Scholar] [CrossRef]
- Schwenn, O. Ocular pulse amplitude in patients with open angle glaucoma, normal tension glaucoma, and ocular hypertension. Br. J. Ophthalmol. 2002, 86, 981–984. [Google Scholar] [CrossRef]
- Kniestedt, C.; Kanngiesser, H.; Stamper, R.L. Assessment of Pascal dynamic contour tonometer in monitoring IOP after LASIK. J. Cataract. Refract. Surg. 2005, 31, 458–459. [Google Scholar] [CrossRef]
- Siganos, D.S.; Papastergiou, G.I.; Moedas, C. Assessment of the Pascal dynamic contour tonometer in monitoring intraocular pressure in unoperated eyes and eyes after LASIK. J. Cataract. Refract. Surg. 2004, 30, 746–751. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, E.W.; Choi, W.; Park, C.K.; Kim, S.; Bae, H.W.; Seong, G.J.; Kim, C.Y. Significance of dynamic contour tonometry in evaluation of progression of glaucoma in patients with a history of laser refractive surgery. Br. J. Ophthalmol. 2019, 104, 276–281. [Google Scholar] [CrossRef]
- Kandarakis, A.; Soumplis, V.; Pitsas, C.; Kandarakis, S.; Halikias, J.; Karagiannis, D. Comparison of dynamic contour tonometry and Goldmann applanation tonometry following penetrating keratoplasty. Can. J. Ophthalmol. 2010, 45, 489–493. [Google Scholar] [CrossRef]
- Sales-Sanz, M.; Arranz-Marquez, E.; Arruabarrena, C.; Teus, M.A. Influence of LASEK on Schiøtz, Goldmann and dynamic contour Tonometry. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 256, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.S.; Alencar, L.; Weinreb, R.N.; Tafreshi, A.; Deokule, S.; Vizzeri, G.; Medeiros, F.A. Repeatability and Reproducibility of Goldmann Applanation, Dynamic Contour, and Ocular Response Analyzer Tonometry. J. Glaucoma 2013, 22, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Bochmann, F.; Kaufmann, C.; Theil, M.A. Dynamic contour tonometry versus Goldmann applanation tonometry: Challenging the gold standard. Expert. Rev. Ophthalmol. 2015, 5, 743–749. [Google Scholar] [CrossRef][Green Version]
- Okafor, K.C.; Brandt, J.D. Measuring intraocular pressure. Curr. Opin. Ophthalmol. 2015, 26, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Eklund, A.; Hallberg, P.; Linden, C.; Lindahl, O.A. An Applanation Resonator Sensor for Measuring Intraocular Pressure Using Combined Continuous Force and Area Measurement. Investig. Opthalmol. Vis. Sci. 2003, 44, 3017–3024. [Google Scholar] [CrossRef][Green Version]
- Jóhannesson, G.; Hallberg, P.; Eklund, A.; Lindén, C. Introduction and clinical evaluation of servo-controlled applanation resonance tonometry. Acta. Ophthalmol. 2011, 90, 677–682. [Google Scholar] [CrossRef]
- Hallberg, P.; Eklund, A.; Bäcklund, T.; Lindén, C. Clinical Evaluation of Applanation Resonance Tonometry. J. Glaucoma 2007, 16, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Salvetat, M.L.; Zeppieri, M.; Tosoni, C.; Brusini, P. Repeatability and accuracy of applanation resonance tonometry in healthy subjects and patients with glaucoma. Acta. Ophthalmol. 2013, 92, e66–e73. [Google Scholar] [CrossRef] [PubMed]
- Ottobelli, L.; Fogagnolo, P.; Frezzotti, P.; De Cillà, S.; Vallenzasca, E.; Digiuni, M.; Paderni, R.; Motolese, I.; Bagaglia, S.A.; Motolese, E.; et al. Repeatability and reproducibility of applanation resonance tonometry: A cross-sectional study. BMC Ophthalmol. 2015, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Mulak, M.; Czak, W.A.; Mimier, M.; Kaczmarek, R. A comparison of intraocular pressure values obtained using a Goldmann applanation tonometer and a handheld version of applanation resonance tonometer: A preliminary report. Adv. Clin. Exp. Med. 2018, 27, 481–485. [Google Scholar] [CrossRef] [PubMed]
- Jóhannesson, G.; Hallberg, P.; Eklund, A.; Koskela, T.; Lindén, C. Change in Intraocular Pressure Measurement After Myopic LASEK. J. Glaucoma 2012, 21, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.H.; Kripke, D.F.; Hoffman, R.E.; Twa, M.; Loving, R.T.; Rex, K.M.; Gupta, N.; Weinreb, R.N. Nocturnal elevation of intraocular pressure in young adults. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2707–2712. [Google Scholar]
- Konstas, A.G.P.; Mantziris, D.A.; Stewart, W.C. Diurnal Intraocular Pressure in Untreated Exfoliation and Primary Open-angle Glaucoma. Arch. Ophthalmol. 1997, 115, 182–185. [Google Scholar] [CrossRef]
- Asrani, S.; Zeimer, R.; Wilensky, J.; Gieser, D.; Vitale, S.; Lindenmuth, K. Large Diurnal Fluctuations in Intraocular Pressure Are an Independent Risk Factor in Patients With Glaucoma. J. Glaucoma 2000, 9, 134–142. [Google Scholar] [CrossRef]
- Tan, S.; Baig, N.; Hansapinyo, L.; Jhanji, V.; Wei, S.; Tham, C.Y.C. Comparison of self-measured diurnal intraocular pressure profiles using rebound tonometry between primary angle closure glaucoma and primary open angle glaucoma patients. PLoS ONE 2017, 12, e0173905. [Google Scholar] [CrossRef]
- Barkana, Y.; Anis, S.; Liebmann, J.; Tello, C.; Ritch, R. Clinical Utility of Intraocular Pressure Monitoring Outside of Normal Office Hours in Patients With Glaucoma. Arch. Ophthalmol. 2006, 124, 793–797. [Google Scholar] [CrossRef]
- Hughes, E.; Spry, P.; Diamond, J. 24-Hour Monitoring of Intraocular Pressure in Glaucoma Management: A Retrospective Review. J. Glaucoma 2003, 12, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Konstas, A.G.P.; Quaranta, L.; Bozkurt, B.; Katsanos, A.; Feijoo, J.G.; Rossetti, L.; Shaarawy, T.; Pfeiffer, N.; Miglior, S. 24-h Efficacy of Glaucoma Treatment Options. Adv. Ther. 2016, 33, 481–517. [Google Scholar] [CrossRef]
- Ho, C.H.; Wong, J.K.W. Role of 24-Hour Intraocular Pressure Monitoring in Glaucoma Management. J. Ophthalmol. 2019, 2019, 3632197–3632213. [Google Scholar] [CrossRef] [PubMed]
- Mcmonnies, C.W. The importance of and potential for continuous monitoring of intraocular pressure. Clin. Exp. Optom. 2017, 100, 203–207. [Google Scholar] [CrossRef]
- Ittoop, S.M.; SooHoo, J.R.; Seibold, L.K.; Mansouri, K.; Kahook, M.Y. Systematic Review of Current Devices for 24-h Intraocular Pressure Monitoring. Adv. Ther. 2016, 33, 1679–1690. [Google Scholar] [CrossRef] [PubMed]
- Molaei, A.; Karamzadeh, V.; Safi, S.; Esfandiari, H.; Dargahi, J.; Khosravi, M. Upcoming methods and specifications of continuous intraocular pressure monitoring systems for glaucoma. J. Ophthalmic Vis. Res. 2018, 13, 66–71. [Google Scholar] [CrossRef]
- Dick, H.B.; Schultz, T.; Gerste, R.D. Miniaturization in Glaucoma Monitoring and Treatment: A Review of New Technologies That Require a Minimal Surgical Approach. Ophthalmol. Ther. 2019, 8, 19–30. [Google Scholar] [CrossRef]
- Lee, J.O.; Park, H.; Du, J.; Balakrishna, A.; Chen, O.; Sretavan, D.; Choo, H. A microscale optical implant for continuous in vivo monitoring of intraocular pressure. Microsyst. Nanoeng. 2017, 3, 17057. [Google Scholar] [CrossRef]
- Koutsonas, A.; Walter, P.; Roessler, G.; Plange, N. Implantation of a Novel Telemetric Intraocular Pressure Sensor in Patients with Glaucoma (ARGOS Study): 1-Year Results. Investig. Opthalmol. Vis. Sci. 2015, 56, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Choritz, L.; Mansouri, K.; Bosch, J.V.D.; Weigel, M.; Dick, H.B.; Wagner, M.; Thieme, H.; Rüfer, F.; Szurmann, P.; Wehner, W.; et al. Telemetric Measurement of Intraocular Pressure via an Implantable Pressure Sensor—12-Month Results from the ARGOS-02 Trial. Am. J. Ophthalmol. 2020, 209, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Rodger, D.; Humayun, M.S.; Tai, Y.-C. Unpowered spiral-tube parylene pressure sensor for intraocular pressure sensing. Sens. Actuators A Phys. 2006, 127, 276–282. [Google Scholar] [CrossRef]
- Demeng, L.; Niansong, M.; Zhaofeng, Z. An ultralow power wireless intraocular pressure monitoring system. J. Semicond. 2014, 35, 105014. [Google Scholar]
- Mariacher, S.; Ebner, M.; Januschowski, K.; Hurst, J.; Schnichels, S.; Szurman, P. Investigation of a novel implantable suprachoroidal pressure transducer for telemetric intraocular pressure monitoring. Exp. Eye Res. 2016, 151, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Leuenberger, P.; Bertrand, D.; Bertsch, A.; Renaud, P. First Steps toward Noninvasive Intraocular Pressure Monitoring with a Sensing Contact Lens. Investig. Opthalmol. Vis. Sci. 2004, 45, 3113–3117. [Google Scholar] [CrossRef] [PubMed]
- Hediger, A.; Kniestedt, C.; Zweifel, S.; Knecht, P.; Funk, J.; Kanngiesser, H. Kontinuierliche Augeninnendruckmessung. Der. Ophthalmol. 2009, 106, 1111–1115. [Google Scholar] [CrossRef]
- Twa, M.; Roberts, C.J.; Karol, H.J.; Mahmoud, A.M.; Weber, P.A.; Small, R.H. Evaluation of a Contact Lens-Embedded Sensor for Intraocular Pressure Measurement. J. Glaucoma 2010, 19, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Shaarawy, T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: Initial clinical experience in patients with open angle glaucoma. Br. J. Ophthalmol. 2011, 95, 627–629. [Google Scholar] [CrossRef]
- Mansouri, K.; Weinreb, R.N.; Liu, J.H.K. Efficacy of a Contact Lens Sensor for Monitoring 24-H Intraocular Pressure Related Patterns. PLoS ONE 2015, 10, e0125530. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, K.; Medeiros, F.A.; Tafreshi, A.; Weinreb, R.N. Error in PubMed in: Global Burden of Visual Impairment and Blindness. Arch. Ophthalmol. 2012, 130, 1559. [Google Scholar] [CrossRef]
- Lorenz, K.; Korb, C.; Herzog, N.; Vetter, J.M.; Elflein, H.; Keilani, M.M.; Pfeiffer, N. Tolerability of 24-Hour Intraocular Pressure Monitoring of a Pressure-sensitive Contact Lens. J. Glaucoma 2013, 22, 311–316. [Google Scholar] [CrossRef]
- Dunbar, G.E.; Shen, B.Y.; Aref, A.A. The Sensimed Triggerfish contact lens sensor: Efficacy, safety, and patient perspectives. Clin. Ophthalmol. 2017, 11, 875–882. [Google Scholar] [CrossRef]
- Mottet, B.; Aptel, F.; Romanet, J.-P.; Hubanova, R.; Pépin, J.-L.; Chiquet, C. 24-Hour Intraocular Pressure Rhythm in Young Healthy Subjects Evaluated With Continuous Monitoring Using a Contact Lens Sensor. JAMA Ophthalmol. 2013, 131, 1507–1516. [Google Scholar] [CrossRef]
- Holló, G.; Kóthy, P.; Vargha, P. Evaluation of Continuous 24-Hour Intraocular Pressure Monitoring for Assessment of Prostaglandin-induced Pressure Reduction in Glaucoma. J. Glaucoma 2014, 23, e6–e12. [Google Scholar] [CrossRef]
- Mansouri, K. The Road Ahead to Continuous 24-Hour Intraocular Pressure Monitoring in Glaucoma. J. Ophthalmic Vis. Res. 2014, 9, 260–268. [Google Scholar]
- Tojo, N.; Hayashi, A.; Otsuka, M. Correlation between 24-h continuous intraocular pressure measurement with a contact lens sensor and visual field progression. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Tojo, N.; Hayashi, A.; Otsuka, M.; Miyakoshi, A. Fluctuations of the Intraocular Pressure in Pseudoexfoliation Syndrome and Normal Eyes Measured by a Contact Lens Sensor. J. Glaucoma 2016, 25, e463–e468. [Google Scholar] [CrossRef]
- Pajic, B.; Pajic-Eggspuchler, B.; Haefliger, I. Continuous IOP Fluctuation Recording in Normal Tension Glaucoma Patients. Curr. Eye Res. 2011, 36, 1129–1138. [Google Scholar] [CrossRef]
- Agnifili, L.; Mastropasqua, R.; Frezzotti, P.; Fasanella, V.; Motolese, I.; Pedrotti, E.; Di Iorio, A.; Mattei, P.A.; Motolese, E.; Mastropasqua, L. Circadian intraocular pressure patterns in healthy subjects, primary open angle and normal tension glaucoma patients with a contact lens sensor. Acta. Ophthalmol. 2014, 93, e14–e21. [Google Scholar] [CrossRef] [PubMed]
- Tojo, N.; Abe, S.; Ishida, M.; Yagou, T.; Hayashi, A. The Fluctuation of Intraocular Pressure Measured by a Contact Lens Sensor in Normal-Tension Glaucoma Patients and Nonglaucoma Subjects. J. Glaucoma 2017, 26, 195–200. [Google Scholar] [CrossRef]
- Kim, Y.W.; Kim, J.-S.; Lee, S.Y.; Ha, A.; Lee, J.; Park, Y.J.; Kim, Y.K.; Jeoung, J.W.; Park, K.H. Twenty-four–Hour Intraocular Pressure–Related Patterns from Contact Lens Sensors in Normal-Tension Glaucoma and Healthy Eyes. Ophthalmology 2020, 127, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.W.; Kim, M.J.; Park, K.H.; Jeoung, J.W.; Kim, S.H.; Jang, C.I.; Lee, S.H.; Kim, J.H.; Lee, S.; Kang, J.Y. Preliminary study on implantable inductive-type sensor for continuous monitoring of intraocular pressure. Clin. Exp. Ophthalmol. 2015, 43, 830–837. [Google Scholar] [CrossRef]
- Mansouri, K.; Rao, H.L.; Weinreb, R.N. Short-Term and Long-Term Variability of Intraocular Pressure Measured with an Intraocular Telemetry Sensor in Patients with Glaucoma. Ophthalmology 2021, 128, 227–233. [Google Scholar] [CrossRef]
- Xu, S.C.; Gauthier, A.C.; Liu, J. The Application of a Contact Lens Sensor in Detecting 24-Hour Intraocular Pressure-Related Patterns. J. Ophthalmol. 2016, 2016, 4727423. [Google Scholar] [CrossRef]
- Kouhani, M.H.M.; Wu, J.; Tavakoli, A.; Weber, A.J.; Li, W. Wireless, passive strain sensor in a doughnut-shaped contact lens for continuous non-invasive self-monitoring of intraocular pressure. Lab. Chip. 2020, 20, 332–342. [Google Scholar] [CrossRef]
- Xu, J.; Cui, T.; Hirtz, T.; Qiao, Y.; Li, X.; Zhong, F.; Han, X.; Yang, Y.; Zhang, S.; Ren, T.-L. Highly Transparent and Sensitive Graphene Sensors for Continuous and Non-invasive Intraocular Pressure Monitoring. ACS Appl. Mater. Interfaces 2020, 12, 18375–18384. [Google Scholar] [CrossRef] [PubMed]
- Maeng, B.; Chang, H.-K.; Park, J. Photonic crystal-based smart contact lens for continuous intraocular pressure monitoring. Lab. Chip. 2020, 20, 1740–1750. [Google Scholar] [CrossRef] [PubMed]
- Wasilewicz, R.; Varidel, T.; Simon-Zoula, S.; Schlund, M.; Cerboni, S.; Mansouri, K. First-in-human continuous 24-hour measurement of intraocular pressure and ocular pulsation using a novel contact lens sensor. Br. J. Ophthalmol. 2020, 104, 1519–1523. [Google Scholar] [CrossRef]
- Agaoglu, S.; Diep, P.; Martini, M.; Kt, S.; Baday, M.; Araci, I.E. Ultra-sensitive microfluidic wearable strain sensor for intraocular pressure monitoring. Lab. Chip. 2018, 18, 3471–3483. [Google Scholar] [CrossRef]
- Campigotto, A.; Leahy, S.; Zhao, G.; Campbell, R.J.; Lai, Y. Non-invasive Intraocular pressure monitoring with contact lens. Br. J. Ophthalmol. 2019, 104, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Tu, H.; Zhao, H.; Wei, F.; Yang, Y.; Ren, T. A wearable contact lens sensor for noninvasive in-situ monitoring of intraocular pressure. Nanotechnology 2021, 32, 095106. [Google Scholar] [CrossRef]
- Dou, Z.; Tang, J.; Liu, Z.; Sun, Q.; Wang, Y.; Li, Y.; Yuan, M.; Wu, H.; Wang, Y.; Pei, W.; et al. Wearable Contact Lens Sensor for Non-invasive Continuous Monitoring of Intraocular Pressure. Micromachines 2021, 12, 108. [Google Scholar] [CrossRef] [PubMed]
- Gillmann, K.; Wasilewicz, R.; Hoskens, K.; Simon-Zoula, S.; Mansouri, K. Continuous 24-hour measurement of intraocular pressure in millimeters of mercury (mmHg) using a novel contact lens sensor: Comparison with pneumatonometry. PLoS ONE 2021, 16, e0248211. [Google Scholar] [CrossRef] [PubMed]
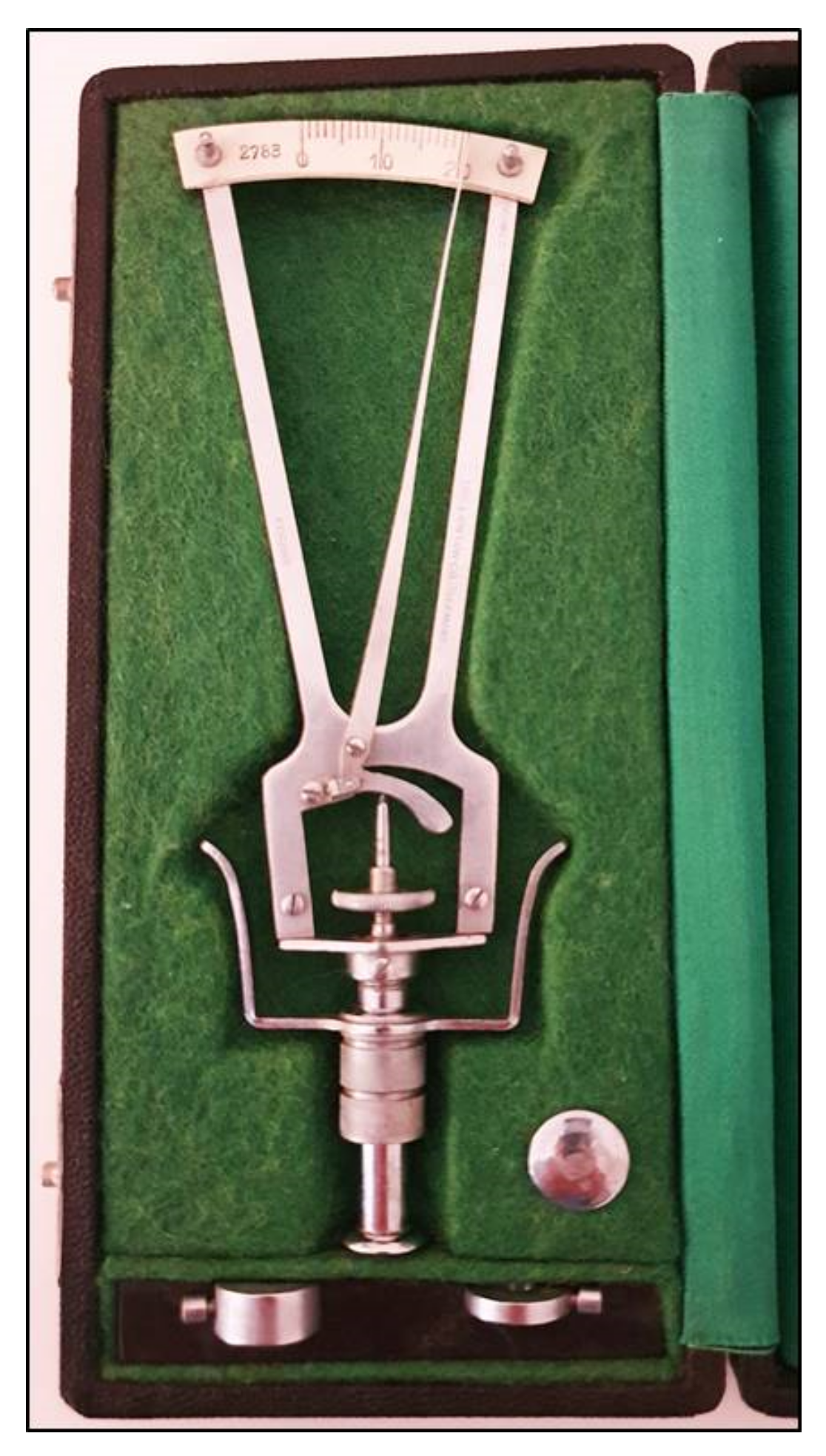
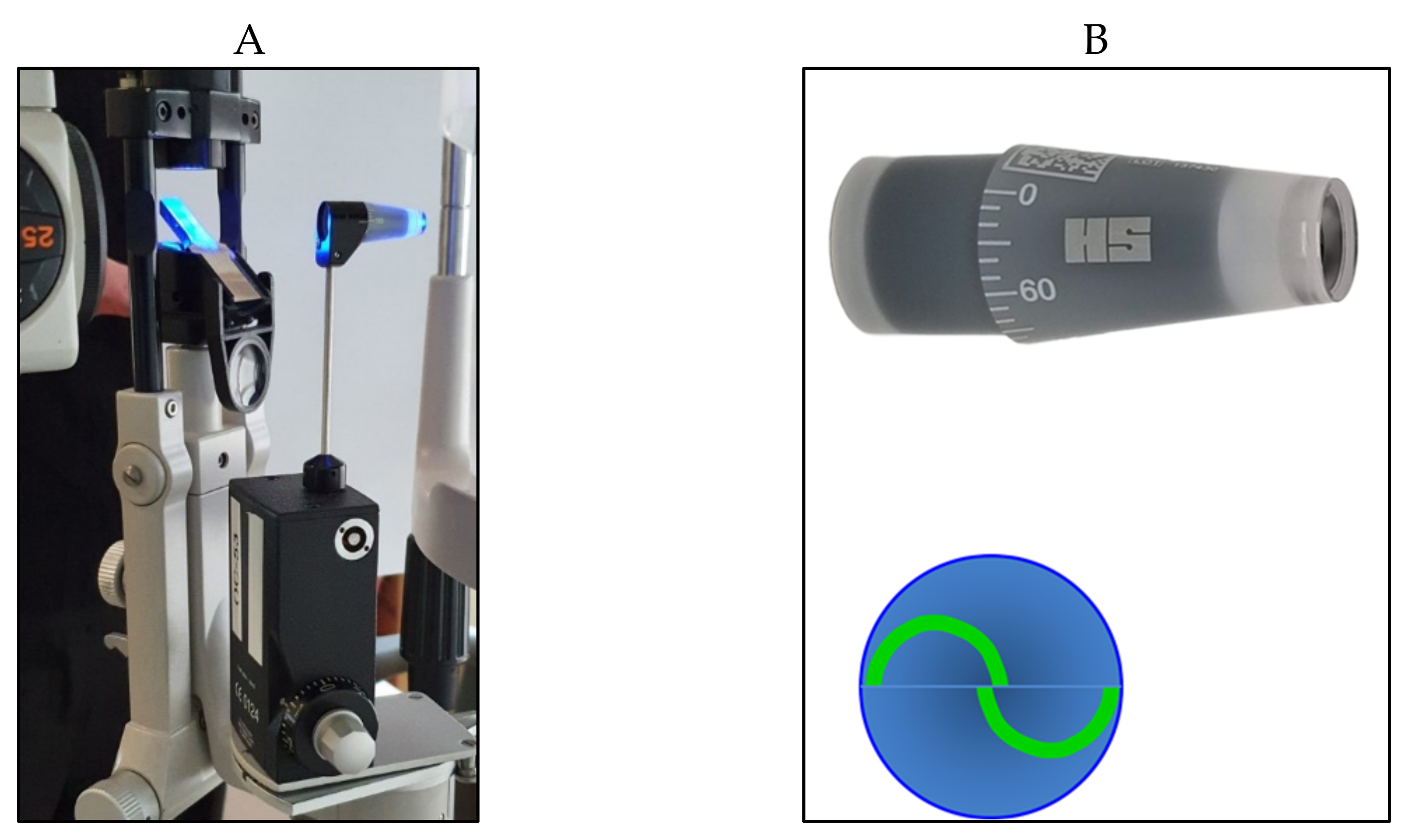
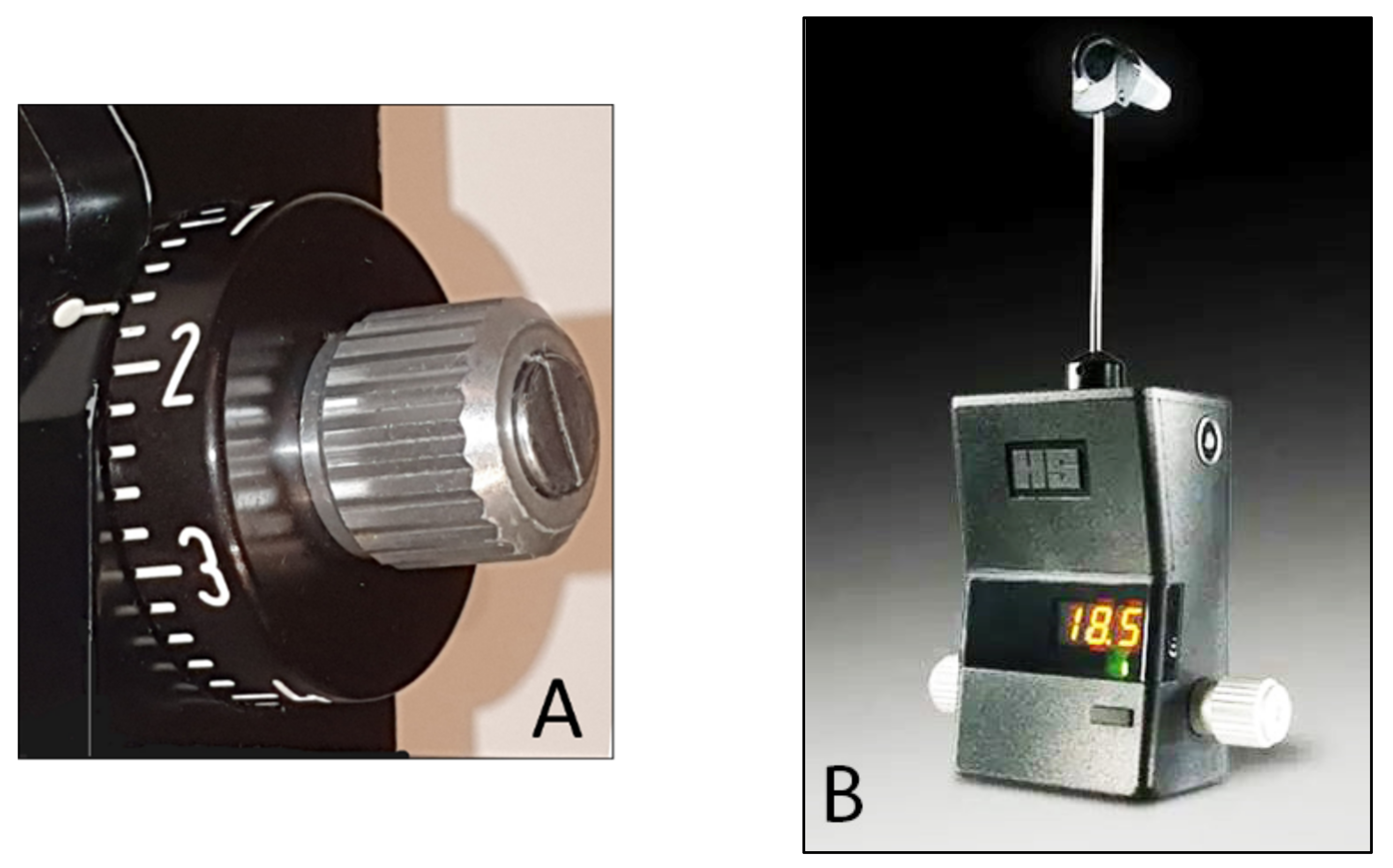
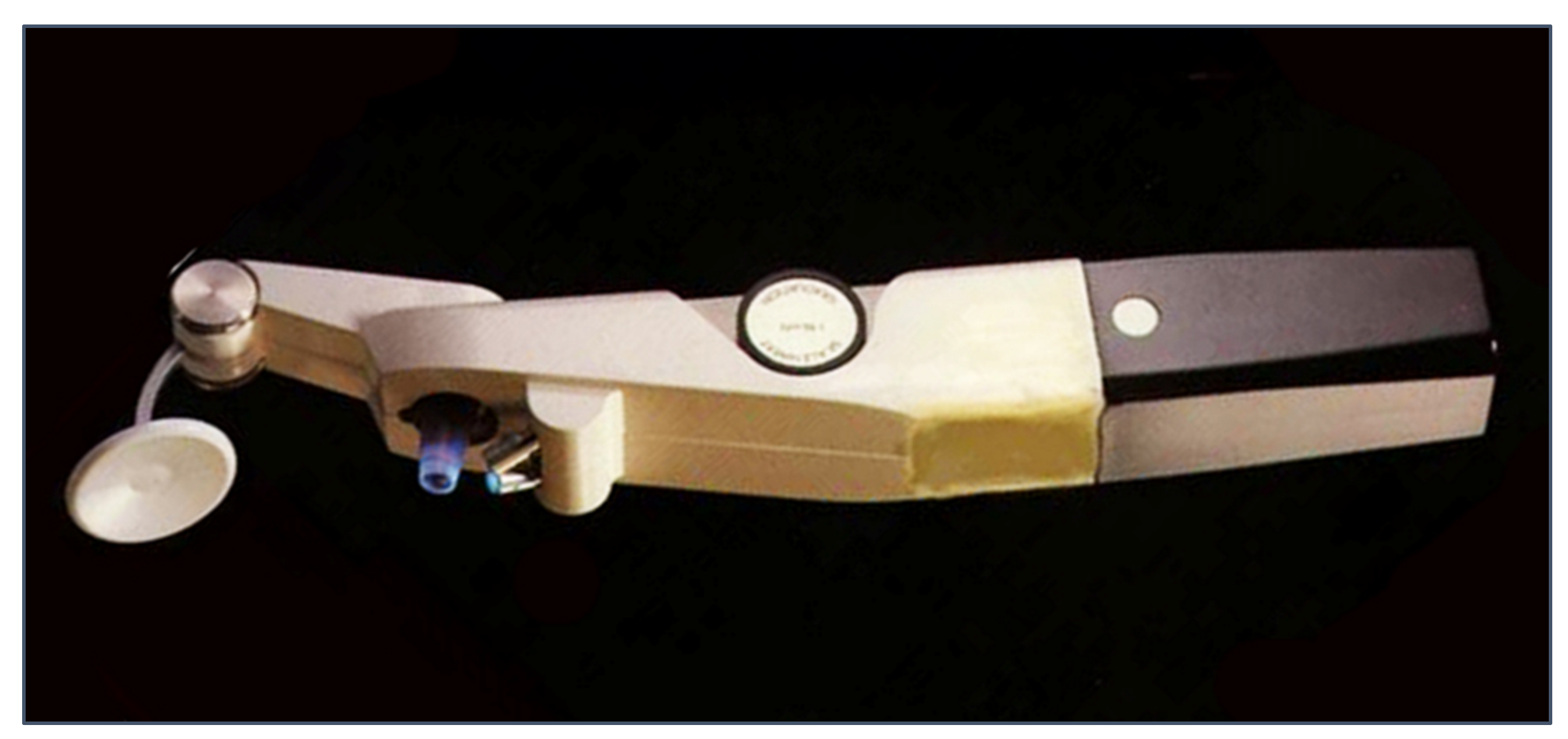




| Tonometer Type | Production Year | Working Principle | Contact/ Noncontact | Advantages | Disadvantages | Clinical Suitability | Cost * |
|---|---|---|---|---|---|---|---|
| Schioetz tonometer | 1905 | indentation | contact | Simple | High variability | still used only in developing countries | feasible |
| Inexpensive | Can be used in a supine position only | ||||||
| No need of slit-lam, electricity or charging batteries | Affected by various sources of error | ||||||
| Topical anesthesia is needed | |||||||
| Goldmann applanation tonometer (GAT) | 1955 | applanation | contact | Quite simple to use | Affected by corneal thickness and other corneal parameters | good | feasible |
| Accurate and reproducible measurements | The accuracy depends on the clinician’s experience | ||||||
| It is currently considered as the gold standard in IOP measurement | Needs to be used with a slit-lamp | ||||||
| Can be used in the upright position only | |||||||
| Topical anesthesia and fluorescein are needed | |||||||
| Perkins tonometer | 1965 | applanation | contact | Can be used in a supine position too | Affected by corneal thickness and other corneal parameters | good | feasible |
| Topical anesthesia and fluorescein are needed | |||||||
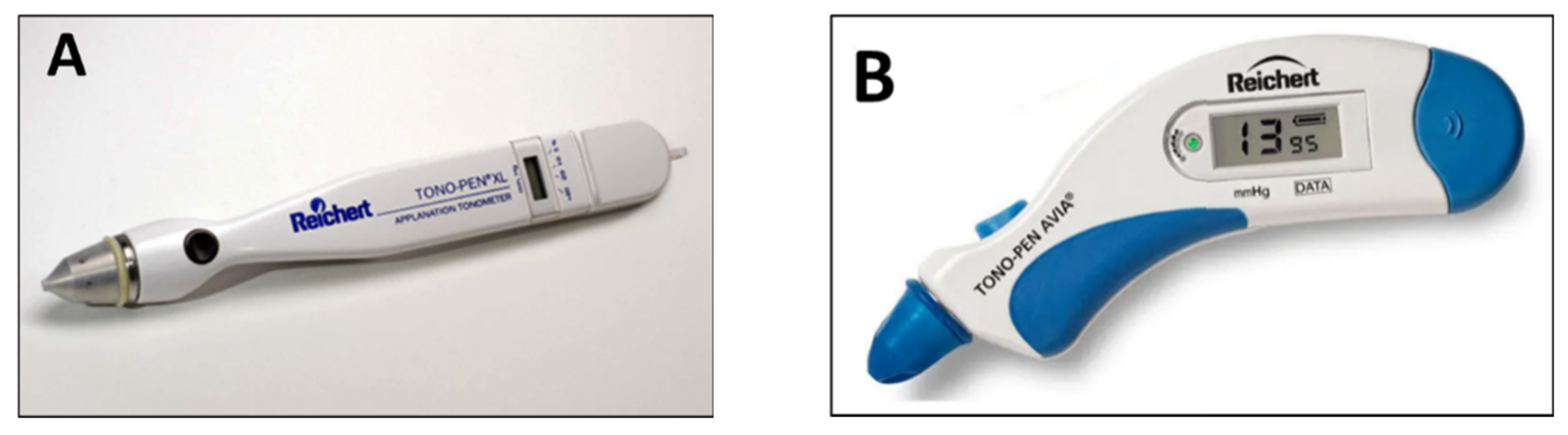
| TonoPen | 1989 | indentation/ applanation | contact | Lightweight and portable | Hight variability and quite poor repeatability | moderate | feasible |
| Quick and simple to use | Can underestimate IOP values | ||||||
| Can be used in any position | Topical anesthesia is needed | ||||||
| No need of slit-lamp or electricity | Influenced by corneal parameters | ||||||
| Self-calibration, provides quality index | |||||||
| Air-puff tonometers | 1973 | applanation | noncontact | Easy and fast to use | Need of regular calibration | ideal as a screening tool | medium |
| No need to touch the cornea | Readings are device-dependent | ||||||
| No need of topical anesthesia and fluorescein | Possible germs aerosol | ||||||
| Can be used by paramedical staff | Influenced by corneal parameters | ||||||
| Less accurate when IOP > 20 mmHg | |||||||
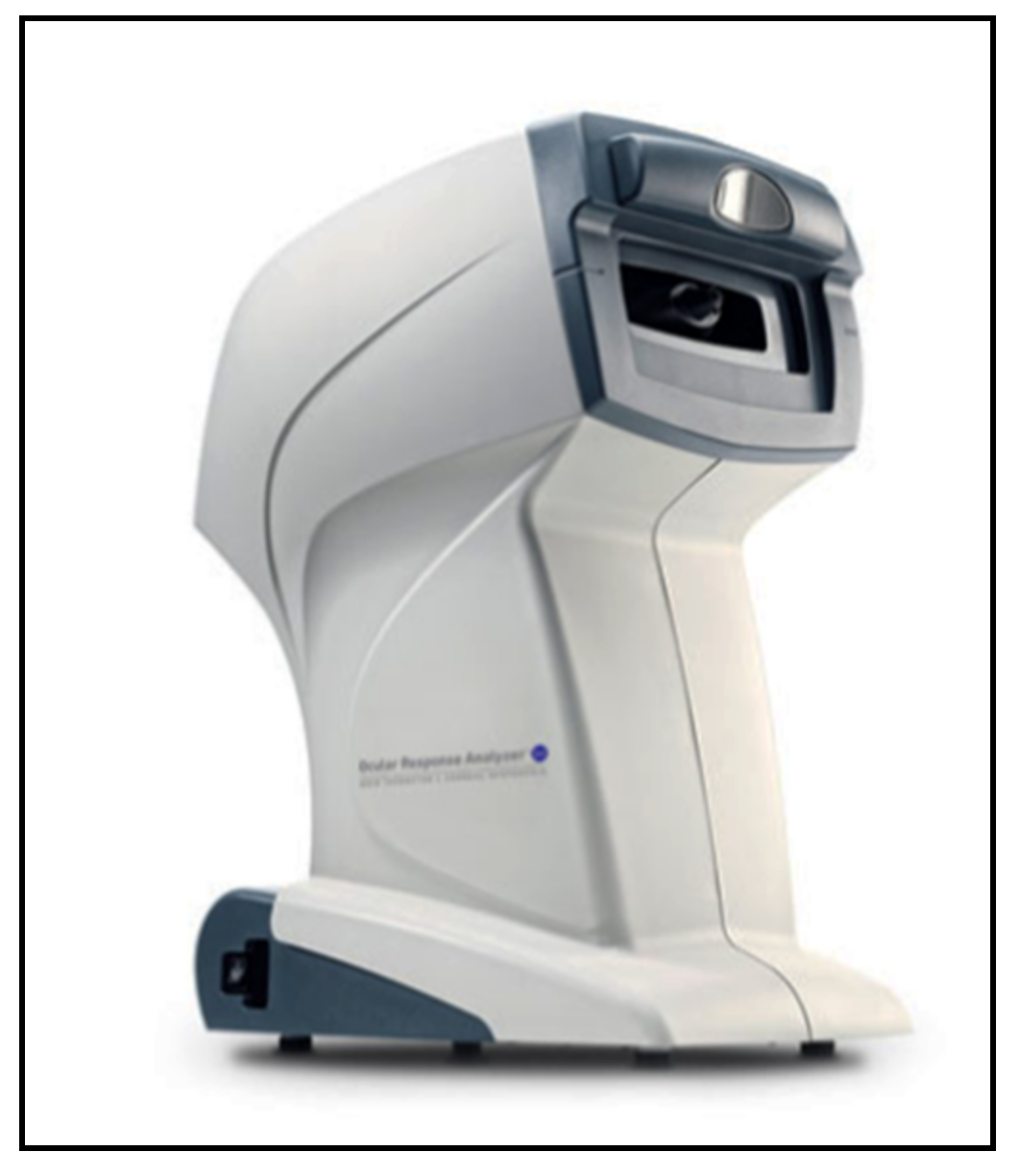
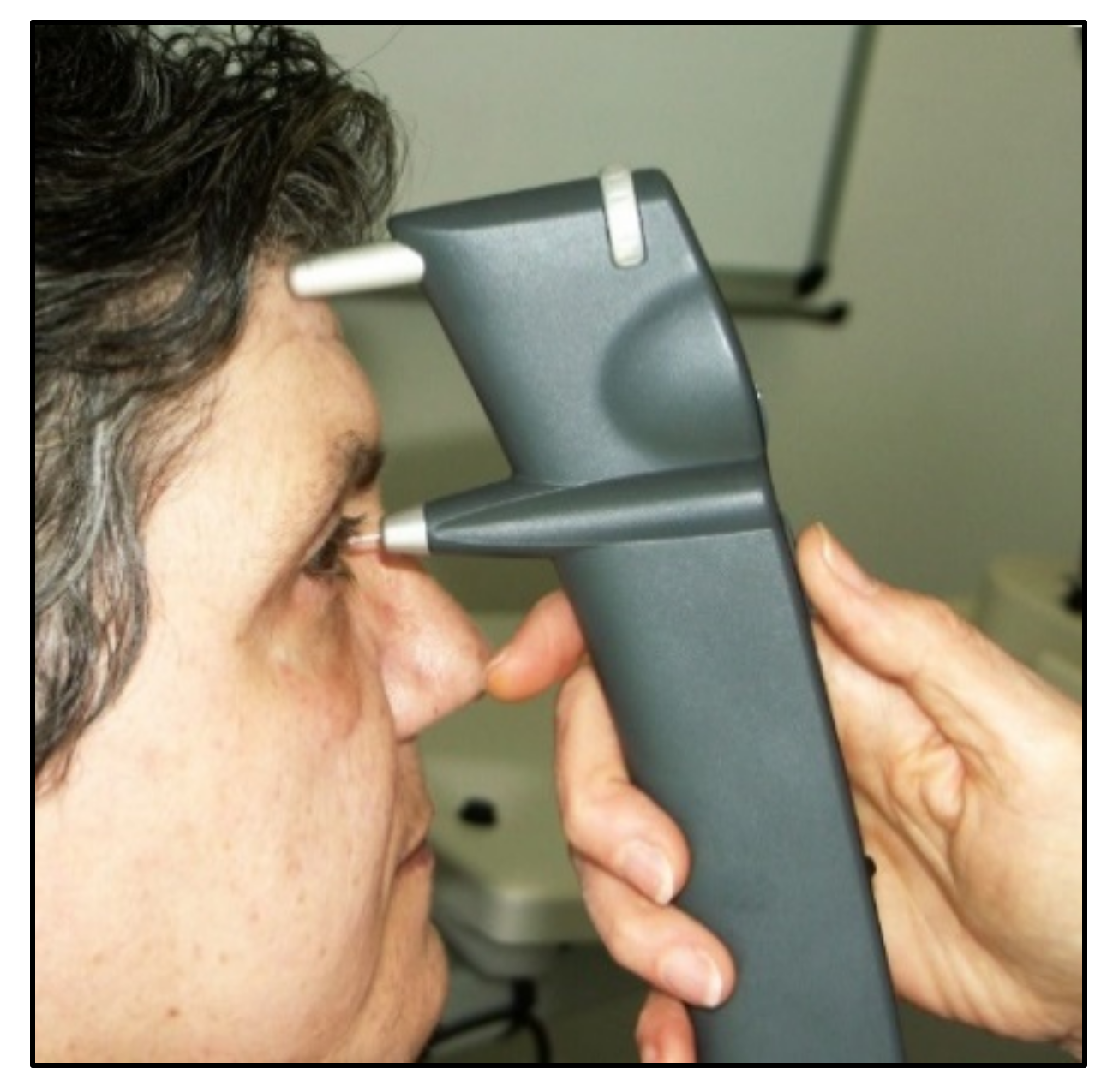
| Ocular Response Analyzer | 2005 | applanation | noncontact | Simple to use, self-calibration, provides quality index | Possible germs aerosol | good | expensive |
| No need of topical anesthesia and fluorescein | |||||||
| Can be used by paramedical staff | |||||||
| Provides additional information (corneal central thickness and biomechanics) | |||||||
| IOP correction for corneal biomechanical parameters | |||||||
| Useful after corneal refractive surgery | |||||||
| Detection of corneal diseases | |||||||
| Corvis ST | 2011 | indentation/ applanation | noncontact | Simple to use, self-calibration, provides quality index | Tends to underestimate the GAT IOP values | good | expensive |
| No need of fluorescein and topical anesthesia | |||||||
| Can be used by paramedical staff | |||||||
| Provides additional information (corneal central thickness and biomechanics) | |||||||
| IOP correction for corneal biomechanical parameters | |||||||
| Useful after corneal refractive surgery | |||||||
| Detection of corneal diseases | |||||||
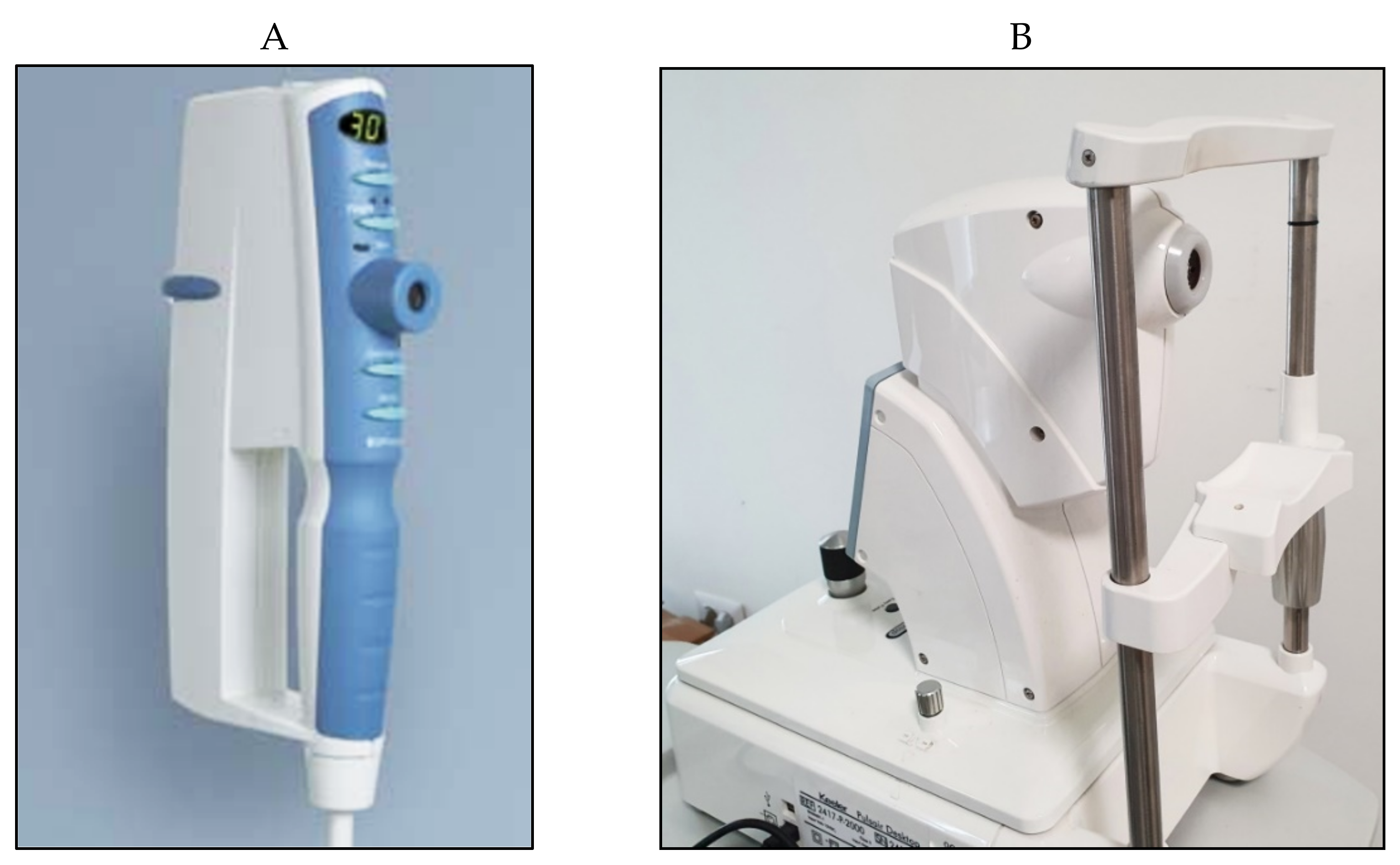
| Pneumotonometers | 1969 | applanation | contact | OBF provides information on the ocular blood pulse | Affected by corneal thickness | controversial | expensive |
| Overestimates IOP values | |||||||
| iCare tonometer | 1997 | ballistic probe (rebound) | contact | Ease of use with a short learning curve | Needs a proper central positioning of the tip | excellent | feasible |
| Portable and self-calibrated | Influenced by corneal thickness | ||||||
| No slit-lamp or topical anesthesia or fluorescein dye required | |||||||
| Can be used by trained non-medical staff | |||||||
| Can be used in supine positions (iCare PRO and iCare 200) | |||||||
| Minimal corneal trauma (useful in post-operative patients) | |||||||
| The home version can be useful in self-twenty-four-hour monitoring of IOP | |||||||
| Dynamic contour tonometer (DCT, PASCAL) | 2005 | contour matching | contact | No need of fluorescein, disposable probes | Need of slit lamp and topical anesthesia | poor | medium |
| Self-calibration, provides quality index | Difficult to use | ||||||
| Independent from corneal properties | Need of highly cooperative patients | ||||||
| Useful after corneal refractive surgery | |||||||
| High precision | |||||||
| Additional information (ocular pulse amplitude) | |||||||
| BioResonator ART | 2003 | applanation | contact | No need of fluorescein | Need of slitlamp and local anesthetic | moderate | medium |
| Self-calibration, provides quality index | Need of probe disinfection | ||||||
| High reliability (median of repeated IOP measurements) | Required training to use | ||||||
| Affected by corneal properties | |||||||
| Sensimed Triggerfish | 2004 | corneal curvature monitoring | contact | Continuous measurements over a 24-hour period | It does not provide direct IOP values | moderate | feasible |
| Good tolerability | IOP estimation accuracy not known | ||||||
| High reproducibility |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brusini, P.; Salvetat, M.L.; Zeppieri, M. How to Measure Intraocular Pressure: An Updated Review of Various Tonometers. J. Clin. Med. 2021, 10, 3860. https://doi.org/10.3390/jcm10173860
Brusini P, Salvetat ML, Zeppieri M. How to Measure Intraocular Pressure: An Updated Review of Various Tonometers. Journal of Clinical Medicine. 2021; 10(17):3860. https://doi.org/10.3390/jcm10173860
Chicago/Turabian StyleBrusini, Paolo, Maria Letizia Salvetat, and Marco Zeppieri. 2021. "How to Measure Intraocular Pressure: An Updated Review of Various Tonometers" Journal of Clinical Medicine 10, no. 17: 3860. https://doi.org/10.3390/jcm10173860
APA StyleBrusini, P., Salvetat, M. L., & Zeppieri, M. (2021). How to Measure Intraocular Pressure: An Updated Review of Various Tonometers. Journal of Clinical Medicine, 10(17), 3860. https://doi.org/10.3390/jcm10173860







