New Insights in Microbial Species Predicting Lung Function Decline in CF: Lessons from the MucoFong Project
Abstract
:1. Introduction
2. Materials and Methods
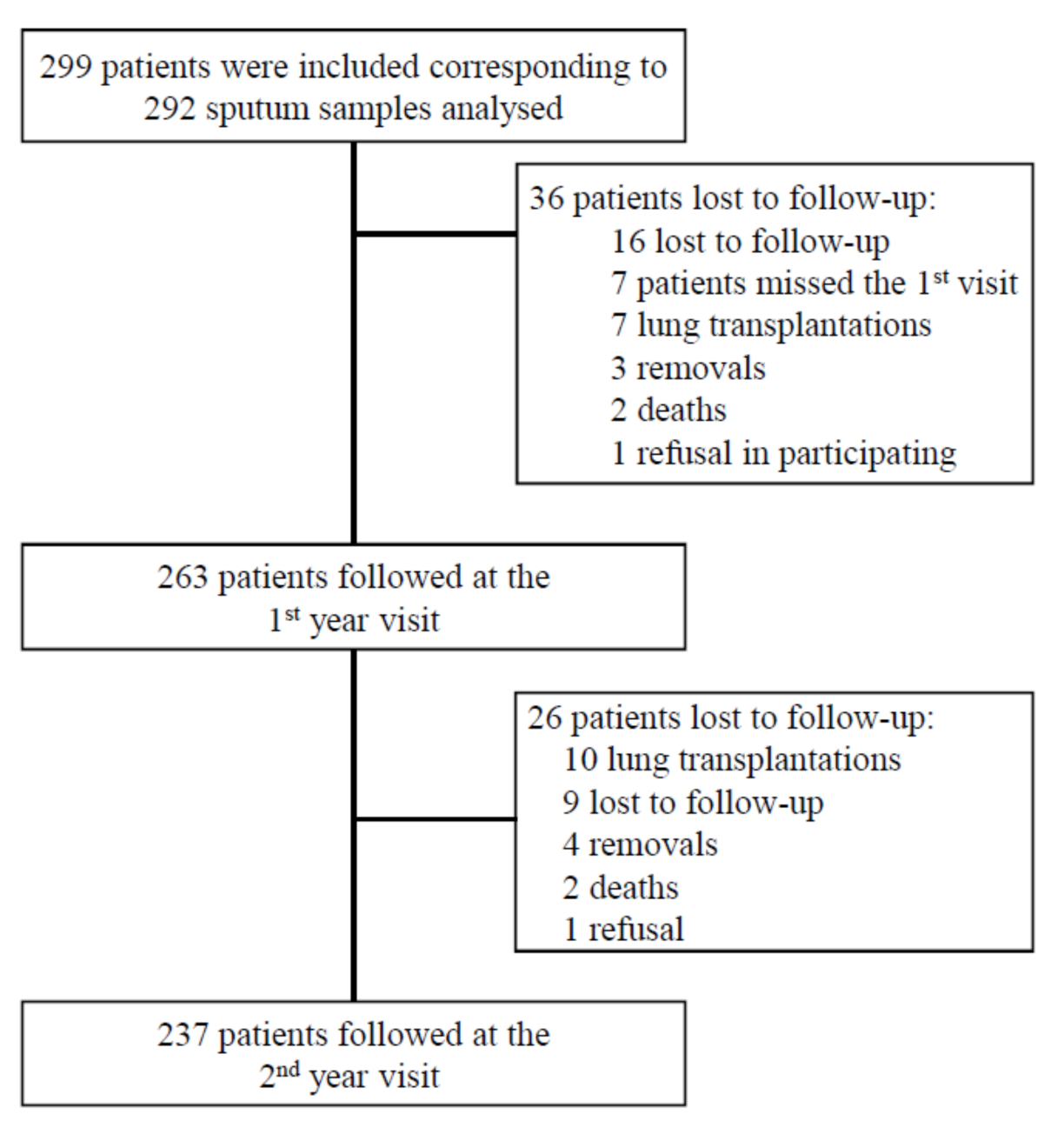
2.1. Study Design and Population
2.2. Ethics
2.3. Microbial Analysis
2.4. Outcome Assessment
2.5. Prognostic Score Assessment
2.6. Statistical Analysis
2.7. Model Robustness
3. Results
3.1. Patients’ Characteristics
3.2. ppFEV1 Characteristics and Univariate Analysis
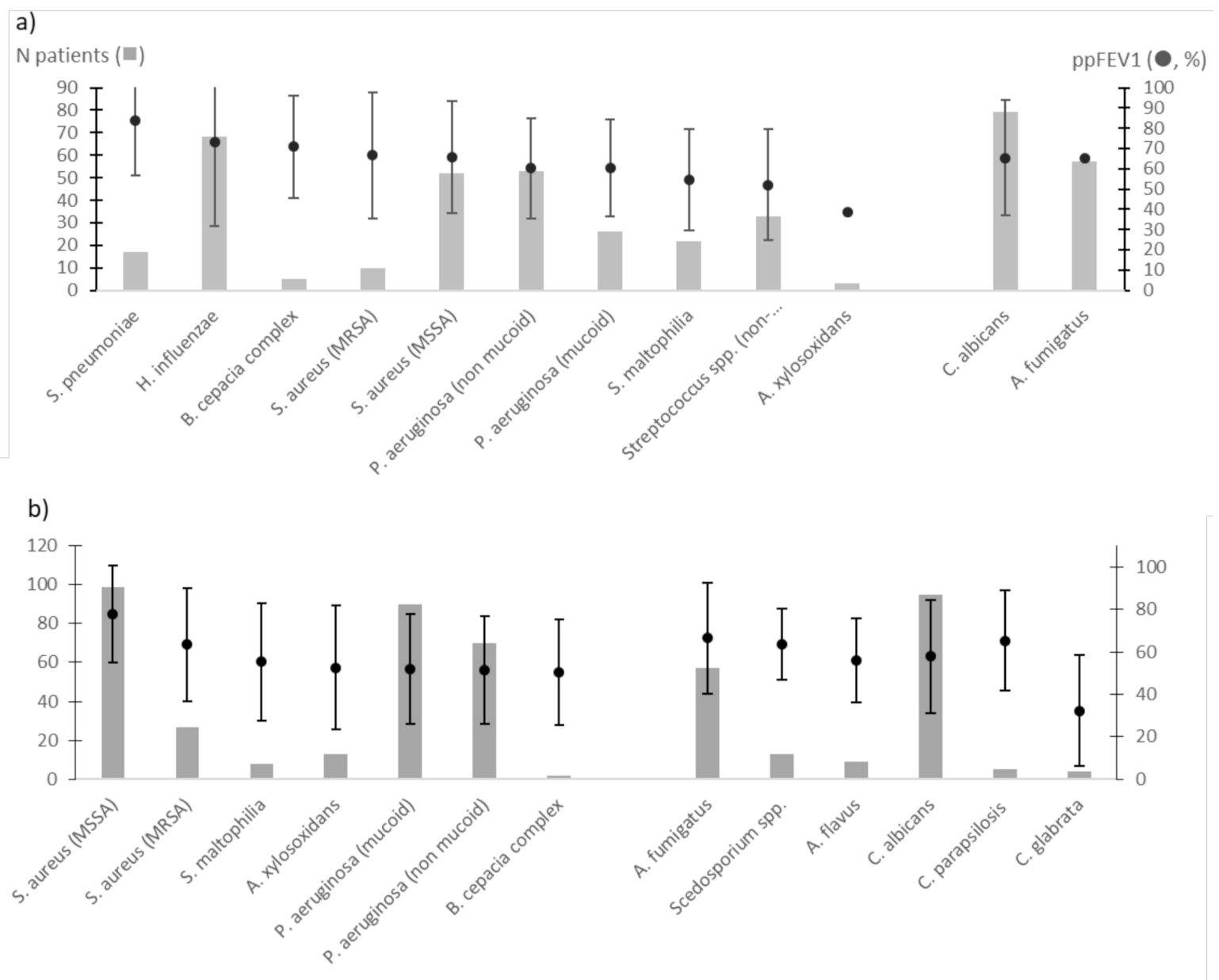
3.3. Bacterial and Fungal Colonisations Predicted ppFEV1 Level
3.4. Assessment of the Performance of our Mixed Model Using the Prognostic Score
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Delhaes, L.; Touati, K.; Faure-Cognet, O.; Cornet, M.; Botterel, F.; DannaouiI, E.; Morio, F.; Le Pape, P.; Grenouillet, F.; Favennec, L.; et al. Prevalence, Geographic Risk Factor, and Development of a Standardized Protocol for Fungal Isolation in Cystic Fibrosis: Results from the International Prospective Study “MFIP”. J. Cyst. Fibros 2019, 18, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Shwachman, H.; Kulczycki, L.L. Long-Term Study of One Hundred Five Patients with Cystic Fibrosis; Studies Made over a Five- to Fourteen-Year Period. AMA J. Dis. Child 1958, 96, 6–15. [Google Scholar] [CrossRef]
- Liou, T.G.; Adler, F.R.; FitzSimmons, S.C.; Cahill, B.C.; Hibbs, J.R.; Marshall, B.C. Predictive 5-Year Survivorship Model of Cystic Fibrosis. Am. J. Epidemiol. 2001, 153, 345–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flume, P.A.; Strange, C.; Ye, X.; Ebeling, M.; Hulsey, T.; Clark, L.L. Pneumothorax in Cystic Fibrosis. Chest 2005, 128, 720–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtney, J.M.; Bradley, J.; Mccaughan, J.; O’Connor, T.M.; Shortt, C.; Bredin, C.P.; Bradbury, I.; Elborn, J.S. Predictors of Mortality in Adults with Cystic Fibrosis. Pediatr. Pulmonol. 2007, 42, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, R.; Alicandro, G.; Minicucci, L.; Notarnicola, S.; Furnari, M.L.; Giordano, G.; Lucidi, V.; Montemitro, E.; Raia, V.; Magazzù, G.; et al. Validation of a Predictive Survival Model in Italian Patients with Cystic Fibrosis. J. Cyst. Fibros. 2012, 11, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, C.; Dimitrov, B.D.; Meurling, I.J.; Gunaratnam, C.; McElvaney, N.G. The CF-ABLE Score: A Novel Clinical Prediction Rule for Prognosis in Patients with Cystic Fibrosis. Chest 2013, 143, 1358–1364. [Google Scholar] [CrossRef] [PubMed]
- Kerem, E.; Viviani, L.; Zolin, A.; MacNeill, S.; Hatziagorou, E.; Ellemunter, H.; Drevinek, P.; Gulmans, V.; Krivec, U.; Olesen, H.; et al. Factors Associated with FEV1 Decline in Cystic Fibrosis: Analysis of the ECFS Patient Registry. Eur. Respir. J. 2014, 43, 125–133. [Google Scholar] [CrossRef] [Green Version]
- MacKenzie, T.; Gifford, A.H.; Sabadosa, K.A.; Quinton, H.B.; Knapp, E.A.; Goss, C.H.; Marshall, B.C. Longevity of Patients with Cystic Fibrosis in 2000 to 2010 and Beyond: Survival Analysis of the Cystic Fibrosis Foundation Patient Registry. Ann. Intern. Med. 2014, 161, 233–241. [Google Scholar] [CrossRef]
- Aaron, S.D.; Stephenson, A.L.; Cameron, D.W.; Whitmore, G.A. A Statistical Model to Predict One-Year Risk of Death in Patients with Cystic Fibrosis. J. Clin. Epidemiol. 2015, 68, 1336–1345. [Google Scholar] [CrossRef]
- Szczesniak, R.; Heltshe, S.L.; Stanojevic, S.; Mayer-Hamblett, N. Use of FEV1 in Cystic Fibrosis Epidemiologic Studies and Clinical Trials: A Statistical Perspective for the Clinical Researcher. J. Cyst. Fibros. 2017, 16, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Nkam, L.; Lambert, J.; Latouche, A.; Bellis, G.; Burgel, P.R.; Hocine, M.N. A 3-Year Prognostic Score for Adults with Cystic Fibrosis. J. Cyst. Fibros. 2017, 16, 702–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keogh, R.H.; Szczesniak, R.; Taylor-Robinson, D.; Bilton, D. Up-to-Date and Projected Estimates of Survival for People with Cystic Fibrosis Using Baseline Characteristics: A Longitudinal Study Using UK Patient Registry Data. J. Cyst. Fibros. 2018, 17, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marsteller, N.L.; Nussbaum, E.; Morphew, T.; Randhawa, I.S. Cystic Fibrosis Patients at Risk for Disease Progression Marked by Decline in FEV1% Predicted: Development of the Cystic Fibrosis Risk of Disease Progression Score. J. Thorac. Dis. 2019, 11, 5557–5565. [Google Scholar] [CrossRef]
- Liou, T.G.; Kartsonaki, C.; Keogh, R.H.; Adler, F.R. Evaluation of a Five-Year Predicted Survival Model for Cystic Fibrosis in Later Time Periods. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed]
- Paganin, P.; Fiscarelli, E.V.; Tuccio, V.; Chiancianesi, M.; Bacci, G.; Morelli, P.; Dolce, D.; Dalmastri, C.; De Alessandri, A.; Lucidi, V.; et al. Changes in Cystic Fibrosis Airway Microbial Community Associated with a Severe Decline in Lung Function. PLoS ONE 2015, 10, e0124348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reece, E.; Segurado, R.; Jackson, A.; McClean, S.; Renwick, J.; Greally, P. Co-Colonisation with Aspergillus Fumigatus and Pseudomonas Aeruginosa Is Associated with Poorer Health in Cystic Fibrosis Patients: An Irish Registry Analysis. BMC Pulm. Med. 2017, 17. [Google Scholar] [CrossRef] [Green Version]
- Soret, P.; Vandenborght, L.-E.; Francis, F.; Coron, N.; Enaud, R.; Avalos, M.; Schaeverbeke, T.; Berger, P.; Fayon, M.; Thiebaut, R.; et al. Respiratory Mycobiome and Suggestion of Inter-Kingdom Network during Acute Pulmonary Exacerbation in Cystic Fibrosis. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coron, N.; Pihet, M.; Fréalle, E.; Lemeille, Y.; Pinel, C.; Pelloux, H.; Gargala, G.; Favennec, L.; Accoceberry, I.; Durand-Joly, I.; et al. Toward the Standardization of Mycological Examination of Sputum Samples in Cystic Fibrosis: Results from a French Multicenter Prospective Study. Mycopathologia 2018, 183, 101–117. [Google Scholar] [CrossRef]
- Cantón, R.; Cobos, N.; de Gracia, J.; Baquero, F.; Honorato, J.; Gartner, S.; Alvarez, A.; Salcedo, A.; Oliver, A.; García-Quetglas, E. Antimicrobial Therapy for Pulmonary Pathogenic Colonisation and Infection by Pseudomonas Aeruginosa in Cystic Fibrosis Patients. Clin. Microbiol. Infect. 2005, 11, 690–703. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Clemente, M.; de la Rosa, D.; Máiz, L.; Girón, R.; Blanco, M.; Olveira, C.; Canton, R.; Martinez-García, M.A. Impact of Pseudomonas Aeruginosa Infection on Patients with Chronic Inflammatory Airway Diseases. J. Clin. Med. 2020, 9, 3800. [Google Scholar] [CrossRef] [PubMed]
- To, K.; Cao, R.; Yegiazaryan, A.; Owens, J.; Venketaraman, V. General Overview of Nontuberculous Mycobacteria Opportunistic Pathogens: Mycobacterium Avium and Mycobacterium Abscessus. J. Clin. Med. 2020, 9, 2541. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.; Dupuis, A.; Aaron, S.D.; Ratjen, F. The Effect of Chronic Infection with Aspergillus Fumigatus on Lung Function and Hospitalization in Patients with Cystic Fibrosis. Chest 2010, 137, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, R.; Viegi, G.; Brusasco, V.; Crapo, R.O.; Burgos, F.; Casaburi, R.; Coates, A. Interpretative Strategies for Lung Function Tests. Eur. Respir. J. 2005, 26, 948–968. [Google Scholar] [CrossRef] [PubMed]
- Albert, P.S.; Shih, J.H. An approach for jointly modeling multivariate longitudinal measurements and discrete time-to-event data. Ann. Appl. Stat. 2010, 4, 1517–1532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerson, J.; Rosenfeld, M.; McNamara, S.; Ramsey, B.; Gibson, R.L. Pseudomonas Aeruginosa and Other Predictors of Mortality and Morbidity in Young Children with Cystic Fibrosis. Pediatr. Pulmonol. 2002, 34, 91–100. [Google Scholar] [CrossRef]
- VandenBranden, S.L.; McMullen, A.; Schechter, M.S.; Pasta, D.J.; Michaelis, R.L.; Konstan, M.W.; Wagener, J.S.; Morgan, W.J.; McColley, S.A. Lung Function Decline from Adolescence to Young Adulthood in Cystic Fibrosis. Pediatr. Pulmonol. 2012, 47, 135–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubert, D.; Réglier-Poupet, H.; Sermet-Gaudelus, I.; Ferroni, A.; Le Bourgeois, M.; Burgel, P.-R.; Serreau, R.; Dusser, D.; Poyart, C.; Coste, J. Association between Staphylococcus Aureus Alone or Combined with Pseudomonas Aeruginosa and the Clinical Condition of Patients with Cystic Fibrosis. J. Cyst. Fibros. 2013, 12, 497–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasenbrook, E.C.; Merlo, C.A.; Diener-West, M.; Lechtzin, N.; Boyle, M.P. Persistent Methicillin-Resistant Staphylococcus Aureus and Rate of FEV1 Decline in Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2008, 178, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.; Rainisio, M.; Harms, H.K.; Hodson, M.E.; Koch, C.; Mastella, G.; Strandvik, B.; McKenzie, S.G. Factors Associated with Poor Pulmonary Function: Cross-Sectional Analysis of Data from the ERCF. European Epidemiologic Registry of Cystic Fibrosis. Eur. Respir. J. 2001, 18, 298–305. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Keogh, R.; Clancy, J.P.; Szczesniak, R.D. Flexible Semiparametric Joint Modeling: An Application to Estimate Individual Lung Function Decline and Risk of Pulmonary Exacerbations in Cystic Fibrosis. Emerg. Themes Epidemiol. 2017, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Andrinopoulou, E.R.; Clancy, J.P.; Szczesniak, R.D. Multivariate Joint Modeling to Identify Markers of Growth and Lung Function Decline That Predict Cystic Fibrosis Pulmonary Exacerbation Onset. BMC Pulm. Med. 2020, 20, 142. [Google Scholar] [CrossRef] [PubMed]
- Cogen, J.; Emerson, J.; Sanders, D.B.; Ren, C.; Schechter, M.S.; Gibson, R.L.; Morgan, W.; Rosenfeld, M. Risk Factors for Lung Function Decline in a Large Cohort of Young Cystic Fibrosis Patients. Pediatr. Pulmonol. 2015, 50, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Chotirmall, S.H.; O’Donoghue, E.; Bennett, K.; Gunaratnam, C.; O’Neill, S.J.; McElvaney, N.G. Sputum Candida Albicans Presages FEV₁ Decline and Hospital-Treated Exacerbations in Cystic Fibrosis. Chest 2010, 138, 1186–1195. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Colombo, C.; Tosco, A.; Montemitro, E.; Volpi, S.; Ruggiero, L.; Lelii, M.; Bisogno, A.; Pelucchi, C.; Principi, N. Streptococcus Pneumoniae Oropharyngeal Colonization in Children and Adolescents with Cystic Fibrosis. J. Cyst. Fibros. 2016, 15, 366–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.E.; O’Toole, G.A. The Yin and Yang of Streptococcus Lung Infections in Cystic Fibrosis: A Model for Studying Polymicrobial Interactions. J. Bacteriol. 2019, 201, e00115-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, E.M. The Emergence of Streptococcus Anginosus Group as a Cystic Fibrosis Pathogen. Clin. Microbiol. Newsl. 2017, 39, 143–147. [Google Scholar] [CrossRef]
- Sibley, C.D.; Parkins, M.D.; Rabin, H.R.; Duan, K.; Norgaard, J.C.; Surette, M.G. A polymicrobial perspective of pulmonary infections exposes an enigmatic pathogen in cystic fibrosis patients. Proc Natl. Acad. Sci. USA 2008, 105, 15070–15075. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Schloss, P.D.; Kalikin, L.M.; Carmody, L.A.; Foster, B.K.; Petrosino, J.F.; Cavalcoli, J.D.; VanDevanter, D.R.; Murray, S.; Li, J.Z.; et al. Decade-long bacterial community dynamics in cystic fibrosis airways. Proc. Natl. Acad. Sci. USA 2012, 109, 5809–5814. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, S.; Fothergill, J.L. The role of multispecies social interactions in shaping Pseudomonas aeruginosa pathogenicity in the cystic fibrosis lung. FEMS Microbiol. Lett. 2017, 364, fnx128. [Google Scholar] [CrossRef] [PubMed]
- Jorth, P.; Ehsan, Z.; Rezayat, A.; Caldwell, E.; Pope, C.; Brewington, J.J.; Goss, C.H.; Benscoter, D.; Clancy, J.P.; Singh, P.K. Direct Lung Sampling Indicates That Established Pathogens Dominate Early Infections in Children with Cystic Fibrosis. Cell Rep. 2019, 27, 1190–1204.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennings, M.T.; Riekert, K.A.; Boyle, M.P. Update on Key Emerging Challenges in Cystic Fibrosis. Med. Princ. Pract. 2014, 23, 393–402. [Google Scholar] [CrossRef]
- Noni, M.; Katelari, A.; Dimopoulos, G.; Doudounakis, S.-E.; Tzoumaka-Bakoula, C.; Spoulou, V. Aspergillus Fumigatus Chronic Colonization and Lung Function Decline in Cystic Fibrosis May Have a Two-Way Relationship. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Ratjen, F.; Bell, S.C.; Rowe, S.M.; Goss, C.H.; Quittner, A.L.; Bush, A. Cystic Fibrosis. Nat. Rev. Dis. Primers 2015, 1, 15010. [Google Scholar] [CrossRef] [PubMed]
- Kent, L.; Reix, P.; Innes, J.A.; Zielen, S.; Le Bourgeois, M.; Braggion, C.; Lever, S.; Arets, H.G.; Brownlee, K.; Bradley, J.M.; et al. Lung clearance index: Evidence for use in clinical trials in cystic fibrosis. J. Cyst. Fibros. 2014, 13, 123–138. [Google Scholar] [CrossRef] [Green Version]
- Stahl, M.; Wielpütz, M.O.; Graeber, S.Y.; Joachim, C.; Sommerburg, O.; Kauczor, H.U.; Puderbach, M.; Eichinger, M.; Mall, M.A. Comparison of Lung Clearance Index and Magnetic Resonance Imaging for Assessment of Lung Disease in Children with Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 2017, 195, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Graeber, S.Y.; Boutin, S.; Wielpütz, M.O.; Joachim, C.; Frey, D.L.; Wege, S.; Sommerburg, O.; Kauczor, H.-U.; Stahl, M.; Dalpke, A.H.; et al. Effects of Lumacaftor-Ivacaftor on Lung Clearance Index, Magnetic Resonance Imaging, and Airway Microbiome in Phe508del Homozygous Patients with Cystic Fibrosis. Ann. Am. Thorac. Soc. 2021, 18, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Conrad, D.J.; Bailey, B.A.; Hardie, J.A.; Bakke, P.S.; Eagan, T.M.L.; Aarli, B.B. Median Regression Spline Modeling of Longitudinal FEV1 Measurements in Cystic Fibrosis (CF) and Chronic Obstructive Pulmonary Disease (COPD) Patients. PLoS ONE 2017, 12, e0190061. [Google Scholar] [CrossRef] [Green Version]
- Boutin, S.; Weitnauer, M.; Hassel, S.; Graeber, S.Y.; Stahl, M.; Dittrich, A.S.; Mall, M.A.; Dalpke, A.H. One time quantitative PCR detection of Pseudomonas aeruginosa to discriminate intermittent from chronic infection in cystic fibrosis. J. Cyst. Fibros. 2018, 17, 348–355. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Missing Data | n or Median or Mean | % or [IQR] or (SD) |
|---|---|---|---|
| Age (years) | 0 | 23.7 | (10.9) |
| Gender (female) | 0 | 143 | 47.8 |
| CFTR mutations | 0 | ||
| F508del/F508del | 119 | 39.8 | |
| F508del/other | 136 | 45.5 | |
| Other/other or unknown | 44 | 14.7 | |
| Comorbidities | 0 | ||
| Exocrine pancreatic insufficiency | 235 | 78.6 | |
| Diabetes mellitus | 83 | 27.8 | |
| Gastroesophageal reflux | 73 | 24.4 | |
| ABPA | 69 | 23.1 | |
| Chronic sinusitis | 58 | 19.4 | |
| Haemoptysis | 55 | 18.4 | |
| Concomitant asthma | 34 | 11.4 | |
| Pneumothorax | 9 | 3.0 | |
| Pulmonary hypertension | 5 | 1.7 | |
| BMI (kg/m2) | 1 | 19.9 | [17.6–21.3] |
| Shwachman–Kulczycki score | 28 | 74.6 | [65–90] |
| Number of days of hospitalization during the last 12 months * | 10 | 7 | [1–115] |
| Sputum bacterial cultures positive with | |||
| Staphylococcus aureus (methicillin susceptible) | 80 | 27.4 | |
| Pseudomonas aeruginosa (non-mucoid strains) | 75 | 26.4 | |
| Pseudomonas aeruginosa (mucoid strains) | 77 | 22.6 | |
| Haemophilus influenzae | 28 | 9.6 | |
| Staphylococcus aureus (methicillin resistant) | 22 | 7.5 | |
| Non-pneumoniae Streptococcus species | 19 | 6.5 | |
| Stenotrophomonas maltophilia | 16 | 5.5 | |
| Alkaligenes xylosoxidans | 12 | 4.1 | |
| Streptococcus pneumoniae | 5 | 1.7 | |
| Bulkhoderia cepatia complex | 3 | 1.0 | |
| Mycobacterium avium complex | 4 | 1.4 | |
| Mycobacterium abscessus complex | 5 | 1.7 | |
| Sputum fungal cultures positive with | 10 | ||
| Candida albicans | 136 | 45.5 | |
| Aspergillus fumigatus | 80 | 26.8 | |
| Other moulds (Cladosporium sp., Penicillium sp.) | 20 | 6.7 | |
| Scedosporium species | 10 | 4.0 | |
| Aspergillus flavus | 9 | 3.0 | |
| Candida parapsilosis | 5 | 1.7 | |
| Other Aspergillus species | 4 | 1.3 | |
| Candida glabrata | 3 | 1.0 | |
| Other Candida species | 3 | 1.0 | |
| Exophiala dermatitidis | 2 | 0.7 | |
| Lomentospora prolificans | 1 | 0.3 | |
| Medications | 0 | ||
| Non-invasive ventilation | 21 | 7.2 | |
| Long-term oxygen | 32 | 10.7 | |
| Nebulized rhDNase | 178 | 59.5 | |
| Inhaled antibiotics | 86 | 28.8 | |
| Inhaled steroids | 168 | 56.2 | |
| Azithromycin | 162 | 54.2 | |
| Oral antibiotic courses during the last 12 months | |||
| 0 | 75 | 25.1 | |
| 1 to 3 | 161 | 53.8 | |
| >3 | 63 | 21.1 | |
| Intravenous antibiotics during the last 12 months | 153 | 51.2 | |
| Mean number of antibiotic courses | 1 | [0–2] | |
| Systemic corticosteroids during the last 12 months | 70 | 23.4 | |
| Other immunosuppressive treatment | 3 | 1.0 | |
| Antifungal treatment during the last 6 months | 61 | 20.4 |
| Variables Expressed as Median [IQR] or Mean (±SD)—(MV) | Inclusion | 1st Follow-Up (Year 1) | 2nd Follow-Up (Year 2) |
|---|---|---|---|
| ppFEV1 | 67 [43–91]—(4) | 70 [41–92]—(36) | 69 [47–91]—(68) |
| Outpatient visits (n) | 4 [3–6]—(1) | 4 [3–5]—(5) | 4 [1–5]—(13) |
| Hospital admissions (n) | 0 [0–1]—(1) | 0 [0–2]—(6) | 0 [0–1]—(13) |
| Days of hospitalization (n) | 12.2 (±16.6)—(10) | 13.0 (±18.3)—(59) | 12.7(±16.8)—(22) |
| Microorganisms | Type of Colonisation (Number of Patients) | β Coefficient | 95%CI | p-Value |
|---|---|---|---|---|
| H. influenzae | Transient (68) | 5.23 | [1.47; 11.94] | 0.13 |
| S. pneumoniae | Transient (17) | 14.79 | [3.47; 26.11] | 0.01 |
| non-pneumoniae Streptococcus | Transient (33) | −6.93 | [−18.95; 5.09] | 0.25 |
| S. aureus (methicillin susceptible) | Transient (53) | 4.60 | [−2.84; 12.04] | 0.17 |
| Chronic (99) | 5.69 | [−0.71; 12.10] | ||
| S. aureus (methicillin resistant) | Transient (10) | 1.60 | [−13.09; 16.28] | 0.38 |
| Chronic (27) | −6.44 | [−15.72; 2.84 | ||
| P. aeruginosa (non-mucoid strains) | Transient (53) | −9.77 | [−16.78; −2.76] | <0.0001 |
| Chronic (70) | −15.41 | [−22.08; −8.75] | ||
| P. aeruginosa (mucoid strains) | Transient (26) | −19.45 | [−28.74; −10.16] | <0.0001 |
| Chronic (90) | −13.15 | [−19.42; −6.89] | ||
| S. maltophilia | Transient (22) | −14.19 | [−24.22; −4.16] | 0.01 |
| Chronic (8) | −10.26 | [−26.44; 5.93] | ||
| B. cepacia complex | Transient (5) | 18.60 | [−2.02; 39.23] | 0.15 |
| Chronic (2) | −13.15 | [−44.69; 18.40] | ||
| A. xylosoxidans | Transient (3) | −6.32 | [−32.99; 20.35] | 0,03 |
| Chronic (13) | −17.97 | [−31.32; −4.62] | ||
| A. fumigatus | Transient (57) | −2.99 | [−10.05; 4.07] | 0.69 |
| Chronic (57) | −1.53 | [−8.65; 5.60] | ||
| Scedosporium species | Transient (0) | / | / | 0.22 |
| Chronic (10) | −7.42 | [−22.56; 7.52] | ||
| C. albicans | Transient (79) | −4.74 | [−11.23; 1.74] | |
| Chronic (85) | −11.03 | [−17.62; −4.44] | 0.005 |
| Microorganisms | Type of Colonisation (Number of Patients) | β Coefficient | 95%CI | p-Value |
|---|---|---|---|---|
| S. pneumoniae | Transient (17) | 10.92 | 0.45; 21.40 | 0.04 |
| non-pneumoniae Streptococcus species | Transient (33) | −13.62 | −25.12; −2.12 | 0.02 |
| A. xylosoxidans | Transient (3) | −3.61 | −26.95; −2.30 | 0.06 |
| Chronic (13) | −14.62 | −32.08; 24.86 | ||
| S. maltophilia | Transient (22) | −11.93 | −21.38; −2.49 | 0.02 |
| Chronic (8) | −11.02 | −26.83; 4.79 | ||
| P. aeruginosa (non-mucoid strains) | Transient (53) | −7.54 | −14.30; −0.79 | 0.01 |
| Chronic (70) | −9.63 | −16.61; −2.65 | ||
| P. aeruginosa (mucoid strains) | Transient (26) | −18.26 | −27.20; −9.31 | <0.0001 |
| Chronic (90) | −10.41 | −16.96; −3.87 | ||
| C. albicans | Transient (79) | −5.12 | −11.17; 0.93 | <0.01 |
| Chronic (85) | −10.26 | −16.50; −4.02 |
| Microorganisms | Type of Colonisation (Number of Patients) | β Coefficient | 95%CI | p-Value |
|---|---|---|---|---|
| S. pneumoniae | Transient (17) | −0.05 | −0.81; 0.71 | 0.90 |
| non-pneumoniae Streptococcus spp. | Transient (33) | 0.53 | −0.23; 1.30 | 0.17 |
| A. xylosoxidans | Transient (3) | 0.49 | 0.84; 2.59 | 0.001 |
| Chronic (13) | 1.72 | −1.27; 2.25 | ||
| S. maltophilia | Transient (22) | 0.90 | 0.21; 1.55 | 0.01 |
| Chronic (8) | 0.88 | −0.18; 1.99 | ||
| P. aeruginosa (non-mucoid strains) | Transient (53) | 0.95 | 0.48; 1.43 | <0.0001 |
| Chronic (70) | 1.30 | 0.80; 1.81 | ||
| P. aeruginosa (mucoid strains) | Transient (26) | 0.91 | 0.26; 1.56 | 0.01 |
| Chronic (90) | 0.41 | −0.07; 0.88 | ||
| C. albicans | Transient (79) | 0.21 | −0.23; 0.65 | 0.63 |
| Chronic (85) | 0.12 | −0.32; 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francis, F.; Enaud, R.; Soret, P.; Lussac-Sorton, F.; Avalos-Fernandez, M.; MucoFong Investigation Group; Bui, S.; Fayon, M.; Thiébaut, R.; Delhaes, L. New Insights in Microbial Species Predicting Lung Function Decline in CF: Lessons from the MucoFong Project. J. Clin. Med. 2021, 10, 3725. https://doi.org/10.3390/jcm10163725
Francis F, Enaud R, Soret P, Lussac-Sorton F, Avalos-Fernandez M, MucoFong Investigation Group, Bui S, Fayon M, Thiébaut R, Delhaes L. New Insights in Microbial Species Predicting Lung Function Decline in CF: Lessons from the MucoFong Project. Journal of Clinical Medicine. 2021; 10(16):3725. https://doi.org/10.3390/jcm10163725
Chicago/Turabian StyleFrancis, Florence, Raphael Enaud, Perrine Soret, Florian Lussac-Sorton, Marta Avalos-Fernandez, MucoFong Investigation Group, Stéphanie Bui, Michael Fayon, Rodolphe Thiébaut, and Laurence Delhaes. 2021. "New Insights in Microbial Species Predicting Lung Function Decline in CF: Lessons from the MucoFong Project" Journal of Clinical Medicine 10, no. 16: 3725. https://doi.org/10.3390/jcm10163725
APA StyleFrancis, F., Enaud, R., Soret, P., Lussac-Sorton, F., Avalos-Fernandez, M., MucoFong Investigation Group, Bui, S., Fayon, M., Thiébaut, R., & Delhaes, L. (2021). New Insights in Microbial Species Predicting Lung Function Decline in CF: Lessons from the MucoFong Project. Journal of Clinical Medicine, 10(16), 3725. https://doi.org/10.3390/jcm10163725







