Thoracoscopy for Spontaneous Pneumothorax
Abstract
1. Introduction
2. Overview on the Management of Spontaneous Pneumothorax
Should Thoracoscopic Surgery Be Offered for Every First Episode of PSP?
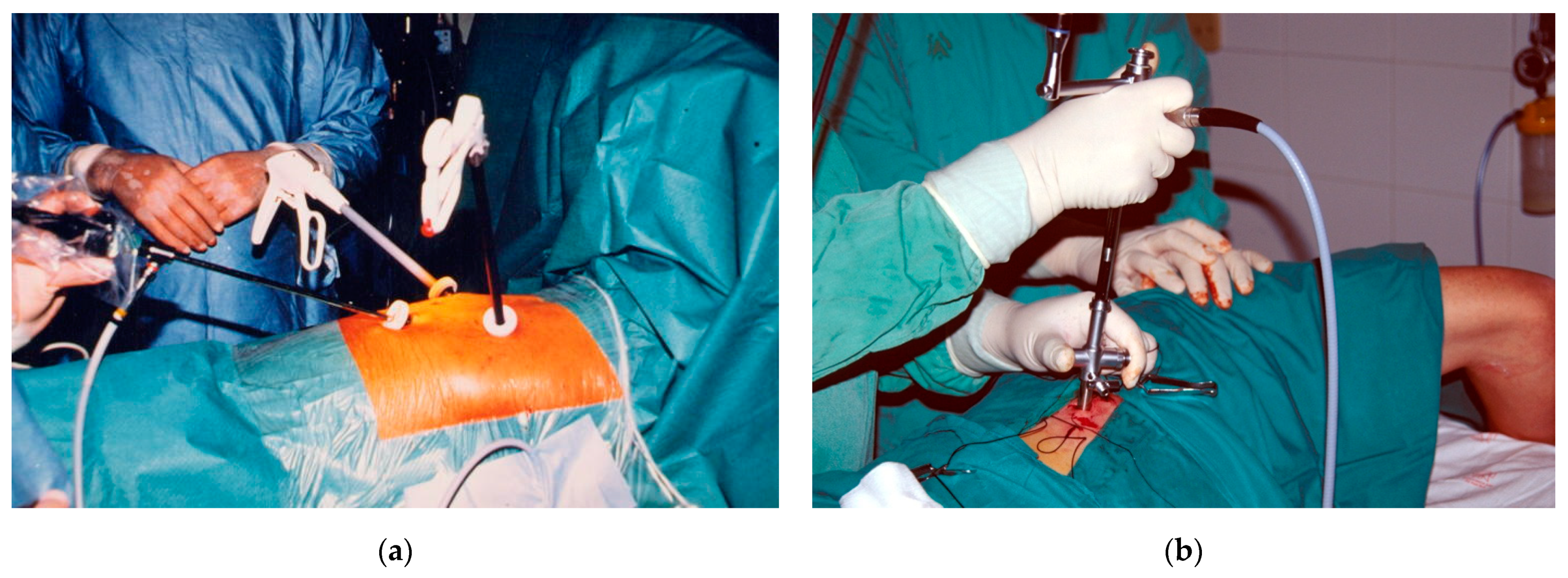
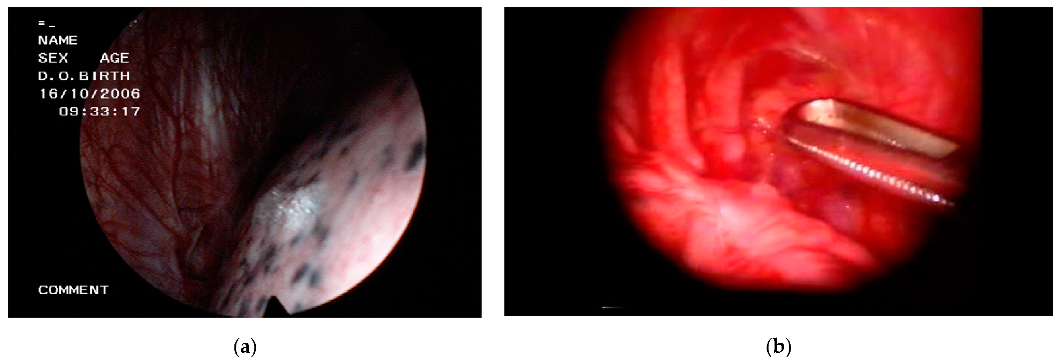
3. Thoracoscopic Techniques
4. VATS for Spontaneous Pneumothorax
4.1. VATS or Medical Thoracoscopy?
4.2. VATS or Open Thoracotomy?
4.3. Uniportal or Multiportal VATS?
4.4. VATS or Needlescopic VATS?
4.5. Intubated or Nonintubated VATS?
4.6. VATS Pleurodesis
4.6.1. Mechanical or Chemical Pleurodesis?
4.6.2. Mechanical Pleurodesis: Abrasion or Pleurectomy?
4.6.3. Chemical Pleurodesis: Talc
4.7. Staple Line Coverage
4.8. Redo-VATS
5. Conclusions
Funding
Conflicts of Interest
References
- Porcel, J.M. Phenotyping primary spontaneous pneumothorax. Eur. Respir. J. 2018, 52, 1801455. [Google Scholar] [CrossRef]
- Walker, S.P.; Bibby, A.C.; Halford, P.; Stadon, L.; White, P.; Maskell, N.A. Recurrence rates in primary spontaneous pneumothorax: A systematic review and meta-analysis. Eur. Respir. J. 2018, 52, 1800864. [Google Scholar] [CrossRef] [PubMed]
- Hallifax, R.J.; Goldacre, R.; Landray, M.J.; Rahman, N.M.; Goldacre, M.J. Trends in the Incidence and Recurrence of Inpatient-Treated Spontaneous Pneumothorax, 1968–2016. JAMA 2018, 320, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Tschopp, J.-M.; Bintcliffe, O.; Astoul, P.; Canalis, E.; Driesen, P.; Janssen, J.; Krasnik, M.; Maskell, N.; Van Schil, P.; Tonia, T.; et al. ERS task force statement: Diagnosis and treatment of primary spontaneous pneumothorax. Eur. Respir. J. 2015, 46, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Hallifax, R.; Janssen, J.P. Pneumothorax—Time for New Guidelines? Semin. Respir. Crit. Care Med. 2019, 40, 314–322. [Google Scholar] [CrossRef] [PubMed]
- Mendogni, P.; Vannucci, J.; Ghisalberti, M.; Anile, M.; Aramini, B.; Congedo, M.T.; Nosotti, M.; Bertolaccini, L.; D’Ambrosio, A.E.; De Vico, A.; et al. Epidemiology and management of primary spontaneous pneumothorax: A systematic review. Interact. Cardiovasc. Thorac. Surg. 2019, 30, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Chiu, H.-Y.; Ho, Y.-C.; Yang, P.-C.; Chiang, C.-M.; Chung, C.-C.; Wu, W.-C.; Lin, Y.-C.; Chen, C.-Y.; Wu, Y.-C. Recommendation for management of patients with their first episode of primary spontaneous pneumothorax, using video-assisted thoracoscopic surgery or conservative treatment. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Olesen, W.H.; Katballe, N.; Sindby, J.E.; Titlestad, I.L.; Andersen, P.E.; Lindahl-Jacobsen, R.; Licht, P.B. Surgical treatment versus conventional chest tube drainage in primary spontaneous pneumothorax: A randomized controlled trial. Eur. J. Cardio-Thoracic Surg. 2018, 54, 113–121. [Google Scholar] [CrossRef]
- Lee, P.; Folch, E. Thoracoscopy: Advances and Increasing Role for Interventional Pulmonologists. Semin. Respir. Crit. Care Med. 2018, 39, 693–703. [Google Scholar] [CrossRef]
- Shaikh, F.; Lentz, R.J.; Feller-Kopman, D.; Maldonado, F. Medical thoracoscopy in the diagnosis of pleural disease: A guide for the clinician. Expert Rev. Respir. Med. 2020, 14, 987–1000. [Google Scholar] [CrossRef]
- Vanderschueren, R.G.J.R.A. The role of thoracoscopy in the evaluation and management of pneumothorax. Lung 1990, 168, 1122–1125. [Google Scholar] [CrossRef]
- Schnell, J.; Beer, M.; Eggeling, S.; Gesierich, W.; Gottlieb, J.; Herth, F.J.; Hofmann, H.-S.; Jany, B.; Kreuter, M.; Ley-Zaporozhan, J.; et al. Management of Spontaneous Pneumothorax and Post-Interventional Pneumothorax: German S3 Guideline. Respiration 2018, 97, 370–402. [Google Scholar] [CrossRef]
- Cardillo, G.; Bintcliffe, O.J.; Carleo, F.; Carbone, L.; Di Martino, M.; Kahan, B.C.; Maskell, N.A. Primary spontaneous pneumothorax: A cohort study of VATS with talc poudrage. Thorax 2016, 71, 847–853. [Google Scholar] [CrossRef]
- Sudduth, C.L.; Shinnick, J.K.; Geng, Z.; McCracken, C.E.; Clifton, M.S.; Raval, M.V. Optimal surgical technique in spontaneous pneumothorax: A systematic review and meta-analysis. J. Surg. Res. 2017, 210, 32–46. [Google Scholar] [CrossRef]
- Ramos-Izquierdo, R.; Moya, J.; Macia, I.; Rivas, F.; Ureña, A.; Rosado, G.; Escobar, I.; Saumench, J.; Cabrera, A.; Delgado, M.A.; et al. Treatment of primary spontaneous pneumothorax by videothoracoscopic talc pleurodesis under local anesthesia: A review of 133 procedures. Surg. Endosc. 2009, 24, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Vohra, H.A.; Adamson, L.; Weeden, D.F. Does video-assisted thoracoscopic pleurectomy result in better outcomes than open pleurectomy for primary spontaneous pneumothorax? Interact. Cardiovasc. Thorac. Surg. 2008, 7, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zhang, Z.; Wang, Q.; Li, J.; Peng, W.; Ge, G. A systematic review and meta-analysis of video-assisted thoracoscopic surgery treating spontaneous pneumothorax. J. Thorac. Dis. 2021, 13, 3093–3104. [Google Scholar] [CrossRef] [PubMed]
- Barker, A.; Maratos, E.C.; Edmonds, L.; Lim, E. Recurrence rates of video-assisted thoracoscopic versus open surgery in the prevention of recurrent pneumothoraces: A systematic review of randomised and non-randomised trials. Lancet 2007, 370, 329–335. [Google Scholar] [CrossRef]
- Goto, T.; Kadota, Y.; Mori, T.; Yamashita, S.-I.; Horio, H.; Nagayasu, T.; Iwasaki, A. Video-assisted thoracic surgery for pneumothorax: Republication of a systematic review and a proposal by the guideline committee of the Japanese Association for Chest Surgery 2014. Gen. Thorac. Cardiovasc. Surg. 2015, 63, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Pagès, P.-B.; Delpy, J.-P.; Falcoz, P.-E.; Thomas, P.; Filaire, M.; Le Pimpec-Barthes, F.; Dahan, M.; Bernard, A. Videothoracoscopy Versus Thoracotomy for the Treatment of Spontaneous Pneumothorax: A Propensity Score Analysis. Ann. Thorac. Surg. 2015, 99, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Abougergi, M.S.; Li, S.; Kazmierski, D.; Patel, P.; Sharma, N.; Ochieng, P. Recurrence Prophylaxis in Secondary Spontaneous Pneumothorax: A Nationwide Readmission Database Analysis. Chest 2020, 158, 2474–2484. [Google Scholar] [CrossRef]
- Qin, S.-L.; Huang, J.-B.; Yang, Y.-L.; Xian, L. Uniportal versus three-port video-assisted thoracoscopic surgery for spontaneous pneumothorax: A meta-analysis. J. Thorac. Dis. 2015, 7, 2274–2287. [Google Scholar] [CrossRef]
- Masmoudi, H.; Etienne, H.; Sylvestre, R.; Evrard, D.; Ouede, R.; Le Roux, M.; Giol, M.; Assouad, J. Three Hundred Fifty-One Patients With Pneumothorax Undergoing Uniportal (Single Port) Video-Assisted Thoracic Surgery. Ann. Thorac. Surg. 2017, 104, 254–260. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kocaturk, C.I.; Akın, H.; Erdogan, S.; Bilen, S.; Karapinar, K.; Sezen, C.B.; Saydam, O.; Kutluk, A.C.; Akin, H. Which is the Best Minimal Invasive Approach for the Treatment of Spontaneous Pneumothorax? Uniport, Two, or Three Ports: A Prospective Randomized Trail. Thorac. Cardiovasc. Surg. 2018, 66, 589–594. [Google Scholar] [CrossRef]
- Yang, H.C.; Kim, S.; Yum, S.; Cho, S.; Kim, K.; Jheon, S. Learning curve of single-incision thoracoscopic surgery for primary spontaneous pneumothorax. Surg. Endosc. 2016, 31, 1680–1687. [Google Scholar] [CrossRef] [PubMed]
- Chou, S.-H.; Chuang, I.-C.; Huang, M.-F.; Chang, S.-J.; Li, H.-P.; Lee, J.-Y.; Lee, Y.-L.; Chiang, H.-H. Comparison of needlescopic and conventional video-assisted thoracic surgery for primary spontaneous pneumothorax. Minim. Invasive Ther. Allied Technol. 2011, 21, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Gelzinis, T. The Anesthetic Management of Patients Undergoing Nonintubated Video-Assisted Thoracic Surgery. Curr. Anesthesiol. Rep. 2021, 16, 1–9. [Google Scholar]
- Pompeo, E.; Tacconi, F.; Mineo, D.; Mineo, T.C. The role of awake video-assisted thoracoscopic surgery in spontaneous pneumothorax. J. Thorac. Cardiovasc. Surg. 2007, 133, 786–790. [Google Scholar] [CrossRef]
- Hwang, J.; Shin, J.S.; Son, J.H.; Min, T.J. Non-intubated thoracoscopic bullectomy under sedation is safe and comfortable in the perioperative period. J. Thorac. Dis. 2018, 10, 1703–1710. [Google Scholar] [CrossRef]
- Liu, J.; Liang, H.; Cui, F.; Liu, H.; Zhu, C.; Liang, W.; He, J.; Wang, W.; Jiang, S.; Dong, Q.; et al. Spontaneous versus mechanical ventilation during video-assisted thoracoscopic surgery for spontaneous pneumothorax: A randomized trial. J. Thorac. Cardiovasc. Surg. 2021, 3. [Google Scholar] [CrossRef]
- Sim, S.K.R.; Nah, S.A.; Loh, A.H.P.; Ong, L.Y.; Chen, Y. Mechanical versus Chemical Pleurodesis after Bullectomy for Primary Spontaneous Pneumothorax: A Systemic Review and Meta-Analysis. Eur. J. Pediatr. Surg. 2020, 30, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Asban, A.; Raza, S.; McLeod, C.; Donahue, J.; Wei, B. Mechanical or chemical and mechanical pleurodesis for spontaneous pneumothorax: What is the most effective approach in preventing recurrence? A systematic review and meta-analysis. Eur. J. Cardio-Thoracic Surg. 2020, 58, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Curtis, H.J.; Bourke, S.J.; Dark, J.H.; Corris, P.A. Lung Transplantation Outcome in Cystic Fibrosis Patients with Previous Pneumothorax. J. Heart Lung Transplant. 2005, 24, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Machuca, T.; Losso, M.; Camargo, S.; Schio, S.; Melo, I.; Hochhegger, B.; Felicetti, J.; Camargo, J. Lung Transplantation for Lymphangioleiomyomatosis: Single-Center Brazilian Experience with No Chylothorax. Transplant. Proc. 2011, 43, 236–238. [Google Scholar] [CrossRef]
- Ling, Z.-G.; Wu, Y.-B.; Ming, M.-Y.; Cai, S.-Q.; Chen, Y.-Q. The Effect of Pleural Abrasion on the Treatment of Primary Spontaneous Pneumothorax: A Systematic Review of Randomized Controlled Trials. PLoS ONE 2015, 10, e0127857. [Google Scholar] [CrossRef] [PubMed]
- Hallifax, R.J.; Yousuf, A.; Jones, H.; Corcoran, J.P.; Psallidas, I.; Rahman, N.M. Effectiveness of chemical pleurodesis in spontaneous pneumothorax recurrence prevention: A systematic review. Thorax 2017, 72, 1121–1131. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.R.; Cho, S.; Huh, D.M.; Lee, E.B.; Ryu, K.M.; Cho, D.G.; Paik, H.C.; Kim, D.K.; Lee, S.-H.; et al. Staple Line Coverage After Bullectomy for Primary Spontaneous Pneumothorax: A Randomized Trial. Ann. Thorac. Surg. 2014, 98, 2005–2011. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-H.; Liu, Y.-H.; Chen, H.-Y.; Chen, P.-H.; Chen, K.-C.; Hsieh, M.-J.; Lin, M.-W.; Kuo, S.-W.; Huang, P.-M.; Chao, Y.-K.; et al. Vicryl Mesh Coverage Reduced Recurrence After Bullectomy for Primary Spontaneous Pneumothorax. Ann. Thorac. Surg. 2021, 1. [Google Scholar] [CrossRef]
- Mao, Y.; Zhang, Z.; Zeng, W.; Zhang, W.; Zhang, J.; You, G.; Wei, Y. A clinical study of efficacy of polyglycolic acid patch in surgery for pneumothorax:a systematic review and meta-analysis. J. Cardiothorac. Surg. 2020, 15, 117. [Google Scholar] [CrossRef]
- Cardillo, G.; Facciolo, F.; Regal, M.; Carbone, L.; Corzani, F.; Ricci, A.; Martelli, M. Recurrences following videothoracoscopic treatment of primary spontaneous pneumothorax: The role of redo-videothoracoscopy. Eur. J. Cardio-Thoracic Surg. 2001, 19, 396–399. [Google Scholar] [CrossRef]
- Chen, J.-S.; Hsu, H.-H.; Kuo, S.-W.; Huang, P.-M.; Lee, J.-M.; Lee, Y.-C. Management of recurrent primary spontaneous pneumothorax after thoracoscopic surgery: Should observation, drainage, redo thoracoscopy, or thoracotomy be used? Surg. Endosc. 2009, 23, 2438–2444. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Jheon, S.; Kim, D.K.; Kim, H.R.; Huh, D.M.; Lee, S.; Ryu, K.M.; Cho, D.G. Results of repeated video-assisted thoracic surgery for recurrent pneumothorax after primary spontaneous pneumothorax. Eur. J. Cardio-Thoracic Surg. 2017, 53, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Cattoni, M.; Rotolo, N.; Mastromarino, M.G.; Cardillo, G.; Nosotti, M.; Mendogni, P.; Rizzi, A.; Raveglia, F.; Siciliani, A.; Rendina, E.A.; et al. Analysis of pneumothorax recurrence risk factors in 843 patients who underwent videothoracoscopy for primary spontaneous pneumothorax: Results of a multicentric study. Interact. Cardiovasc. Thorac. Surg. 2020, 31, 78–84. [Google Scholar] [CrossRef] [PubMed]
| Clinical Scenario | Management | |
|---|---|---|
| A. | First episode of PSP, no dyspnea, and small size on chest radiograph 1 | Observation with supplemental oxygen |
| B. | First episode of PSP, dyspnea, or large size on chest radiograph | Needle aspiration or chest catheter/tube |
| C. | First episode of PSP, and severe dyspnea or hemodynamic instability regardless of size | Immediate drainage with chest catheter/tube. Emergent needle decompression if tension physiology |
| D. | First episode of bilateral PSP | Chest catheter/tube |
| E. | First episode of PSP, with no resolution after observation or needle aspiration | Chest catheter/tube |
| F. | First episode of PSP, associated with a pleural effusion | Chest catheter/tube |
| G. | Recurrent PSP | Chest catheter/tube |
| H. | SSP | Chest catheter/tube |
| Type of Spontaneous Pneumothorax | Indications for Definitive Therapy |
|---|---|
| PSP, first episode | Tension pneumothorax Persistent air leak >5–7 days High risk professions or hobbies 1 Bilateral pneumothorax Patient desire for definitive therapy Concomitant indication for thoracoscopy 2 |
| PSP, second episode | All cases |
| SPP | All cases 3 |
| Feature | Medical Thoracoscopy | VATS |
|---|---|---|
| Proceduralist | Interventional pulmonologist | Thoracic surgeon |
| Location | Endoscopy suite or operating room | Operating room |
| Anesthesia | Local, conscious sedation 1 | General, single lung ventilation, double-lumen endotracheal tube |
| Entry ports | One | Two or three |
| Instruments | Rigid 2, flex-rigid | Rigid |
| Technical variants | Mini-thoracoscopy 3, flex-rigid thoracoscopy 4 | Uniportal 5, needlescopic 6, nonintubated |
| Indications in SP patients | Pleurodesis, electrocoagulation of blebs | Bullectomy/blebectomy, pleurodesis, staple line coverage |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Porcel, J.M.; Lee, P. Thoracoscopy for Spontaneous Pneumothorax. J. Clin. Med. 2021, 10, 3835. https://doi.org/10.3390/jcm10173835
Porcel JM, Lee P. Thoracoscopy for Spontaneous Pneumothorax. Journal of Clinical Medicine. 2021; 10(17):3835. https://doi.org/10.3390/jcm10173835
Chicago/Turabian StylePorcel, José M., and Pyng Lee. 2021. "Thoracoscopy for Spontaneous Pneumothorax" Journal of Clinical Medicine 10, no. 17: 3835. https://doi.org/10.3390/jcm10173835
APA StylePorcel, J. M., & Lee, P. (2021). Thoracoscopy for Spontaneous Pneumothorax. Journal of Clinical Medicine, 10(17), 3835. https://doi.org/10.3390/jcm10173835






