Postoperative Analgesia after Open Liver Surgery: Systematic Review of Clinical Evidence
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
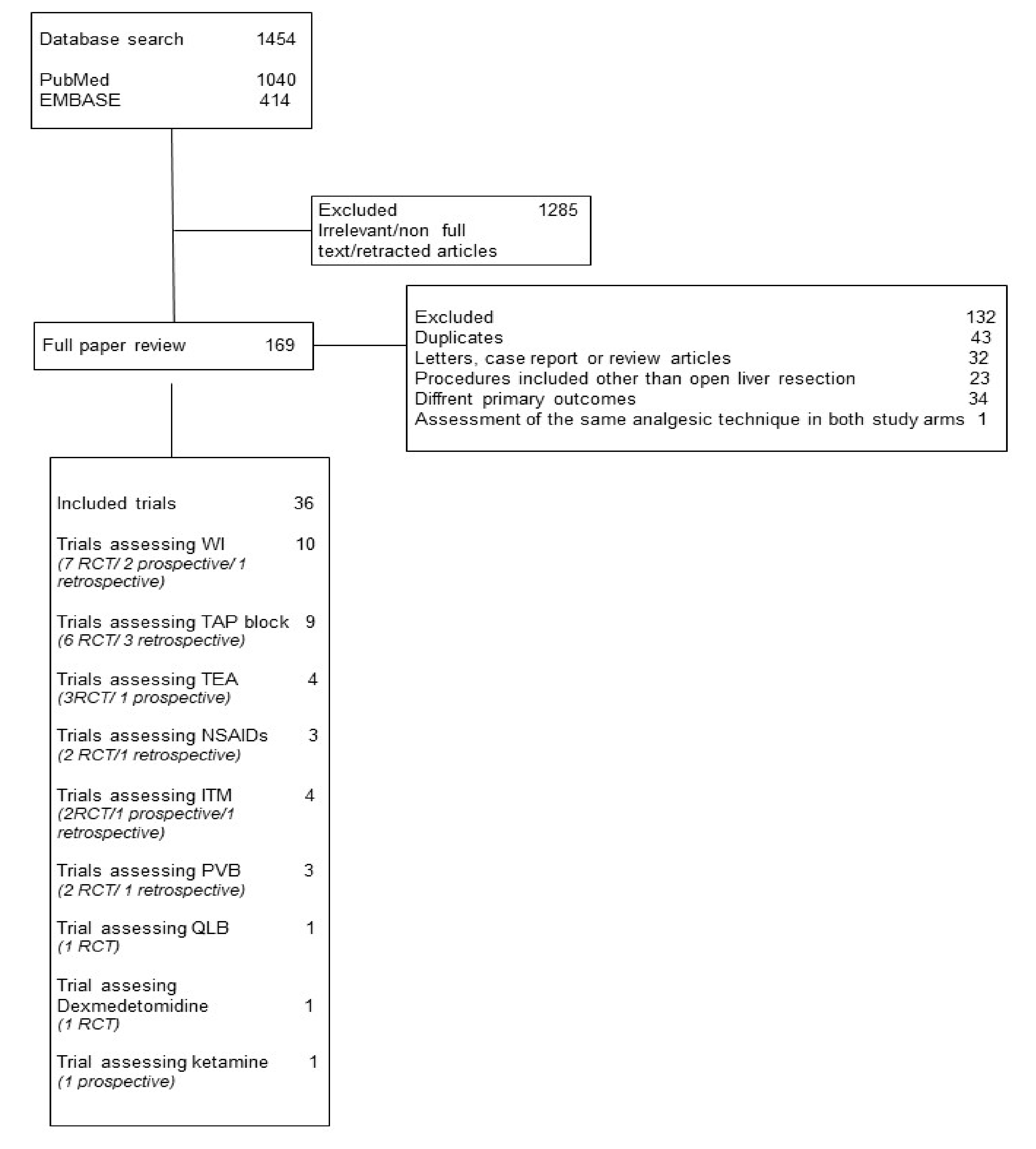
2.2. Study Selection and Inclusion Criteria
2.3. Outcomes
2.4. Data Extraction and Data Analysis
2.5. Risk of Bias
3. Results
3.1. Trials Assessing WI
3.2. Trials Assessing TAP Block
3.3. Trials Assessing TEA
3.4. Trials Assessing NSAIDs
3.5. Trial Assessing ITM
3.6. Trials Assessing PVB
3.7. Trial Assessing QLB
3.8. Trial Assessing Dexmedetomidine
3.9. Trial Assessing Ketamine
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Carr, D.B.; Goudas, L.C. Acute pain. Lancet 1999, 353, 2051–2058. [Google Scholar] [CrossRef]
- Kelly, D.J.; Ahmad, M.; Brull, S.J. Preemptive analgesia I: Physiological pathways and pharmacological modalities. Can. J. Anesth. Can. d’anesthésie 2001, 48, 1000–1010. [Google Scholar] [CrossRef]
- Soliz, J.M.; Gebhardt, R.; Feng, L.; Dong, W.; Reich, M.; Curley, S. Comparing epidural analgesia and ON-Q infiltrating catheters for pain management after hepatic resection. Open J Anesth. 2013, 3, 3–7. [Google Scholar] [CrossRef]
- Lentschener, C.; Ozier, Y. Anaesthesia for elective liver resection: Some points should be revisited. Eur. J. Anaesthesiol. 2002, 19, 780. [Google Scholar] [CrossRef]
- Milan, Z.B.; Kalami, T.; Harbis, A.; Hooper, J.; Westwood, P.; Milic, N.; Bellamy, M. An audit on postoperative pain in liver resection surgery following epidural catheter removal. J. Pain Manag. 2011, 4, 381–386. [Google Scholar]
- Chou, R.; Gordon, D.B.; de Leon-Casasola, O.A.; Rosenberg, J.M.; Bickler, S.; Brennan, T.; Carter, T.; Cassidy, C.L.; Chittenden, H.; Degenhardt, E.; et al. Guidelines on the management of postoperative pain. J. Pain 2016, 17, 131–157. [Google Scholar] [CrossRef]
- Apfelbaum, J.L.; Chen, C.; Mehta, S.S.; Gan, T.J. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth. Analg. 2003, 97, 534–540. [Google Scholar] [CrossRef]
- Sommer, M.; De Rijke, J.M.; Van Kleef, M.; Kessels, A.G.H.; Peters, M.L.; Geurts, J.W.J.M.; Gramke, H.F.; Marcus, M.A.E. The prevalence of postoperative pain in a sample of 1490 surgical inpatients. Eur. J. Anaesthesiol. 2008, 25, 267–274. [Google Scholar] [CrossRef]
- Kim, B.J.; Soliz, J.M.; Aloia, T.A.; Vauthey, J.N. What is the best pain control after major hepatopancreatobiliary surgery? Adv. Surg. 2018, 52, 235–246. [Google Scholar] [CrossRef]
- Wrighton, L.J.; O’Bosky, K.R.; Namm, J.P.; Senthil, M. Postoperative management after hepatic resection. J. Gastrointest. Oncol. 2012, 3, 41–47. [Google Scholar] [CrossRef]
- Redai, I.; Emond, J.; Brentjens, T. Anesthetic considerations during liver surgery. Surg. Clin. North Am. 2004, 84, 401–411. [Google Scholar] [CrossRef]
- Zhou, L.; Huang, J.; Chen, C. Most effective pain-control procedure for open liver surgery: A network meta-analysis. ANZ J. Surg. 2018, 88, 1236–1242. [Google Scholar] [CrossRef]
- Salicath, J.H.; Yeoh, E.C.Y.; Bennett, M.H. Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst. Rev. 2018, CD010434. [Google Scholar] [CrossRef]
- Sheth, K.R.; Bernthal, N.M.; Ho, H.S.; Bergese, S.D.; Apfel, C.C.; Stoicea, N.; Jahr, J.S. Perioperative bleeding and non-steroidal anti-inflammatory drugs (NSAIDs): An evidence-based literature review, and current clinical appraisal. Medicine 2020, 99, e20042. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Pourrahmat, M.M.; Vasilyeva, E.; Kim, P.T.W.; Osborn, J.; Wiseman, S.M. Efficacy and safety of patient-controlled analgesia compared with epidural analgesia after open hepatic resection: A systematic review and meta-analysis. Ann. Surg. 2019, 270, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, L.; Scurrah, N.; Gunning, K.; McNicol, L. Postoperative changes in prothrombin time following hepatic resection: Implications for perioperative analgesia. Anaesth. Intensive Care 2006, 34, 438–443. [Google Scholar] [CrossRef] [PubMed]
- Jacquenod, P.; Wallon, G.; Gazon, M.; Darnis, B.; Pradat, P.; Virlogeux, V.; Farges, O.; Aubrun, F. Incidence and risk factors of coagulation profile derangement after liver surgery: Implications for the use of epidural analgesia—A retrospective cohort study. Anesth. Analg. 2018, 126, 1142–1147. [Google Scholar] [CrossRef]
- Shontz, R.; Karuparthy, V.; Temple, R.; Brennan, T.J. Prevalence and risk factors predisposing to coagulopathy in patients receiving epidural analgesia for hepatic surgery. Reg. Anesth. Pain Med. 2009, 34, 308–311. [Google Scholar] [CrossRef]
- Hughes, M.; McNally, S.; McKeown, D.W.; Wigmore, S. Effect of analgesic modality on outcome following open liver surgery: A systematic review of postoperative analgesia. Minerva Anestesiol. 2015, 81, 541–556. [Google Scholar]
- Dieu, A.; Huynen, P.; Lavand’homme, P.; Beloeil, H.; Freys, S.M.; Pogatzki-Zahn, E.M.; Joshi, G.P.; Van de Velde, M. Pain management after open liver resection: Procedure-specific postoperative pain management (PROSPECT) recommendations. Reg. Anesth. Pain Med. 2021, 46, 433–445. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; Antes, G.; Atkins, D.; Barbour, V.; Barrowman, N.; Berlin, J.A.; et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Schardt, C.; Adams, M.B.; Owens, T.; Keitz, S.; Fontelo, P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med. Inform. Decis. Mak. 2007, 7, 1–6. [Google Scholar] [CrossRef]
- Hughes, M.J.; Harrison, E.M.; Peel, N.J.; Stutchfield, B.; McNally, S.; Beattie, C.; Wigmore, S.J. Randomized clinical trial of perioperative nerve block and continuous local anaesthetic infiltration via wound catheter versus epidural analgesia in open liver resection (LIVER 2 trial). Br. J. Surg. 2015, 102, 1619–1628. [Google Scholar] [CrossRef]
- Bell, R.; Ward, D.; Jeffery, J.; Toogood, G.J.; Lodge, J.A.; Rao, K.; Lotia, S.; Hidalgo, E. A randomized controlled trial comparing epidural analgesia versus continuous local anesthetic infiltration via abdominal wound catheter in open liver resection. Ann. Surg. 2019, 269, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Dalmau, A.; Fustran, N.; Camprubi, I.; Sanzol, R.; Redondo, S.; Ramos, E.; Torras, J.; Sabaté, A. Analgesia with continuous wound infusion of local anesthetic versus saline: Double-blind randomized, controlled trial in hepatectomy. Am. J. Surg. 2018, 215, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Peres-Bachelot, V.; Blanc, E.; Oussaid, N.; Pérol, D.; Daunizeau-Walker, A.L.; Pouderoux, S.; Peyrat, P.; Rivoire, M.; Dupré, A. A 96-hour continuous wound infiltration with ropivacaine reduces analgesic consumption after liver resection: A randomized, double-blind, controlled trial. J. Surg. Oncol. 2019, 119, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.-X.; Bai, K.-Y.; Liu, Y.-F.; Du, G.; Fu, Z.-H.; Zhang, H.; Yang, J.-H.; Wang, B.; Wang, X.-Y.; Jin, B. Effect of local wound infiltration with ropivacaine on postoperative pain relief and stress response reduction after open hepatectomy. World J. Gastroenterol. 2017, 23, 6733–6740. [Google Scholar] [CrossRef]
- Xin, Y.; Hong, Y.; Yong, L.Z. Efficacy of postoperative continuous wound infiltration with local anesthesia after open hepatectomy. Clin. J. Pain 2014, 30, 571–576. [Google Scholar] [CrossRef]
- Wu, Y.F.; Li, X.P.; Yu, Y.B.; Chen, L.; Jiang, C.B.; Li, D.Y.; Chen, M.L. Postoperative local incision analgesia for acute pain treatment in patients with hepatocellular carcinoma. Rev. Assoc. Med. Bras. 2018, 64, 175–180. [Google Scholar] [CrossRef]
- Karanicolas, P.J.; Cleary, S.; McHardy, P.; Kiss, A.; Sawyer, J.; Behman, R.; Ladak, S.; McCluskey, S.A.; Srinivas, C.; Katz, J.; et al. Medial open transversus abdominis plane (MOTAP) catheters reduce opioid requirements and improve pain control following open liver resection: A multicenter, blinded, randomized controlled trial. Ann. Surg. 2018, 268, 233–240. [Google Scholar] [CrossRef]
- Guo, J.-G.; Li, H.-L.; Pei, Q.-Q.; Feng, Z.-Y. The analgesic efficacy of subcostal transversus abdominis plane block with Mercedes incision. BMC Anesthesiol. 2018, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Kıtlık, A.; Erdogan, M.A.; Ozgul, U.; Aydogan, M.S.; Ucar, M.; Toprak, H.I.; Colak, C.; Durmus, M. Ultrasound-guided transversus abdominis plane block for postoperative analgesia in living liver donors: A prospective, randomized, double-blinded clinical trial. J. Clin. Anesth. 2017, 37, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, C.; Uzgul, U.; Uçar, M.; Yalin, R.; Colak, Y.Z.; Çolak, C.; Toprak, H.I. Effect of transversus abdominis plane block in combination with general anesthesia on perioperative opioid consumption, hemodynamics, and recovery in living liver donors: The prospective, double-blinded, randomized study. Clin. Transplant. 2017, 31, e12931. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.-F.F.; Jia, W.-D.D.; Li, Y.-Q.Q.; Lv, J.-G.G.; Zhou, H. Effectiveness of Parecoxib Sodium Combined with Transversus Abdominis Plane Block for Pain Management After Hepatectomy for Hepatocellular Carcinoma: A Prospective Controlled Study. Med. Sci. Monit. 2019, 25, 1053–1060. [Google Scholar] [CrossRef]
- Su, W.; Deng, X.; Li, X.; Deng, J.; Li, P.; Yang, M. Effect of the transversus abdominis plane block on postoperative pain and recovery in patients with hepatic echinococcosis. J. Int. Med. Res. 2018, 46, 3563–3569. [Google Scholar] [CrossRef]
- Hausken, J.; Fretland, Å.A.; Edwin, B.; Andersen, M.H.; Dagenborg, V.J.; Bjørnelv, G.M.W.; Kristiansen, R.; Røysland, K.; Kvarstein, G.; Tønnessen, T.I. Intravenous patient-controlled analgesia versus thoracic epidural analgesia after open liver surgery: A prospective, randomized, controlled, noninferiority trial. Ann. Surg. 2019, 270, 193–199. [Google Scholar] [CrossRef]
- Aloia, T.A.; Kim, B.J.; Segraves-Chun, Y.S.; Cata, J.P.; Truty, M.J.; Shi, Q.; Holmes, A.; Soliz, J.M.; Popat, K.U.; Rahlfs, T.F.; et al. A Randomized Controlled Trial of Postoperative Thoracic Epidural Analgesia Versus Intravenous Patient-controlled Analgesia after Major Hepatopancreatobiliary Surgery. Ann. Surg. 2017, 266, 545–554. [Google Scholar] [CrossRef]
- Aydogan, M.S.; Biçakcioʇlu, M.; Sayan, H.; Durmus, M.; Yilmaz, S.; Bıçakcıoglu, M.; Sayan, H.; Durmus, M. Effects of two different techniques of postoperative analgesia management in liver transplant donors: A prospective, randomized, double-blind study. Transplant. Proc. 2015, 47, 1204–1206. [Google Scholar] [CrossRef]
- Wang, R.D.; Zhu, J.-Y.; Zhu, Y.; Ge, Y.-S.; Xu, G.-L.; Jia, W.-D. Perioperative analgesia with parecoxib sodium improves postoperative pain and immune function in patients undergoing hepatectomy for hepatocellular carcinoma. J. Eval. Clin. Pract. 2020, 26, 992–1000. [Google Scholar] [CrossRef]
- Chen, H.; Liao, Z.; Fang, Y.; Niu, B.; Chen, A.; Cao, F.; Mei, W.; Tian, Y. Continuous right thoracic paravertebral block following bolus initiation reduced postoperative pain after right-lobe hepatectomy: A randomized, double-blind, placebo-controlled trial. Reg. Anesth. Pain Med. 2014, 39, 506–512. [Google Scholar] [CrossRef][Green Version]
- Dichtwald, S.; Ben-Haim, M.; Papismedov, L.; Hazan, S.; Cattan, A.; Matot, I. Intrathecal morphine versus intravenous opioid administration to impact postoperative analgesia in hepato-pancreatic surgery: A randomized controlled trial. J. Anesth. 2017, 31, 237–245. [Google Scholar] [CrossRef]
- Niewiński, G.; Figiel, W.; Grąt, M.; Dec, M.; Morawski, M.; Patkowski, W.; Zieniewicz, K. A comparison of intrathecal and intravenous morphine for analgesia after hepatectomy: A randomized controlled trial. World J. Surg. 2020, 44, 2340–2349. [Google Scholar] [CrossRef]
- Schreiber, K.L.; Chelly, J.E.; Scott Lang, R.; Abuelkasem, E.; Geller, D.A.; Wallis Marsh, J.; Tsung, A.; Sakai, T. Epidural versus paravertebral nerve block for postoperative analgesia in patients undergoing open liver resection a randomized clinical trial. Reg. Anesth. Pain Med. 2016, 41, 460–468. [Google Scholar] [CrossRef]
- Chen, M.-T.T.; Jin, B.; Da Du, S.-D.; Pei, L.-J.J.; Zhu, B.; Yan, L.; Chi, T.-Y.Y.; Xu, H.-F.F.; Zheng, Y.-C.C.; Xu, Y.-Y.Y.; et al. Role of a selective cyclooxygenase-2 inhibitor on pain and enhanced recovery after open hepatectomy: A randomized controlled trial. Transl. Cancer Res. 2017, 6, 806–814. [Google Scholar] [CrossRef]
- Zhu, Q.; Li, L.; Yang, Z.; Shen, J.; Zhu, R.; Wen, Y.; Cai, W.; Liu, L. Ultrasound guided continuous quadratus lumborum block hastened recovery in patients undergoing open liver resection: A randomized controlled, open-label trial. BMC Anesthesiol. 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, G.; Liu, X.; Wang, T.L.; Chi, P. The opioid-sparing effect of perioperative dexmedetomidine combined with oxycodone infusion during open hepatectomy: A randomized controlled trial. Front. Pharmacol. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wong-Lun-Hing, E.M.; van Dam, R.M.; Welsh, F.K.S.; Wells, J.K.G.; John, T.G.; Cresswell, A.B.; Dejong, C.H.C.; Rees, M. Postoperative pain control using continuous i.m. bupivacaine infusion plus patient-controlled analgesia compared with epidural analgesia after major hepatectomy. HPB 2014, 16, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Katz, J.; Montbriand, J.; Ladak, S.; McCluskey, S.; Srinivas, C.; Ko, R.; Grant, D.; Bradbury, A.; LeManach, Y.; et al. Surgically placed abdominal wall catheters on postoperative analgesia and outcomes after living liver donation. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2015, 21, 478–486. [Google Scholar] [CrossRef]
- Che, L.; Lu, X.; Pei, L. Efficacy and safety of a continuous wound catheter in open abdominal partial hepatectomy. Chin. Med. Sci. J. 2017, 32, 171–176. [Google Scholar] [CrossRef]
- Hernandez, M.C.; Panchamia, J.; Finnesgard, E.J.; Leiting, J.L.; Franssen, B.; Saleem, H.; Kendrick, M.L.; Nagorney, D.M.; Truty, M.J.; Smoot, R.L. Transversus abdominis plane blocks with liposomal bupivacaine after open major hepatectomy is associated with reduced early patient-reported pain scores and opioid administration. Surgery 2018, 164, 1251–1258. [Google Scholar] [CrossRef]
- Amundson, A.W.; Olsen, D.A.; Smith, H.M.; Torsher, L.C.; Martin, D.P.; Heimbach, J.K.; Findlay, J.Y. Acute benefits after liposomal bupivacaine abdominal wall blockade for living liver donation: A retrospective review. Mayo Clin. Proc. Innov. Qual. Outcomes 2018, 2, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Shibata, S.C.; Wada, H.; Marubashi, S.; Kamibayashi, T.; Eguchi, H.; Fujino, Y. The efficacy of continuous subcostal transversus abdominis plane block for analgesia after living liver donation: A retrospective study. J. Anesth. 2016, 30, 39–46. [Google Scholar] [CrossRef]
- Ganapathi, S.; Roberts, G.; Mogford, S.; Bahlmann, B.; Ateleanu, B.; Kumar, N. Epidural analgesia provides effective pain relief in patients undergoing open liver surgery. Br. J. Pain 2015, 9, 78–85. [Google Scholar] [CrossRef]
- Lim, K.I.; Liu, C.K.; Chen, C.L.; Wang, C.H.; Huang, C.J.; Cheng, K.W.; Wu, S.C.; Shih, T.H.; Yang, S.C.; Lee, Y.E.; et al. Transitional study of patient-controlled analgesia morphine with ketorolac to patient-controlled analgesia morphine with parecoxib among donors in adult living donor liver transplantation: A single-center experience. Transplant. Proc. 2016, 48, 1074–1076. [Google Scholar] [CrossRef]
- Tang, J.; Churilov, L.; Tan, C.O.; Hu, R.; Pearce, B.; Cosic, L.; Christophi, C.; Weinberg, L. Intrathecal morphine is associated with reduction in postoperative opioid requirements and improvement in postoperative analgesia in patients undergoing open liver resection. BMC Anesthesiol. 2020, 20, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kasivisvanathan, R.; Abbassi-Ghadi, N.; Prout, J.; Clevenger, B.; Fusai, G.K.; Mallett, S. V A prospective cohort study of intrathecal versus epidural analgesia for patients undergoing hepatic resection. HPB 2014, 16, 768–775. [Google Scholar] [CrossRef]
- Mistry, K.; Hutchins, J.; Leiting, J.; Mangalick, K.; Pruett, T.; Chinnakotla, S. Continuous paravertebral infusions as an effective adjunct for postoperative pain management in living liver donors: A retrospective observational study. Transplant. Proc. 2017, 49, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Masgoret, P.; Gomar, C.; Tena, B.; Taurá, P.; Ríos, J.; Coca, M.; Taura, P.; Rios, J.; Coca, M. Incidence of persistent postoperative pain after hepatectomies with 2 regimes of perioperative analgesia containing ketamine. Medicine 2017, 96, e6624. [Google Scholar] [CrossRef] [PubMed]
- Terrazina, S.; Robba, C.; Prette, A.; Sergi, P.; Bilotta, F. Prevention and treatment of postoperative pain after lumbar spine procedures: A systematic review. Pain Pract. 2018, 18, 925–945. [Google Scholar] [CrossRef]
- Tsaousi, G.; Logan, S.; Bilotta, F. Postoperative pain control following craniotomy: A systematic review of recent clinical literature. Pain Pract. 2017, 17, 968–981. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, A.; Cohen, S.; Yang, S.; Ochroch, E.A. Preventing and treating pain after thoracic surgery. Anesthesiology 2006, 104, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Ramsay, M.A. Postoperative Pain Management: Is the Surgical Team Approach Finally Getting It Right? Ann. Surg. 2019, 270, 209–210. [Google Scholar] [CrossRef] [PubMed]
| Authors | Sequence Generation | Allocation Concealment | Blinding of Participants, Personnel and Outcome Assessor | Incomplete Outcome Data | Selective Outcome Reporting | Others Criteria |
|---|---|---|---|---|---|---|
| Hughes et al. [24] | U | U | H | L | L | H |
| Bell et al. [25] | L | L | H | L | L | L |
| Dalmau et al. [26] | L | L | L | L | L | L |
| Peres-Bachelot et al. [27] | L | L | L | U | L | U |
| Sun et al. [28] | L | U | L | L | L | L |
| Xin et al. [29] | L | L | L | L | L | L |
| Wu et al. [30] | L | L | U | L | L | L |
| Karanicalas et al. [31] | L | L | L | L | L | L |
| Guo et al. [32] | L | L | L | L | L | L |
| Kıtlık et al. [33] | L | L | L | L | H | U |
| Erdogan et al. [34] | L | L | H | L | H | U |
| Qiao et al. [35] | L | L | H | L | H | L |
| Su et al. [36] | U | L | U | L | H | H |
| Hausken et al. [37] | L | U | H | L | L | L |
| Aloia et al. [38] | U | U | H | L | L | L |
| Aydogan et al. [39] | U | U | U | L | L | U |
| Wang et al. [40] | L | L | L | L | L | L |
| Chen H et al. [41] | L | L | L | L | L | U |
| Dichtwald et al. [42] | L | L | U | H | L | U |
| Niewiński et al. [43] | L | U | U | L | L | H |
| Schreiber et al. [44] | L | L | H | L | L | U |
| Chen MT et al. [45] | L | L | U | L | L | L |
| Zhu et al. [46] | L | U | H | L | L | L |
| Zhang et al. [47] | L | L | L | L | L | L |
| Authors | Bias Due to Confounding | Bias in Selection of Participants into the Study | Bias in Measurement of Interventions | Bias Due to Departures from Intended Interventions | Bias due to Missing Data | Bias in Measurement of Outcomes | Bias in Selection of the Reported Result |
|---|---|---|---|---|---|---|---|
| Wong-Lun-Hing et al. [48] | U | U | L | M | S | L | L |
| Khan et al. [49] | L | M | L | L | H | L | L |
| Che et al. [50] | S | S | L | L | L | U | L |
| Hernandez et al. [51] | S | S | S | U | M | L | L |
| Amundson et al. [52] | S | S | S | M | S | M | L |
| Maeda et al. [53] | S | S | S | L | U | L | L |
| Ganapathi et al. [54] | U | U | M | N.A | U | M | M |
| Lim et al. [55] | L | L | L | U | L | M | L |
| Tang et al. [56] | S | S | U | U | L | L | L |
| Kasivisvanathan et al. [57] | L | L | L | L | L | L | M |
| Mistry et al. [58] | S | S | S | U | S | M | M |
| Masgoret et al. [59] | L | U | L | N.A | M | L | L |
| Authors | Surgery/ Operation | Study Type/ Number of Patients (N)/ Tested Analgesic Techniques and Doses | Postoperative Follow-Up | Primary Endpoint | Secondary Endpoint | Key Message |
|---|---|---|---|---|---|---|
| Wong-Lun-Hing et al. | Elective open liver resection | Prospective study N = 498 WI: n = 429 At the end of surgery, 10 mL bolus of 0.25% bupivacaine, and then 0.25% bupivacaine, at a rate of 3 mL was continued for 72 h. Additionally IV-PCA with morphine or fentanyl. TEA: n = 69 During surgery 20 mL of 0.25% bupivacaine and then 0.1% bupivacaine with 2 µg/mL fentanyl at a rate of 5–15 mL/h for 48 h. | Postoperative days: 1, 2, 3 | VRS score at rest and on movement during the first 48 h Total opioid consumption | Need for rescue opioid Side effects of analgesia | No differences in VRS scores between the groups. Lower total opioid consumption in the WI group than in the TEA group. Increased need for rescue opioid in WI group on PoD0 than in the TEA group. Higher sedation scores on PoD0 in WI group than in TEA group. No differences in other side effects of analgesia rate between the groups. No reported cases of epidural hematoma, abscess formation, or paralysis in the TEA group. No difference in complications rate. Shorter LOS in the WI group than in the TEA group. |
| Khan et al. | Living donor hepatectomy | Retrospective study N = 319 WI: n = 84 At the end of surgery a bolus of 0.125% bupivacaine, 0.2 mL/kg per side. Postoperatively, a bolus of bupivacaine 0.125%, 0.2 mL/kg per catheter twice a day. Additionally IV-PCA with morphine. TEA: n = 68 Intraoperatively 0.1% bupivacaine with 0.015 mg/mL hydromorphone at a rate 5 mL/h. Postoperatively 0.1% bupivacaine solution with 0.015 mg/mL hydromorphone at an infusion rate of 5 mL/h with 3 mL bolus IV-PCA: n = 167 with morphine or hydromorphone. | Postoperative hours: 6, 12, 18, 24, 30, 36, 42, 48, 54, 60, 66, 72 | NRS scores Total opioid consumption | Side effects of analgesia: PONV, sedation, pruritus. Time to full diet Time to ambulation LOS | Higher NRS scores in the WI group than in the TEA group and similar to the IV PCA. Lower total opioid consumption in the WI group than in the IV-PCA group. Lower incidence of pruritus and sedation in the WI group compared to the TEA group. No differences in time to ambulation, incidences of PONV, and LOS between the groups. |
| Hughes et al. | Elective open liver resection | RCT N = 95 WI: n = 49 At the end of surgery 40 mL 0.125% levobupivacaine and then 0.375% levobupivacaine at a rate of 4 mL/h for 48 h. TEA: n = 44 10 mL levobupivacaine with 100 µg of fentanyl to establish epidural block than an infusion of 0.1% levobupivacaine with 2 µg/mL fentanyl for 48 h | Postoperative hours: 2, 6, 12, 24, 48, 72 | Functional recovery time: independent mobilization, tolerating full diet and oral analgesics, blood tests normal and patient willing to go home. | VAS score at rest and on movement complication rates inflammatory response CVP during transection | No differences in VAS scores, morbidity, inflammatory response, and CVP during transection between the groups. Greater opioid consumption in the WI group up to PoD 1. After PoD 1 TEA group received a greater amount of opioids. Shorter functional recovery time in the WI group than in the TEA group. WI group spent less time in the HDU than the TEA group. Greater volume of iv crystalloid being administered in the TEA group on PoD 1. |
| Bell et al. | Elective open liver resection and living donor hepatectomy | RCT N = 83 WI: n = 42 20 mL 0.5% bupivacaine bolus followed by an infusion of 0.25% bupivacaine at 4 mL/h per catheter for 60 h. Additionally IV-PCA with morphine or oxycodone for breakthrough pain. TEA: n = 41 0.15% bupivacaine with 2 µg/mL of fentanyl at 6–10 mL/h and continued for 60 h postoperatively. | Postoperative hours: 6, 24,36, 48, 60 | LOS | NRS score at rest Functional recovery time Peak flow Vasopressor and fluid requirements Complication rates | Higher NRS scores on PoD 0, afternoon of PoD 1, and morning of PoD 2 in the WI group than in the TEA group. Greater opioid consumption in the WI group on PoD 0, 1, and 2. No differences in side effects of analgesia and complication rates between the groups. No difference in LOS and functional recovery time between the groups. No difference in the volume of intraoperative fluid between the groups. Vasopressor support often required in the TEA group. No difference in baseline peak flow between the groups, but change in peak flow from the baseline level was worse in WI group. |
| Che et al. | Elective open liver resection | Prospective study N = 80 WI: n = 10 300 mL of 0.4% lidocaine for 72 h TEA: n = 22 Infusion of 0.2% ropivacaine at 4 mL/h with a bolus of 4 mL IV PCA: n = 48 morphine 0.25 mg/mL or sufentanil 0.6 mcg/mL at a rate of 4 mL/h, with a bolus of 4 mL. | Postoperative hours: 4, 12, 48, 72 | VAS score at rest and on movement | Need of rescue analgesic side effects of analgesia complication rates | No difference in the VAS scores at rest or on movement between the WI group and all other groups. Higher incidences of need for rescue analgesia at 4, 12 h after surgery in the WI group than in the TEA and IV-PCA groups. No differences in the incidence of PONV and functional recovery time between the groups. No severe adverse effects associated with WI. |
| Dalmau et al. | Elective open liver resection | RCT N = 99 WI: n = 53 0.23% ropivacaine at 5 mL/h for 48 h. Control group: n = 46 with placebo In both groups: before abdominal closure, iv 50 mg of dexketoprofen, iv acetaminophen (1 g every 6 h) and iv 0.05 mg/kg of morphine. Postoperatively: IV-PCA pump with morphine, iv 50 mg of dexketoprofen and iv 1 g of acetaminophen twice a day during the 48 h. | Postoperative hours: 0, 6, 12, 24, 48 | Total opioid consumption | NRS score Perioperative blood transfusion time to sit in a chair and walk Time to solid-food intake Side effects of analgesia wound complication LOS | Lower NRS score at 6 h in the WI group than in the control group. No differences in opioid consumption between the groups. No difference in transfusion requirements, solid food intake, ambulation, or LOS between the groups. Patients in the control group could sit in a chair earlier than those in the WI group. No wound complication was recorded. |
| Peres- Bachelot et al. | Elective open liver resection | RCT N = 85 WI: n = 42 40 mL of 0.375% ropivacaine on closure of the wound, followed by 8 mL/h continuous infusion of 0.2% ropivacaine for 96 h Control group: n = 43 with placebo Intraoperatively continuous iv remifentanil infusion in both groups. Acetaminophen 1 g and single morphine dose (0.2 mg/kg) were administered 1 h before the end of surgery. Postoperative analgesia: IV-PCA with morphine in both groups. Acetaminophen and nefopam as a rescue analgesia. | Postoperative hours: first 96 | Total opioid consumption | VAS scores daily opioid, acetaminophen, and nefopam consumptions Time of recovery of GI function Adverse events (PONV, psychiatric disorders, tachycardia, hypotension, residual pain, wound status) LOS | No difference in VAS scores, pain management satisfaction, hemodynamic parameters, recovery of GI function, wound complications, and LOS between the groups. Lower opioid consumption in WI group compared to placebo. Lower acetaminophen requirement during the first 96 postoperative hours in the WI group compared with the control group. No difference in the consumption of nefopam during the 96 postoperative hours between the groups. |
| Sun et al. | Elective open liver resection | RCT N = 53 WI: n = 26 Single shot of 20 mL of 0.75% ropivacaine at the end of the surgery Control group: n = 27 with saline Postoperative analgesia for both groups: IV-PCA with sufentanil at a rate of 2 μg/h and a bolus of 0.5 μg. | Postoperative hours: 0, 6, 12, 24, 48 | VAS score at rest and on movement | Total opioid consumption, MAP, HR, time to bowel recovery LOS PONV Concentration of epinephrine, norepinephrine, and cortisol in serum plasma | Lower VAS scores at rest and on movement at 0, 6, and 12 h postop in the WI group compared with the control group. No differences in VAS scores at rest and on movement at 24 h and 48 h between the two groups. Lower MAP and HR, total opioid consumption, shortened time to bowel recovery and LOS in the WI group than in the control group. No difference in the incidence of PONV between the groups. Lower levels of epinephrine, norepinephrine, and cortisol in the WI group than in the control group. |
| Xin et al. | Elective open liver resection | RCT N = 39 WI: n = 19 At the end of surgery 20 mL of 0.5% ropivacaine and then 0.3% ropivacaine at a rate of 2 mL/h per side. Control group: n = 20 with placebo Postoperative analgesia for both groups: PCA with sufentanil with no constant infusion | Postoperative hours: first 48 | NRS score at rest and on movement | Opioid consumption PONV Sedation score Time to bowel recovery Liver function change Patient satisfaction LOS | Lower pain scores at rest after 8 and 16 postoperative hours in the WI group than in the control group. No differences in NRS scores on movement at any time in postoperative period between the groups. Reduced opioid consumption, time to bowel recovery, incidences of PONV and LOS in the WI group. Comparable sedation score and liver function change in the groups. No differences in patient satisfaction between the groups. |
| Wu et al. | Elective open liver resection | RCT N = 60 WI: n = 20 50 mL 0.25% ropivacaine on closure of the wound followed by 5 mL/h constant flow for 48 h IV-PCA: n = 20 With fentanyl Control group: n = 20 with tramadol injection according to the NRS scoring system. | Postoperative hours: 6, 12, 24, 48 | NRS score | Side effects of analgesia Hepatic dysfunction (ALT value) Indicators of rehabilitation Wound healing | Lower NRS scores, reduced rate of analgesic usage, ambulation time, and GI function recovery time in the WI group than in the control group. Lower NRS scores at 12 postoperative hours in WI group than in IV-PCA group with no differences at the later time points. Increased mean survival time in WI and IV-PCA groups than in the control group. More side effects of analgesia and hepatic dysfunction in the IV-PCA group than in the WI and control groups with no differences between the WI and the control group. Higher incidences of incision exudation in the WI group than in the IV-PCA group and the control group. |
| Hernandez et al. | Elective open liver resection | Retrospective study N = 232 TAP block alone: n = 16 Open TAP block 30 mL 0.25% bupivacaine and 20 mL 1.3% liposomal bupivacaine Neuraxial (TEA or intrathecal opioid) alone: n = 66 Intrathecal hydromorphone 75–150 µg or TEA an infusion of bupivacaine 0.075% + hydromorphone 5 µg/mL at 8–12 mL/h. IV-PCA alone: n = 35 Combined neuraxial + TAP block: n = 115 | Postoperative hours: 24, 48 | Median NRS score | Opioid consumption Time to first postoperative opioid administration PONV Complications related to the neuraxial anesthetics LOS | Lower NRS scores in the patients with TAP block than in the IV-PCA alone and neuraxial alone groups. Higher NRS scores in the group with systemic opioids alone than in the other groups. Lower NRS score in TEA+ TAP block than in intrathecal+ TAP block group. Lower opioid consumption in TAP + TEA/intrathecal than in systemic opioid alone group. No difference in incidences of PONV in the first 24 h between the groups. No differences in rate of complications related to the neuraxial technique between TEA+ TAP and intrathecal+ TAP. Longer LOS in systemic opioid alone group. |
| Amundson et al. | Living donor hepatectomy | Retrospective study N = 77 TAP block: n = 29 liposomal bupivacaine (266 mg) mixed with 30 mL of 0.25% plane bupivacaine. Control group: n = 48 Both groups received 100–150 µg intrathecal hydromorphone and 800 mg oral gabapentin. | Postoperative days: 0, 1, 2, 3, 4 | NRS score on PoDs 0 and 1 | NRS score on PoDs 2, 3, and 4. Opioid consumption Treatment of PONV Time to ingestion of clear fluids and a full diet Time to bowel activity LOS | Lower NRS scores and lower opioid consumption on PoD 0 in the TAP block group than in the control group with no differences on subsequent days. Shorter time to full diet, first bowel movement, and flatus in the TAP block group than in the control group. No differences in LOS and incidences of PONV between the groups. |
| Karanicolas et al. | Elective open liver resection | RCT N = 153 MOTAP block: n = 71 40 mL of 0.3% ropivacaine and then 5 mL 0.2% ropivacaine through each catheter for 72 h Control group: n = 82 with placebo. In both groups: IV-PCA and celecoxib | Postoperative hours: 12, 24, 36, 48, 60, 72 | Total opioid consumption over the first 48 postoperative hours | NRS score at rest and on coughing Complications rates LOS | Lower NRS at rest and with coughing at all time points in MOTAP block group than in the control group. Lower opioid consumption in MOTAP block group than in the control group. Shorter LOS in MOTAP block group than in the control group. No difference in complications rate between the groups. |
| Guo et al. | Elective open liver resection | RCT N = 70 OSTAP block: n = 35 40 mL of 0.375% ropivacaine Control group: n = 35 with placebo In both groups: Intraoperatively sufentanil and dexmedetomidine with a loading dose iv administration. Postoperatively IV-PCA with sufentanil and iv parecoxib. | 5 min after extubation and postoperative hours: 2, 4, 12, 24, 48 | Total opioid consumption 24 h after surgery | NRS score at rest and on coughing PONV Time to extubate Side effects of analgesia | Lower NRS scores at rest at 2 h and 4 h postoperatively, and on coughing at all time points in the OSTAP block group than in the control group. Lower opioid consumption at all time points up to 24 h in OSTAP block group. No difference in opioid consumption at 48 h between the groups. Lower incidence of PONV between 4 h and 8 h in OSTAP block group. Reduced extubation time OSTAP block group. No difference in complications rates between the groups. |
| Kıtlık et al. | Living donor hepatectomy | RCT N = 50 TAP block: n = 25 1.5 mg/kg of 0.5% bupivacaine with saline to reach a total volume of 40 mL. IV-PCA: n = 25 In both groups: Intraoperatively remifentanil infusion. IV-PCA with morphine and iv acetaminophen postoperatively. | Postoperative hours: 0, 2, 4, 6, 12 and 24 | Total opioid consumption | VAS score at rest and on movement sedation scores PONV Need for antiemetic medication | Lower VAS scores at rest and movement and lower opioid consumption in the TAP block group than in the control group. No difference between the two groups in terms of PONV and sedation scores. |
| Erdogan et al. | Living donor hepatectomy | RCT N = 44 TAP block: n = 22 1.5 mg/kg of 0.5% bupivacaine with saline to reach a total volume of 40 mL. IV-PCA: n = 22 Intraoperatively remifentanil infusion. In both groups: Postoperatively IV-PCA and iv acetaminophen. | Intraoperative and postoperative 24 h | Intraoperative and postoperative opioid consumption. | Difference in mean MAP and HR Anesthesia recovery time LOS | Reduced perioperative and postoperative opioid consumption in TAP block group No difference in HR and MAP between groups at any time. Shorter anesthesia recovery time in TAP group. Shorter LOS in TAP block group than in the control group. |
| Maeda et al. | Living donor hepatectomy | Retrospective N = 32 TAP block: n = 16 0.25% levobupivacaine 10 mL for each side at the end of surgery and then infusion of 0.125% levobupivacaine at 6 mL/h per side for 48 h IV-PCA: n = 16 With fentanyl In both groups: IV-PCA | Postoperative hours: 3, 6, 12, 24, 48 | Total opioid consumption | VRS score Time to rescue analgesia PONV LOS | Lower VRS scores at 3 and 6 h in the TAP block group than in the control group. No differences in VRS scores at 12, 24, 48 h between the groups. Lower opioid consumption in the TAP block group than in the control group. Longer time and lower incidence of rescue analgesia requests in the TAP block group than in the control group. Lower total number of requests for supplemental analgesia in the TAP block group than in the control group. Lower incidence of PONV in the TAP block group than in the control group. No difference in LOS between the groups. |
| Qiao et al. | Elective open liver resection | Single blind RCT N = 100 TAP block+ parecoxib: n = 51 40 mg of parecoxib 30 min before induction, and 150 mg of 0.375% ropivacaine with 5 mg dexamethasone, before closing the abdominal incision. Control group: n = 49 placebo 30 min before induction, without TAP block. In both groups: Postoperatively IV-PCA with sufentanil and 40 mg of parecoxib every 12 h for 72 h. | Postoperative hours: 24, 48, and 72 | VAS score at rest and on coughing | Adverse events: PONV, pruritus urinary retention, hypotension, respiratory depression. Postoperative ambulation LOS | Lower VAS scores in the study group than in the control group. No differences between the groups in terms of adverse events. Improved ambulation in the study group than in the control group. Shorter LOS in the study group than in the control group. |
| Su et al. | Elective open liver resection | RCT N = 58 TAP block: n = 29 open TAP block 0.2% Ropivacaine 10 mL per side. WI: n = 29 Subcutaneous injection of 20 mL of 0.2% ropivacaine at the incision. In both groups: postoperatively IV-PCA with sufentanil | Postoperative hours: 24, 48 | Total opioid consumption | VAS score Time to first flatus PONV LOS | No differences in VAS score between the groups. Lower opioid consumption in the first 24 postoperative hours in the TAP group than in the WI group. Shorter time to first flatus in the TAP block group than in the WI group. No differences in incidence of PONV and LOS. |
| Hausken et al. | Elective open liver resection | RCT N = 143 TEA: n = 77 0.1% bupivacaine with fentanyl 2 mcg/mL and epinephrine 2 mcg/mL at a rate of 5 to 15 mL/h, with 2 boluses of 5 mL allowed per hour. iv acetaminophen (1 g every 6 h) IV-PCA: n = 66 with Ketomebidone 1 mg boluses with an 8 min lockout interval (max. 7 mg/h) with no basal infusion. iv acetaminophen (1 g every 6 h) and iv ketorolac (30 mg every 8 h) on POD 0 to POD 2. Wound infiltration with 20 mL 0.5% bupivacaine before skin closure | Postoperative days: 0, 1, 2, 3, 4, 5 | Mean NRS score | Opioid consumption on PODs 0 to 2; side effects of analgesia, intraoperative blood loss, fluid requirements; need for vasoactive medication; days until discontinuation of TEA or IV-PCA; time in operating room and PACU; surgical complications, LOS | No difference in mean NRS score between the groups. Lower NRS scores in the TEA group on PODs 0 and 1, but higher or equal on PODs 2 and 5 when compared to the IV-PCA group. Lower total opioid consumption in the first 3 days in the IV-PCA group. Earlier discontinuation of pumps in the IV-PCA group than in the TEA group. No difference in bleeding, intraoperative fluid requirements, and blood transfusions between the groups. No incidences of postoperative liver failure. Greater incidence of pruritus in the TEA group. Shorter LOS for patients in the IV PCA group than in the TEA group. |
| Aloia et al. | Elective open liver resection (n = 136)/pancreatic surgery (n = 4) | RCT N = 140 TEA: n = 106 3–10 mL of 2% lidocaine before surgical incision. Continuous infusion of bupivacaine 0.075%+ 0.5% hydromorphone at 5–8 mL/h. Postoperatively infusion rate: 5–8 mL/h. IV-PCA: n = 34 Intraoperative iv opioids and then IV-PCA with hydromorphone infusion with no basal rate, 0.2 mg every 10 min of demand dosing, and a 0.5 mg nursing bolus every 1 h as needed for additional pain control. | Postoperative hours: first 48 | NRS/VAS scores severe pain event rates (pain scores >7) | Patient satisfaction Total opioid consumption till PoD5 Surgical complications Side effects of analgesia Patients satisfaction LOS | Lower pain scores and severe pain event rates in the TEA group than in the IV-PCA group. Lower opioid consumption in the TEA group than in the control group. Greater patient satisfaction with pain control in the TEA group than in the IV-PCA group. No difference in rates of side effects of analgesia, surgical complication rates, and LOS between the groups. |
| Ganapathi et al. | Elective open liver resection | Prospective study N = 70 TEA 0.1% bupivacaine with fentanyl 2 μg/mL infusions intraoperatively and then TEA–PCA with extra 3 mL bolus and continuous infusion rate of 3–12 mL/h. All patients received iv acetaminophen (1 g every 6 h) | Postoperative days: 0, 1, 2, 3 | Success rate of epidural catheter placement | VDS score Postoperative chest infection LOS | TEA success rate of 91%. Pain relief was effective in 91% of patients with successful TEA placement. 7% had chest infection. No difference in LOS based on the success in epidural analgesia. |
| Aydogan et al. | Living donor hepatectomy | RCT N = 40 TEA: n = 20 Intraoperative iv remifentanil infusion and TEA with morphine 2 mg 15 minbefore the completion of surgery. Postoperatively TEA-PVA infusion with no basal infusion. IV-PCA: n = 20 Intraoperative iv remifentanil infusion and iv morphine 5 mg 15 min before the completion of surgery. Postoperatively IV-PCA with morphine infusion with no basal infusion. | Postoperative hours: 2, 4, 12, 24 | VAS score at rest and on movement | Total opioid consumption | Lower VAS scores at rest and at movement in TEA group than in the IV-PCA group. Lower total opioid consumption in TEA group than in IV-PCA group. |
| Wang et al. | Elective open liver resection | RCT N = 80 Parecoxib+ IV-PCA: n = 40 40 mg parecoxib 30 min before induction, followed by 40 mg every 12 h for 48 h after surgery. Control group: n = 40 with saline In both groups: IV-PCA with fentanyl. | Postoperative hours: 2, 6, 12, 24, 48 | VAS score at rest and on coughing | Opioid consumption, side effects of analgesia Immune response | Lower VAS scores at 2, 6, 12, and 24 h after surgery in parecoxib group than in the control group. No differences in VAS scores between the two groups at 48 h after surgery. Lower total opioid consumption in the parecoxib group than in the control group. No differences in the incidence of side effects of analgesia between the groups. Longer median disease-free survival time of patients in the parecoxib group than in the control group. No difference in overall survival between the groups. Higher percentages of CD3+ T cells at 24 h after surgery in the parecoxib group than that in control group. Higher percentages of NK cells in the parecoxib group than that of control group. |
| Chen MT et al. | Elective open liver resection | RCT N = 56 Parecoxib+ IV-PCA: n = 28 40 mg parecoxib before induction followed by 40 mg every 12 h and IV-PCA with sufentanil. Control group: n = 28 Saline+ IV-PCA with sufentanil in the same regimen | Postoperative hours: 6, 18, 30, 42, 54, 66 | VAS score at rest and on movement | Opioid consumption, rescue analgesic, Side effects of analgesia Time to first flatus and exercise on floor. | Lower VAS scores on 30–54 postoperative hours, lower opioid consumption, rescue analgesic usage and rate of incidence of PONV in the parecoxib group than in the control group. No differences in time to first flatus and exercise on floor between the groups. Greater decrease in systemic erythrocyte sedimentation rate, IL-4 concentrations at 48 h after surgery in parecoxib group than in the control group. Higher level of TGF-beta after surgery in the parecoxib group than in the control group. |
| Lim et al. | Living donor hepatectomy | Retrospective study N = 50 Ketorolac+ IV-PCA: n = 29 PCA with morphine and ketorolac 1.87 mg/mL postoperatively. Bolus: 0.8 to 1.0 mL with the 4-h maximal dose 16 to 20 mL. Parecoxib+ IV-PCA: n = 21 Single dose of IV parecoxib 40 mg 30 min before the end of surgery and then plain PCA with morphine postoperatively. | Postoperative days: 1, 2, 3 | VAS score | Opioid consumption, need for rescue analgesia Side effects of analgesia Satisfaction score | No difference in the VAS scores between the groups. No difference in total opioid consumption, satisfactory score, the incidence of side effects, and the need of rescue analgesia between the groups. |
| Tang et al. | Elective open liver resection | Retrospective study N = 216 ITM: n = 125 150–500 µg of morphine with bupivacaine and clonidine intrathecally before skin incision Control group: n = 91 Postoperatively both groups received PCA with morphine | Postoperative days: 1, 2, 3 | Opioid consumption on POD 1 | NRS scores at rest and on movement over the first 24 postoperative hours Opioid consumption on POD 1, 2 and 3. Side effects of analgesia LOS | Lower NRS scores at rest and on movement on POD 1 in the ITM group than in the IV-PCA group with no differences afterward. Lower opioids consumption on POD 1 in the ITM group than in the IV-PVA group with no differences afterward. No differences in time to full ward diet and mobilization, side effects of analgesia, complications, LOS, and 30-day readmission between the groups. Higher hospital costs in the ITM group. |
| Dichtwald et al. | Elective open liver resection/pancreatic surgery | RCT N = 49 ITM: n = 23 4 µg/kg of morphine intrathecally before skin incision and intraoperatively iv remifentanil infusion IV-PCA: n = 26 remifentanil infusion during surgery followed by iv bolus of morphine 0.15 mg/kg before the end of surgery Postoperatively in both groups: PCA with morphine | Postoperative hours: 12, 24, 36, 48, 60, 72 | NRS score at rest and on coughing | Total opioid consumption Need for rescue analgesic drugs Side effects of analgesic technique Functional recovery time | Lower NRS scores in the ITM group than in the IV-PCA group. No difference in total opioid consumption between the groups. Need for additional rescue opioid often in IV-PCA group. No differences in complication and side effects related to the analgesia and recovery parameters between the groups. |
| Niewiński et al. | Elective open liver resection | RCT N = 36 ITM: n = 18 0.4 mg of morphine before skin incision and intraoperatively iv remifentanil infusion IV-PCA: n = 18 Intraoperatively iv remifentanil infusion and single dose iv morphine (0.15 mg/kg) 30 min before extubation. Postoperatively in both groups: PCA with morphine, iv acetaminophen (1 g every 6 h) and iv dexketoprofen (50 mg every 8 h) | Postoperative hours: 12, 24, 36, 48, 60, 72 | NRS score at rest | NRS score on coughing Total opioid consumption Functional recovery time sedation grade Complication rate LOS | Lower NRS scores at rest at 12 and 24 h postoperative hour in the ITM group with no differences afterward and on coughing. No differences in total opioid consumption, sedation grade, complication rate, and functional recovery time. Shorter LOS in the ITM group than in the IV-PCA. |
| Kasivisvanathan et al. | Elective open liver resection | Prospective study N = 73 ITM: n = 37 5 µg/kg of morphine before skin incision in combination 2.5–3 mL of 0.5% heavy bupivacaine+ postoperative IV-PCA with fentanyl TEA: n = 36 7–10 mL of 0.125% bupivacaine with 2 mcg/mL fentanyl and then 5–10 mL/h continuous infusion. Postoperatively in both groups: Iv acetaminophen and tramadol. | Postoperative hours: 12, 24, 36, 48, 60, 72, 84, 96 | LOS | VAS score on coughing Opioid requirements Blood loss CVP Fluid requirements Hemodynamic stability Time to fluid/solid intake Mobilization Quality of recovery | Lower VAS score on coughing in the first 12 postoperative hours in TEA group than in the ITM group with no differences afterward. Shorter LOS in ITM group than in the TEA group. Higher opioid consumption, intraoperative CVP and blood loss in ITM group than in the TEA group. Faster mobilization, lower iv fluid administration, and vasopressors requirement in ITM group than in the TEA group. No difference in quality of recovery and mortality and morbidity between the groups. |
| Schreiber et al. | Elective open liver resection | RCT N = 80 TEA: n = 41 Intraoperative iv remifentanil infusion then TEA with 0.2% ropivacaine infusion at 5–6 mL/h starting after completion of the liver resection portion of surgery and continued postoperatively with max infusion rate of 8 mL/h. btPVB: n = 39 0.5% ropivacaine 15 mL each side before the surgery. Intraoperative iv remifentanil infusion and btPVB with 0.2% ropivacaine infusion at 7 mL/h each side starting after completion of the liver resection portion of surgery and continued postoperatively with max infusion rate of 12 mL/h each. Postoperatively in both group: IV-PCA with hydromorphone to maintain VRS < score 6. Adjuvant analgesics: iv ketorolac, acetaminophen, or low-dose ketamine infusion (5–10 mg·h−1) | Postoperative hours: 24, 48 | VRS score at rest and with postoperative incentive spirometry | Total opioid consumption inspired volumes during incentive spirometry Measures of hemodynamic stability (intraoperative and postoperative fluid and vasopressor requirement) Side effects of analgesia LOS | Lower VRS score in the TEA group than in the btPVB group. No difference in total opioid consumption between the groups. No differences in rate of side effects of analgesia and LOS. No difference in maximal tidal volumes between the groups. Greater decrease in MAP 24 h-postoperatively compared with baseline in the TEA group than in the bTPVB group. No difference in vasopressive drugs and fluids given between the groups. |
| Chen H et al. | Elective right- lobe hepatectomy | RCT N = 44 Continuous right tPVB: n = 22 right T7 with 10 mL bolus of 0.2% ropivacaine before emergence, then continuous infusion of 6 mL/h for 24 h. Control group: n = 22 with saline. In both groups: remifentanil infusion during the surgery. Postoperatively IV-PCA with sufentanil. | Postoperative hours: 24 | Total opioid consumption during | NRS score at rest and on coughing at 1, 4, 8, 16, and 24 postoperative hours Need of the rescue analgesia side effects of analgesia patients Satisfaction scores LOS | Lower opioid consumption at 24 postoperative hours in the PVB group than in the control group. Lower NRS scores in the PVB group than in the control group at rest and with coughing for the first 24 h. Higher patients satisfaction score in the PVB group than in the control group. No differences in the incidence of need for rescue analgesia, PONV, bloating, excessive sedation, and the LOS between the groups. |
| Mistry et al. | Living donor hepatectomy | Retrospective study N = 26 Right PVB: n = 16 0.2% ropivacaine infusion at a rate of 10–14 mL/h up to 7 days. Non-PVB: n = 10 Postoperatively both groups received PCA with hydromorphone | Postoperative hours: 24, 48, 72 | Total opioid consumption | NRS score Side effects of analgesia | No difference in NRS between the groups. Lower opioid consumption in PVB group than in the control group. No difference in rates of side effects of analgesia. |
| Zhu et al. | Elective open liver resection | RCT N = 63 QLB: n = 32 30 min before induction 0.4% ropivacaine at 0.6 mL/kg and then ropivacaine 0.2%, at 5 mL/h continuous infusion, 5 mL bolus dose IV-PCA: n = 31 with sufentanil | Postoperative hours: 0.5, 2, 6, 12, 24, 48 | NRS score at rest and on coughing | Time to first out-of-bed activity Self-administered analgesic counts Rate of rescue analgesia Total dose of propofol and remifentanil during surgery. Time to recovery after anesthestia | Lower NRS scores on coughing and at all time points in the QLB group than in the IV-PCA group. No differences of postoperative self-administered analgesic counts, rate of rescue analgesic usage, and incidences of analgesic-related side effects between the groups. Lower intraoperative consumption of propofol and remifentanil in QLB group. Faster recovery from anesthesia and earlier time to first out-of-bed activity QLB group. |
| Zhang et al. | Elective open liver resection | RCT N = 52 Dexmedetomidine group (DEX): n = 26 Dexmedetomidine infusion at an initial loading dose of 0.5 µg/kg before intubation then 0.3 µg/kg/h till the end of surgery. After surgery, for 48 h, 60 mg oxycodone and 360 µg dexmedetomidine diluted to 120 mL and administered at a bolus dose of 2 mL, with 5 min lockout interval and a 1 h limit of 20 mL. Control group: n = 26 with saline. Postoperatively: 60 mg Oxycodone alone with the same regimen | Postoperative hours: 1, 4, 8, 12, 24, 48 | Total opioid consumption | VAS score at rest and on movement Requirement of narcotic and vasoactive drugs, haemodynamic parameters, side effects of analgesia patient satisfaction First exhaust time | Lower VAS scores at rest at 1, 4, and 8 h postoperative and with cough at 24, and 48 h after surgery in the DEX group than in the control group. Lower opioid consumption after surgery in the DEX group than in the control group. Higher patient satisfaction with pain control, shorter time to the first exhaust, and less incidence of PONV in the DEX group than in the control group. No difference in sedation between the groups. Decreased intraoperative consumption of propofol and remifentanil during surgery in the DEX group compared with the control group. |
| Masgoret et al. | Elective open liver resection | Prospective observational study N = 44 TEA with ketamine: n = 23 6 mL of ketamine 0.5 mg/kg + morphine 4 mg + 1% of lidocaine before surgical incision. No infusion during surgery. Before skin closure iv bolus of morphine 0.05 mg/kg and then the TEA–PCA pump 5 mL/h of ketamine 1.5 mg/mL+ morphine 15 µg/mL + ropivacaine 0.15%a till PoD5 IV-PCA: n = 21 iv bolus of ketamine 0.5 mg/kg before surgical incision and iv infusion of morphine 0.025 mg/kg/h during surgery iv bolus of morphine 0.05 mg/kg before skin closure and then IV-PCA 1 mL/h of ketamine 7.5 mg/mL +morphine 1 mg/mL+ ketorolac 1.5 mg/mL till PoD5 Both groups received iv acetaminophen (1 g every 6 h) | Preoperatively and postoperative hours: 2, 24, and after 7 days, 1 month, and 6 months | Persistent postoperative pain: VAS, NPSI, PCS and QST | Side effects: PONV, hemodynamic side effects (new onset arrhythmia or 20% deviation in MAP, cognitive side effects, and need for vasoactive drugs or transfusion. LOS | No differences in VAS scores between the groups. No not-controlled pain (VAS > 3) at 1 or 6 months. No difference in persistent postoperative pain incidence between the groups. Cognitive side effects were higher in IV group. No differences between the groups in other side effects. Median hospital LOS was 10 days in both groups. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, P.; Zawadka, M.; Andruszkiewicz, P.; Gelo, R.; Pugliese, F.; Bilotta, F. Postoperative Analgesia after Open Liver Surgery: Systematic Review of Clinical Evidence. J. Clin. Med. 2021, 10, 3662. https://doi.org/10.3390/jcm10163662
Dudek P, Zawadka M, Andruszkiewicz P, Gelo R, Pugliese F, Bilotta F. Postoperative Analgesia after Open Liver Surgery: Systematic Review of Clinical Evidence. Journal of Clinical Medicine. 2021; 10(16):3662. https://doi.org/10.3390/jcm10163662
Chicago/Turabian StyleDudek, Paula, Mateusz Zawadka, Paweł Andruszkiewicz, Remigiusz Gelo, Francesco Pugliese, and Federico Bilotta. 2021. "Postoperative Analgesia after Open Liver Surgery: Systematic Review of Clinical Evidence" Journal of Clinical Medicine 10, no. 16: 3662. https://doi.org/10.3390/jcm10163662
APA StyleDudek, P., Zawadka, M., Andruszkiewicz, P., Gelo, R., Pugliese, F., & Bilotta, F. (2021). Postoperative Analgesia after Open Liver Surgery: Systematic Review of Clinical Evidence. Journal of Clinical Medicine, 10(16), 3662. https://doi.org/10.3390/jcm10163662





