Severe Myocardial Dysfunction after Non-Ischemic Cardiac Arrest: Effectiveness of Percutaneous Assist Devices
Abstract
:1. Introduction
2. Material and Methods
2.1. Animal Preparation
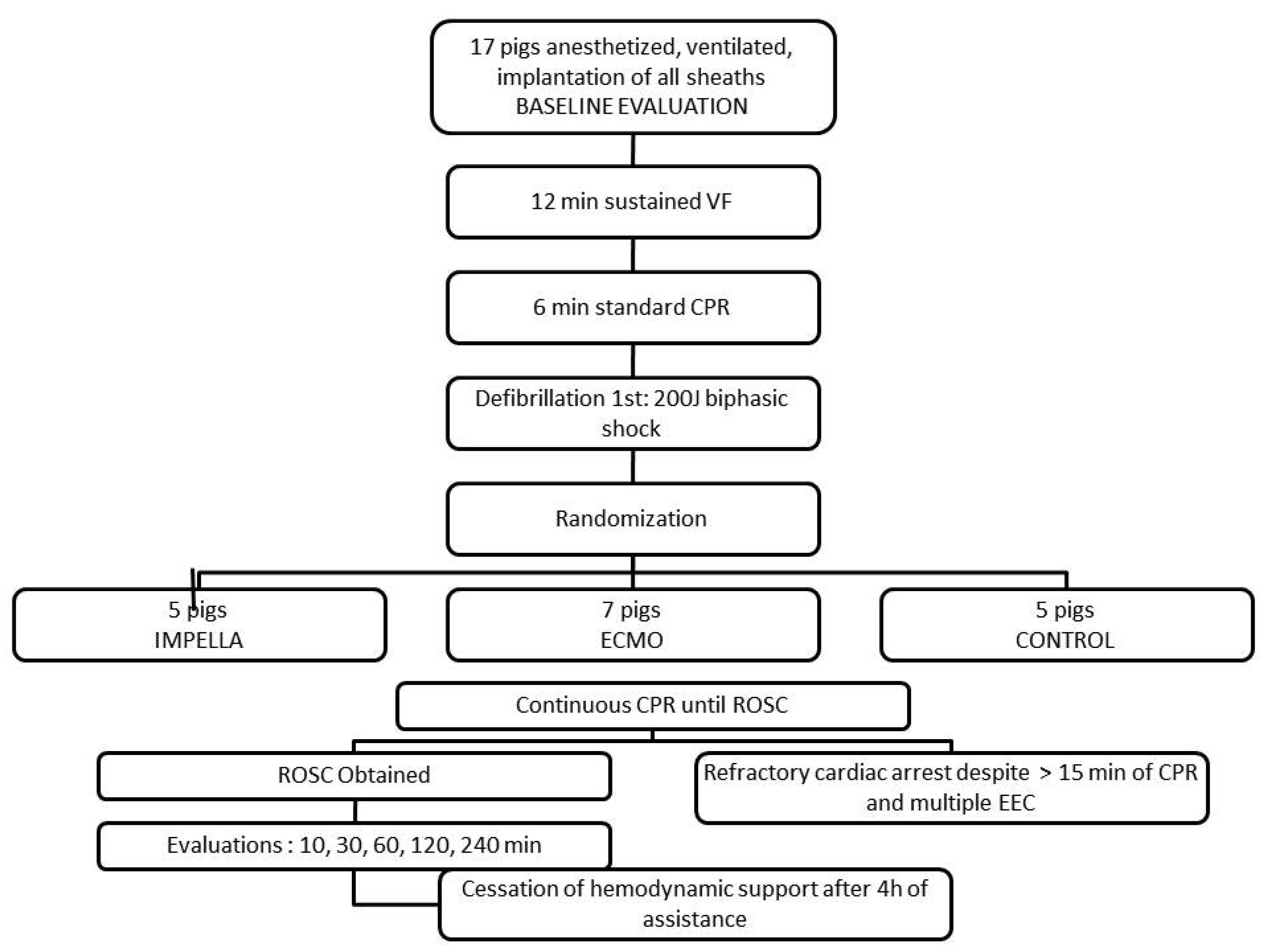
2.2. Experimental Protocol
2.3. Data Analysis
- (1)
- LV function (LVEF) after 4 h post-resuscitation measured by fluoroscopy (RAO 30° incidence) using an injection of 12 mL contrast medium (Visipaque®, GE Healthcare SAS, Boston, MA, USA) [26]
- (2)
- Myocardial function: Left ventricular end-diastolic pressure was measured by the pigtail, cardiac outflow, systolic ejection volume (mL/min), left ventricular systolic and diastolic volumes measured by fluoroscopy (RAO 30° incidence), and calculated using the standard software of the angiography suite;
- (3)
- Hemodynamic efficacy assessed based on systolic, mean and diastolic arterial pressures, central venous pressure, and pulmonary arterial pressure.
2.4. Statistical Analysis
3. Results
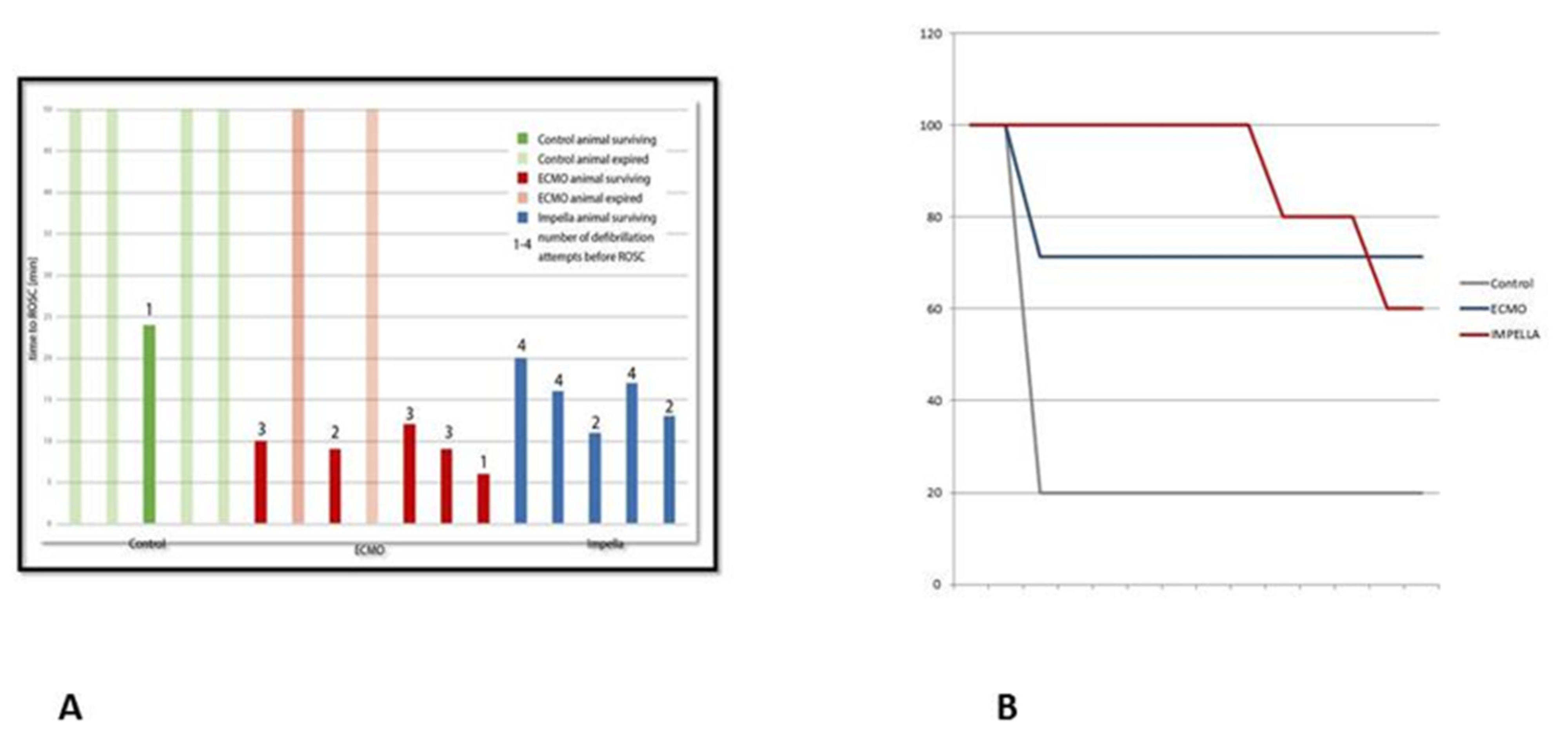
3.1. Resuscitation, ROSC and Survival
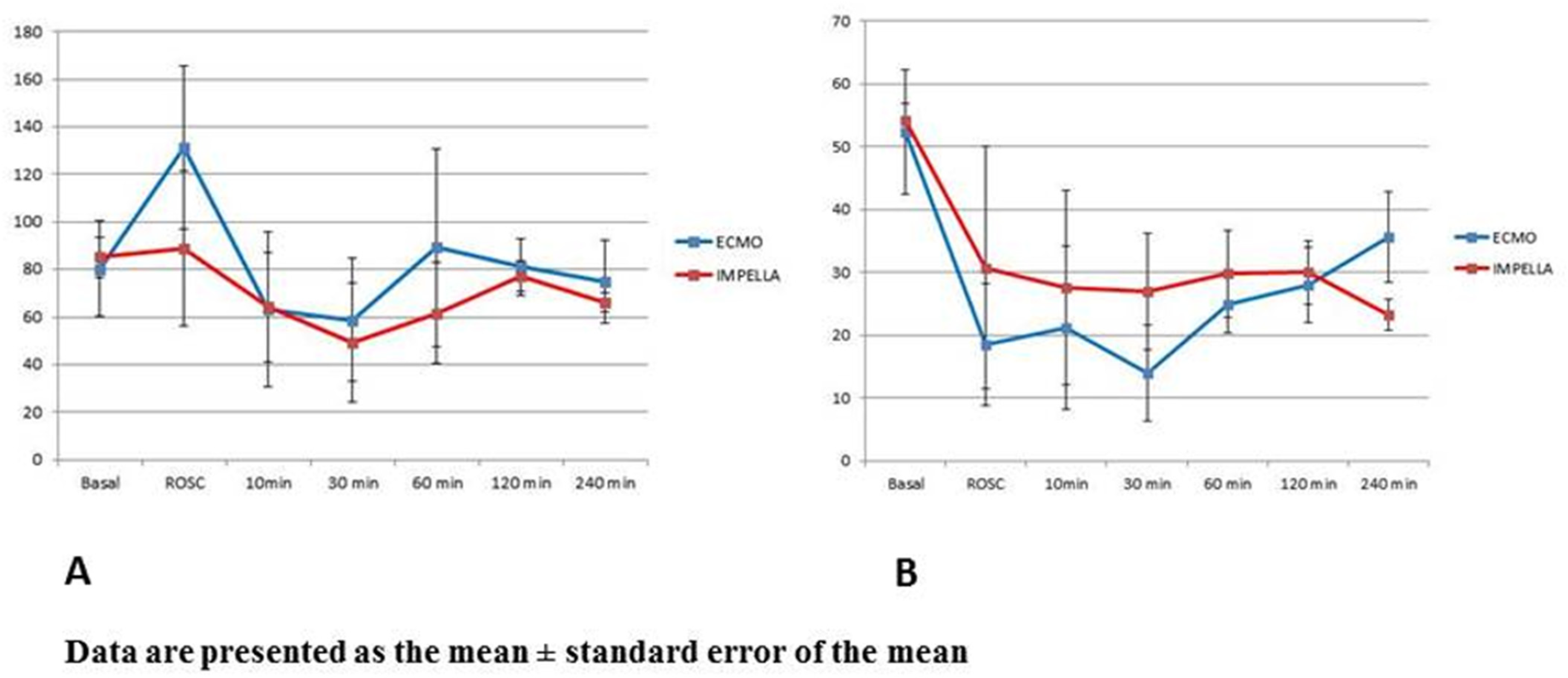
3.2. Mechanical Circulatory Support and Hemodynamic
4. Discussion
4.1. Effectiveness of Prompt Cardiac Assistance
4.2. Hemodynamic Support
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| CA | Cardiac arrest |
| CAD | Coronary artery disease |
| CO | Cardiac output |
| CPR | Cardiopulmonary resuscitation |
| ECMO | Extracorporeal membrane oxygenation |
| FiO2 | Inspired oxygen fraction |
| HR | Heart rate |
| IV | Intravenous |
| LV | left ventricle |
| LVEDP | Left ventricle end diastolic pressure |
| LVEDV | Left ventricle end diastolic volume |
| LVEF | Left ventricle ejection fraction |
| MAP | Mean arterial pressure |
| MPAP | Mean pulmonary artery pressure |
| OHCA | Out hospital cardiac arrest |
| PaCO2 | Arterial carbon dioxide tension |
| PaO2 | Arterial oxygen tension |
| ROSC | Return of spontaneous circulation |
| VF | Ventricular fibrillation. |
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report from the American Heart Association. Circulation 2018, 137, e67–e492. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, M.S.; Mengert, T.J. Cardiac resuscitation. N. Engl. J. Med. 2001, 344, 1304–1313. [Google Scholar] [CrossRef]
- Bunch, T.J.; White, R.D.; Gersh, B.J.; Meverden, R.A.; Hodge, D.O.; Ballman, K.V.; Hammill, S.C.; Shen, W.K.; Packer, D.L. Long-term outcomes of out-of-hospital cardiac arrest after successful early defibrillation. N. Engl. J. Med. 2003, 348, 2626–2633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumas, F.; Cariou, A.; Manzo-Silberman, S.; Grimaldi, D.; Vivien, B.; Rosencher, J.; Empana, J.-P.; Carli, P.; Mira, J.-P.; Jouven, X.; et al. Immediate Percutaneous Coronary Intervention Is Associated with Better Survival After Out-of-Hospital Cardiac Arrest: Insights from the PROCAT (Parisian Region Out of Hospital Cardiac Arrest) Registry. Circ. Cardiovasc. Interv. 2010, 3, 200–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubb, N.R.; Elton, R.A.; Fox, K.A. In-hospital mortality after out-of-hospital cardiac arrest. Lancet 1995, 346, 417–421. [Google Scholar] [CrossRef]
- Herlitz, J.; Engdahl, J.; Svensson, L.; Angquist, K.A.; Silfverstolpe, J.; Holmberg, S. Major differences in 1-month survival between hospitals in Sweden among initial survivors of out-of-hospital cardiac arrest. Resuscitation 2006, 70, 404–409. [Google Scholar] [CrossRef] [PubMed]
- Neumar, R.W.; Nolan, J.P.; Adrie, C.; Aibiki, M.; Berg, R.A.; Bottiger, B.W.; Callaway, C.; Clark, R.S.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008, 118, 2452–2483. [Google Scholar]
- Berdowski, J.; Berg, R.A.; Tijssen, J.G.; Koster, R.W. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation 2010, 81, 1479–1487. [Google Scholar] [CrossRef]
- Wang, H.E.; Devlin, S.M.; Sears, G.K.; Vaillancourt, C.; Morrison, L.J.; Weisfeldt, M.; Callaway, C.W. Regional variations in early and late survival after out-of-hospital cardiac arrest. Resuscitation 2013, 83, 1343–1348. [Google Scholar] [CrossRef] [Green Version]
- Cerchiari, E.L.; Safar, P.; Klein, E.; Cantadore, R.; Pinsky, M. Cardiovascular function and neurologic outcome after cardiac arrest in dogs. The cardiovascular post-resuscitation syndrome. Resuscitation 1993, 25, 9–33. [Google Scholar] [CrossRef]
- Yannopoulos, D.; Matsuura, T.; McKnite, S.; Goodman, N.; Idris, A.; Tang, W.; Aufderheide, T.P.; Lurie, K.G. No assisted ventilation cardiopulmonary resuscitation and 24-hour neurological outcomes in a porcine model of cardiac arrest. Crit. Care Med. 2010, 38, 254–260. [Google Scholar] [CrossRef]
- Kern, K.B.; Hilwig, R.W.; Rhee, K.H.; Berg, R.A. Myocardial dysfunction after resuscitation from cardiac arrest: An example of global myocardial stunning. J. Am. Coll. Cardiol. 1996, 28, 232–240. [Google Scholar] [CrossRef] [Green Version]
- Lamhaut, L.; Jouffroy, R.; Soldan, M.; Phillipe, P.; Deluze, T.; Jaffry, M.; Dagron, C.; Vivien, B.; Spaulding, C.; An, K.; et al. Safety and feasibility of prehospital extra corporeal life support implementation by non-surgeons for out-of-hospital refractory cardiac arrest. Resuscitation 2013, 84, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Yannopoulos, D.; Bartos, J.; Raveendran, G.; Walser, E.; Connett, J.; Murray, T.A.; Collins, G.; Zhang, L.; Kalra, R.; Kosmopoulos, M.; et al. Advanced reperfusion strategies for patients with out-of-hospital cardiac arrest and refractory ventricular fibrillation (ARREST): A phase 2, single centre, open-label, randomised controlled trial. Lancet 2020, 396, 1807–1816. [Google Scholar] [CrossRef]
- Mork, S.R.; Stengaard, C.; Linde, L.; Moller, J.E.; Jensen, L.O.; Schmidt, H.; Riber, L.P.; Andreasen, J.B.; Thomassen, S.A.; Laugesen, H.; et al. Mechanical circulatory support for refractory out-of-hospital cardiac arrest: A Danish nationwide multicenter study. Crit. Care 2021, 25, 174. [Google Scholar] [CrossRef]
- Rihal, C.S.; Naidu, S.S.; Givertz, M.M.; Szeto, W.Y.; Burke, J.A.; Kapur, N.K.; Kern, M.; Garratt, K.N.; Goldstein, J.A.; Dimas, V.; et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J. Am. Coll. Cardiol. 2015, 65, e7–e26. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.S.; Lin, J.W.; Yu, H.Y.; Ko, W.J.; Jerng, J.S.; Chang, W.T.; Chen, W.J.; Huang, S.C.; Chi, N.H.; Wang, C.H.; et al. Cardiopulmonary resuscitation with assisted extracorporeal life-support versus conventional cardiopulmonary resuscitation in adults with in-hospital cardiac arrest: An observational study and propensity analysis. Lancet 2008, 372, 554–561. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Schotborgh, J.V.; Limpens, J.; Sjauw, K.D.; Engstrom, A.E.; Lagrand, W.K.; Cherpanath, T.G.V.; Driessen, A.H.G.; de Mol, B.; Henriques, J.P.S. Extracorporeal life support during cardiac arrest and cardiogenic shock: A systematic review and meta-analysis. Intensiv. Care Med. 2016, 42, 1922–1934. [Google Scholar] [CrossRef] [Green Version]
- Cheng, R.; Hachamovitch, R.; Kittleson, M.; Patel, J.; Arabia, F.; Moriguchi, J.; Esmailian, F.; Azarbal, B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1866 adult patients. Ann. Thorac. Surg. 2014, 97, 610–616. [Google Scholar] [CrossRef]
- Manzo-Silberman, S.; Fichet, J.; Mathonnet, A.; Varenne, O.; Ricome, S.; Chaib, A.; Zuber, B.; Spaulding, C.; Cariou, A. Percutaneous left ventricular assistance in post cardiac arrest shock: Comparison of intra aortic blood pump and IMPELLA Recover LP2.5. Resuscitation 2013, 84, 609–615. [Google Scholar] [CrossRef]
- Panagides, V.; Vase, H.; Shah, S.P.; Basir, M.B.; Mancini, J.; Kamran, H.; Batra, S.; Laine, M.; Eiskjaer, H.; Christensen, S.; et al. Impella CP Implantation during Cardiopulmonary Resuscitation for Cardiac Arrest: A Multicenter Experience. J. Clin. Med. 2021, 10, 339. [Google Scholar] [CrossRef] [PubMed]
- Lamhaut, L.; Hutin, A.; Puymirat, E.; Jouan, J.; Raphalen, J.H.; Jouffroy, R.; Jaffry, M.; Dagron, C.; An, K.; Dumas, F.; et al. A Pre-Hospital Extracorporeal Cardio Pulmonary Resuscitation (ECPR) strategy for treatment of refractory out hospital cardiac arrest: An observational study and propensity analysis. Resuscitation 2017, 117, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Lamhaut, L.; Jouffroy, R.; Kalpodjian, A.; Deluze, T.; Phillipe, P.; Vivien, B.; An, K.; Carli, P. Successful treatment of refractory cardiac arrest by emergency physicians using pre-hospital ECLS. Resuscitation 2012, 83, e177–e178. [Google Scholar] [CrossRef] [PubMed]
- Derwall, M.; Brucken, A.; Bleilevens, C.; Ebeling, A.; Fohr, P.; Rossaint, R.; Kern, K.B.; Nix, C.; Fries, M. Doubling survival and improving clinical outcomes using a left ventricular assist device instead of chest compressions for resuscitation after prolonged cardiac arrest: A large animal study. Crit. Care 2015, 19, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutin, A.; Lamhaut, L.; Lidouren, F.; Kohlhauer, M.; Mongardon, N.; Carli, P.; Berdeaux, A.; Ghaleh, B.; Tissier, R. Early Coronary Reperfusion Facilitates Return of Spontaneous Circulation and Improves Cardiovascular Outcomes After Ischemic Cardiac Arrest and Extracorporeal Resuscitation in Pigs. J. Am. Heart Assoc. 2016, 5, e004588. [Google Scholar] [CrossRef]
- Greene, D.G.; Carlisle, R.; Grant, C.; Bunnell, I.L. Estimation of left ventricular volume by one-plane cineangiography. Circulation 1967, 35, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, N.; Matsuura, T.; Caldwell, E.; Sarraf, M.; McKnite, S.; Zviman, M.; Aufderheide, T.P.; Halperin, H.R.; Lurie, K.G.; Yannopoulos, D. Ischemic postconditioning at the initiation of cardiopulmonary resuscitation facilitates functional cardiac and cerebral recovery after prolonged untreated ventricular fibrillation. Resuscitation 2012, 83, 1397–1403. [Google Scholar] [CrossRef]
- Ong, M.E.H.; Perkins, G.D.; Cariou, A. Out-of-hospital cardiac arrest: Prehospital management. Lancet 2018, 391, 980–988. [Google Scholar] [CrossRef] [Green Version]
- Soar, J.; Donnino, M.W.; Maconochie, I.; Aickin, R.; Atkins, D.L.; Andersen, L.W.; Berg, K.M.; Bingham, R.; Bottiger, B.W.; Callaway, C.W.; et al. 2018 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations Summary. Resuscitation 2018, 133, 194–206. [Google Scholar] [CrossRef] [Green Version]
- Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 1: Introduction to the International Guidelines 2000 for CPR and ECC: A consensus on science. Circulation 2000, 102 (Suppl. 8), I1–I11.
- Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 3: Adult basic life support. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 2000, 102 (Suppl. 8), I22–I59. [Google Scholar]
- Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 6: Advanced cardiovascular life support: Section 3: Adjuncts for oxygenation, ventilation and airway control. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 2000, 102 (Suppl. 8), I95–I104.
- Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 6: Advanced cardiovascular life support: Section 5: Pharmacology I: Agents for arrhythmias. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 2000, 102 (Suppl. 8), I112–I128.
- Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 6: Advanced cardiovascular life support: Section 6: Pharmacology II: Agents to optimize cardiac output and blood pressure. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 2000, 102 (Suppl. 8), I129–I135.
- Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Part 6: Advanced cardiovascular life support: Section 8: Postresuscitation care. The American Heart Association in collaboration with the International Liaison Committee on Resuscitation. Circulation 2000, 102 (Suppl. 8), I166–I171.
- Le Guen, M.; Nicolas-Robin, A.; Carreira, S.; Raux, M.; Leprince, P.; Riou, B.; Langeron, O. Extracorporeal life support following out-of-hospital refractory cardiac arrest. Crit. Care 2011, 15, R29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, K.; Tanno, K.; Hase, M.; Mori, K.; Asai, Y. Extracorporeal cardiopulmonary resuscitation for patients with out-of-hospital cardiac arrest of cardiac origin: A propensity-matched study and predictor analysis. Crit. Care Med. 2013, 41, 1186–1196. [Google Scholar] [CrossRef]
- Chieffo, A.; Dudek, D.; Hassager, C.; Combes, A.; Gramegna, M.; Halvorsen, S.; Huber, K.; Kunadian, V.; Maly, J.; Moller, J.E.; et al. Joint EAPCI/ACVC expert consensus document on percutaneous ventricular assist devices. Eur. Heart J. Acute Cardiovasc. Care 2021, 10, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Brechot, N.; Combes, A. Ten situations in which ECMO is unlikely to be successful. Intensive Care Med. 2016, 42, 750–752. [Google Scholar] [CrossRef]
- Rubertsson, S.; Lindgren, E.; Smekal, D.; Ostlund, O.; Silfverstolpe, J.; Lichtveld, R.A.; Boomars, R.; Ahlstedt, B.; Skoog, G.; Kastberg, R.; et al. Mechanical chest compressions and simultaneous defibrillation vs conventional cardiopulmonary resuscitation in out-of-hospital cardiac arrest: The LINC randomized trial. JAMA 2014, 311, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Fisk, C.A.; Olsufka, M.; Yin, L.; McCoy, A.M.; Latimer, A.J.; Maynard, C.; Nichol, G.; Larsen, J.; Cobb, L.A.; Sayre, M.R. Lower-dose epinephrine administration and out-of-hospital cardiac arrest outcomes. Resuscitation 2018, 124, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Perkins, G.D.; Ji, C.; Deakin, C.D.; Quinn, T.; Nolan, J.P.; Scomparin, C.; Regan, S.; Long, J.; Slowther, A.; Pocock, H.; et al. A Randomized Trial of Epinephrine in Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2018, 379, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Kapur, N.K.; Paruchuri, V.; Urbano-Morales, J.A.; Mackey, E.E.; Daly, G.H.; Qiao, X.; Pandian, N.; Perides, G.; Karas, R.H. Mechanically unloading the left ventricle before coronary reperfusion reduces left ventricular wall stress and myocardial infarct size. Circulation 2013, 128, 328–336. [Google Scholar] [CrossRef] [Green Version]
- Remmelink, M.; Sjauw, K.D.; Henriques, J.P.; de Winter, R.J.; Koch, K.T.; van der Schaaf, R.J.; Vis, M.M.; Tijssen, J.G.; Piek, J.J.; Baan, J., Jr. Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter. Cardiovasc. Interv. 2007, 70, 532–537. [Google Scholar] [CrossRef]
- Manning, J.E. Feasibility of blind aortic catheter placement in the prehospital environment to guide resuscitation in cardiac arrest. J. Trauma Acute Care Surg. 2013, 75 (Suppl. 2), S173–S177. [Google Scholar] [CrossRef] [PubMed]
| CONTROL n = 5 | ECMO n = 7 | IMPELLA n = 5 | p Value ECMO vs. IMPELLA | |
|---|---|---|---|---|
| Epinephrine total dose mg | 6.5 ± 1.9 | 3.6 ± 0.8 | 3.2 ± 0.8 | 0.623 |
| External shock (n) | 4 ± 2.4 | 2.4 ± 0.8 | 3.2 ± 1.1 | 0.2 |
| Total VF Duration (min) | 19 ± 1.7 | 20.7 ± 2.9 | 25 ± 4.8 | 0.13 |
| Total duration chest compressions | 25 ± 11.4 | 10.4 ± 5.8 | 13.4 ± 5.4 | 0.28 |
| ROSC before initiation of assistance n (%) | 1 (20) | 1 (14.3) | 1 (20) | 1 |
| Success n (%) | 1(20) | 5 (71.4) | 5 (100) | 0.6 |
| Basal ECMO = 7 Impella = 5 | ROSC ECMO = 5 Impella = 5 | 10 min ECMO = 5 Impella = 5 | 30 min ECMO = 4 Impella = 5 | 60 min ECMO = 4 Impella = 5 | 120 min ECMO = 4 Impella = 4 | 240 minECMO = 4 Impella = 3 | |
|---|---|---|---|---|---|---|---|
| LVEDV | |||||||
| ECMO = | 45.2 ± 11.2 | 45.9 ± 13.5 | 49.6 ± 12.1 | 56.3 ± 2.9 | 54.8 ± 8.9 | 49.5 ± 6.4 | 41.9 ± 15.8 |
| Impella = | 53.8 ± 18.6 | 35.4 ± 12.8 | 31.9 ± 17.7 | 42.7 ± 15.5 | 44.7 ± 13.1 | 51.9 ± 15.8 | 62.6 ± 13.5 |
| LVESV | |||||||
| ECMO = | 21.0 ± 7.8 | 38.6 ± 15.0 | 40.2 ± 15.9 | 48.6 ± 6.5 | 41.1 ± 8.8 | 36.0 ± 7.2 | 26.9 ± 7.2 |
| Impella = | 24.6 ± 8.7 | 25.7 ± 15.9 | 28.48 ± 9.5 | 31.6 ± 14.0 | 31.7 ± 11.3 | 40.3 ± 15.4 | 48.1 ± 15.4 |
| LVEDP (mmHg) | |||||||
| ECMO = | 5.86 ± 7.9 | 11.6 ± 16.5 | 13.0 ± 10.1 | 14.5 ± 5.3 | 3.75 ± 1.5 | 4.5 ± 4.0 | 4.5 ± 5.8 |
| Impella = | 8.6 ± 6.3 | 20.2 ± 17.9 | 16.4 ± 12.4 | 13.25 ± 9.4 | 11.6 ± 7.8 | 8.75 ± 7.7 | 12.67 ± 10.8 |
| Cardiac Output (L/min) | |||||||
| ECMO = | 6.20 ± 2.36 | 6.15 ± 3.53 | 5.78 ± 3.36 | 3.70 † | 4.0 ± 2.12 | 5.56 ± 0.83 | 7.73 ± 2.75 |
| Impella = | 5.98 ± 1.28 | 4.03 ± 3.21 | 4.47 ± 1.42 | 2.10 † | 4.3 ± 2.49 | 7.68 ± 2.09 | 5.73 ± 1.09 |
| Basal ECMO = 7 Impella = 5 | ROSC ECMO = 5 Impella = 5 | 10 min ECMO = 5 Impella = 5 | 30 min ECMO = 4 Impella = 5 | 60 min ECMO = 4 Impella =5 | 120 min ECMO = 4 Impella = 4 | 240 min ECMO = 4 Impella = 3 | |
|---|---|---|---|---|---|---|---|
| HR (bpm) | |||||||
| ECMO | 141 ± 35 | 131 ± 26 | 128 ± 16 | 127 ± 16 | 147 ± 21 | 150 ± 12 | 147 ± 35 |
| Impella | 118 ± 21 | 131 ± 15 | 157 ± 29 | 120 ± 34 | 126 ± 43 | 155 ± 18 | 163 ± 6 |
| MAP (mmHg) | |||||||
| ECMO = | 84.14 ± 18.2 | 137.8 ± 33.8 | 68.75 ± 34.8 | 67.33 ± 23.8 | 96.25 ± 44.5 | 83.25 ± 12.5 | 79.75 ± 15.4 |
| Impella = | 85.20 ± 8.6 | 88.8 ± 32.6 | 64.20 ± 23.1 | 49.25 ± 24.8 | 61.6 ± 21.2 | 77.25 ± 6.2 | 66.33 ± 4.0 |
| SPAP (mmHg) | |||||||
| ECMO = | 29.9 ± 5.9 | 27.8 ± 14.8 | 24.4 ± 20.7 | 21.0 ± 11.7 | 42.0 ± 26.2 | 35.0 ± 14.8 | 40.5 ± 17.6 |
| Impella = | 25.8 ± 1.8 | 40.6 ± 12.6 | 29.0 ± 7.8 | 35.8 ± 12.0 | 36.6 ± 10.7 | 42.8 ± 5.7 | 36.7 ± 9.3 |
| CVP (mmHg) | |||||||
| ECMO = | 6.0 ± 4.3 | 3.6 ± 3.4 | 3.2 ± 4.3 | 4.0 ± 2.9 | 3.75 ± 2.2 | 4.0 ± 4.2 | 5.0 ± 3.4 |
| Impella = | 5.8 ± 3.6 | 12.2 ± 4.4 | 10.6 ± 4.9 | 8.75 ± 2.9 | 7.4 ± 3.6 | 5.5 ± 2.6 | 4.3 ± 2.1 |
| PaO2 (mmHg) | |||||||
| ECMO = | 485.6 ± 83.4 | 558.8 ± 28.3 | 435.6 ± 215.7 | 349.7 ± 164.2 | 501.7 ± 104.4 | 456.9 ± 69.9 | 373.0 ± 86.9 |
| Impella = | 437.9 ± 61.4 | 238.7 ± 138.2 | 227.9 ± 142.3 | 247.9 ± 163.2 | 339.3 ± 144.8 | 346.2 ± 171.6 | 301.9 ± 230.9 |
| PCo2 (mmHg) | |||||||
| ECMO = | 37.6 ± 7.9 | 29.9 ± 9.9 | 30.1 ± 5.9 | 24.7 ± 6.4 | 27.4 ± 1.8 | 25.6 ± 7.5 | 28.1 ± 10.4 |
| Impella = | 38.6 ± 9.8 | 49.0 ± 16.2 | 49.9 ± 18.6 | 51.0 ± 23.9 | 50.3 ± 10.9 * | 42.9 ± 13.3 | 61.1 ± 16.9 |
| Lactate (mmol/L) | |||||||
| ECMO = | 1.3 ± 0.6 | 7.2 ± 1.7 | 7.2 ± 1.1 | 7.9 ± 2.3 | 9.1 ± 1.9 | 8.4 ± 1.7 | 6.9 ± 2.4 |
| Impella = | 2.4 ± 0.6 | 9.9 ± 2.3 | 9.9 ± 1.8 | 10.7 ± 2.5 | 10.9 ± 1.0 | 9.9 ± 1.3 | 9.6 ± 0.3 |
| pH | |||||||
| ECMO = | 7.45 ± 0.09 | 7.29 ± 0.07 | 7.23 ± 0.14 | 7.26 ± 0.15 | 7.29 ± 0.04 | 7.34 ± 0.14 | 7.34 ± 0.21 |
| Impella = | 7.48 ± 0.09 | 7.13 ± 0.16 | 7.09 ± 0.09 | 7.07 ± 0.12 | 7.08 ± 0.08 | 7.15 ± 0.08 | 7.05 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzo-Silberman, S.; Nix, C.; Goetzenich, A.; Demondion, P.; Kang, C.; Bonneau, M.; Cohen-Solal, A.; Leprince, P.; Lebreton, G. Severe Myocardial Dysfunction after Non-Ischemic Cardiac Arrest: Effectiveness of Percutaneous Assist Devices. J. Clin. Med. 2021, 10, 3623. https://doi.org/10.3390/jcm10163623
Manzo-Silberman S, Nix C, Goetzenich A, Demondion P, Kang C, Bonneau M, Cohen-Solal A, Leprince P, Lebreton G. Severe Myocardial Dysfunction after Non-Ischemic Cardiac Arrest: Effectiveness of Percutaneous Assist Devices. Journal of Clinical Medicine. 2021; 10(16):3623. https://doi.org/10.3390/jcm10163623
Chicago/Turabian StyleManzo-Silberman, Stéphane, Christoph Nix, Andreas Goetzenich, Pierre Demondion, Chantal Kang, Michel Bonneau, Alain Cohen-Solal, Pascal Leprince, and Guillaume Lebreton. 2021. "Severe Myocardial Dysfunction after Non-Ischemic Cardiac Arrest: Effectiveness of Percutaneous Assist Devices" Journal of Clinical Medicine 10, no. 16: 3623. https://doi.org/10.3390/jcm10163623
APA StyleManzo-Silberman, S., Nix, C., Goetzenich, A., Demondion, P., Kang, C., Bonneau, M., Cohen-Solal, A., Leprince, P., & Lebreton, G. (2021). Severe Myocardial Dysfunction after Non-Ischemic Cardiac Arrest: Effectiveness of Percutaneous Assist Devices. Journal of Clinical Medicine, 10(16), 3623. https://doi.org/10.3390/jcm10163623






