Comparison of Mechanical Support with Impella or Extracorporeal Life Support in Post-Cardiac Arrest Cardiogenic Shock: A Propensity Scoring Matching Analysis
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Patients’ Management
2.3. Data Collection and Outcome Variables
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deo, R.; Albert, C.M. Epidemiology and genetics of sudden cardiac death. Circulation 2012, 125, 620–637. [Google Scholar] [CrossRef] [Green Version]
- Neumar, R.W.; Nolan, J.P.; Adrie, C.; Aibiki, M.; Berg, R.A.; Bottiger, B.W.; Callaway, C.; Clark, R.S.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008, 118, 2452–2483. [Google Scholar] [CrossRef] [Green Version]
- Lemiale, V.; Dumas, F.; Mongardon, N.; Giovanetti, O.; Charpentier, J.; Chiche, J.D.; Carli, P.; Mira, J.P.; Nolan, J.; Cariou, A. Intensive care unit mortality after cardiac arrest: The relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013, 39, 1972–1980. [Google Scholar] [CrossRef] [PubMed]
- Rihal, C.S.; Naidu, S.S.; Givertz, M.M.; Szeto, W.Y.; Burke, J.A.; Kapur, N.K.; Kern, M.; Garratt, K.N.; Goldstein, J.A.; Dimas, V.; et al. 2015 SCAI/ACC/HFSA/STS Clinical Expert Consensus Statement on the Use of Percutaneous Mechanical Circulatory Support Devices in Cardiovascular Care: Endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology-Association Canadienne de Cardiologie d’intervention. J. Am. Coll. Cardiol. 2015, 65, e7–e26. [Google Scholar]
- Werdan, K.; Gielen, S.; Ebelt, H.; Hochman, J.S. Mechanical circulatory support in cardiogenic shock. Eur. Heart J. 2014, 35, 156–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzo-Silberman, S.; Fichet, J.; Mathonnet, A.; Varenne, O.; Ricome, S.; Chaib, A.; Zuber, B.; Spaulding, C.; Cariou, A. Percutaneous left ventricular assistance in post cardiac arrest shock: Comparison of intra aortic blood pump and IMPELLA Recover LP2. Resuscitation 2013, 84, 609–615. [Google Scholar] [CrossRef]
- Karatolios, K.; Chatzis, G.; Markus, B.; Luesebrink, U.; Ahrens, H.; Dersch, W.; Betz, S.; Ploeger, B.; Boesl, E.; O’Neill, W.; et al. Impella support compared to medical treatment for post-cardiac arrest shock after out of hospital cardiac arrest. Resuscitation 2018, 126, 104–110. [Google Scholar] [CrossRef] [PubMed]
- De Chambrun, M.P.; Brechot, N.; Lebreton, G.; Schmidt, M.; Hekimian, G.; Demondion, P.; Trouillet, J.L.; Leprince, P.; Chastre, J.; Combes, A.; et al. Venoarterial extracorporeal membrane oxygenation for refractory cardiogenic shock post-cardiac arrest. Intensive Care Med. 2016, 42, 1999–2007. [Google Scholar] [CrossRef]
- Bougouin, W.; Aissaoui, N.; Combes, A.; Deye, N.; Lamhaut, L.; Jost, D.; Maupain, C.; Beganton, F.; Bougle, A.; Karam, N.; et al. Post-cardiac arrest shock treated with veno-arterial extracorporeal membrane oxygenation: An observational study and propensity-score analysis. Resuscitation 2017, 110, 126–132. [Google Scholar] [CrossRef]
- Ouweneel, D.M.; Schotborgh, J.V.; Limpens, J.; Sjauw, K.D.; Engstrom, A.E.; Lagrand, W.K.; Cherpanath, T.G.V.; Driessen, A.H.G.; de Mol, B.; Henriques, J.P.S. Extracorporeal life support during cardiac arrest and cardiogenic shock: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 1922–1934. [Google Scholar] [CrossRef] [Green Version]
- Karatolios, K.; Chatzis, G.; Markus, B.; Luesebrink, U.; Ahrens, H.; Divchev, D.; Syntila, S.; Jerrentrup, A.; Schieffer, B. Comparison of mechanical circulatory support with venoarterial extracorporeal membrane oxygenation or Impella for patients with cardiogenic shock: A propensity-matched analysis. Clin. Res. Cardiol. 2020. [Google Scholar] [CrossRef]
- Wernly, B.; Karami, M.; Engstrom, A.E.; Windecker, S.; Hunziker, L.; Luscher, T.F.; Henriques, J.P.; Ferrari, M.W.; Binnebossel, S.; Masyuk, M.; et al. Impella versus extracorporal life support in cardiogenic shock: A propensity score adjusted analysis. ESC Heart Fail. 2021, 8, 953–961. [Google Scholar] [CrossRef]
- Garan, A.R.; Takeda, K.; Salna, M.; Vandenberge, J.; Doshi, D.; Karmpaliotis, D.; Kirtane, A.J.; Takayama, H.; Kurlansky, P. Prospective Comparison of a Percutaneous Ventricular Assist Device and Venoarterial Extracorporeal Membrane Oxygenation for Patients with Cardiogenic Shock Following Acute Myocardial Infarction. J. Am. Heart Assoc. 2019, 8, e012171. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.B.; Al-Hussaini, A.; Rosser, G.; Salehi, S.; Phylactou, M.; Rajakulasingham, R.; Patel, J.; Elliott, K.; Mohan, P.; Green, R.; et al. Predictors of survival and favorable functional outcomes after an out-of-hospital cardiac arrest in patients systematically brought to a dedicated heart attack center (from the Harefield Cardiac Arrest Study). Am. J. Cardiol. 2015, 115, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Wibrandt, I.; Norsted, K.; Schmidt, H.; Schierbeck, J. Predictors for outcome among cardiac arrest patients: The importance of initial cardiac arrest rhythm versus time to return of spontaneous circulation, a retrospective cohort study. BMC Emerg. Med. 2015, 15, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinell, L.; Nielsen, N.; Herlitz, J.; Karlsson, T.; Horn, J.; Wise, M.P.; Unden, J.; Rylander, C. Early predictors of poor outcome after out-of-hospital cardiac arrest. Crit. Care 2017, 21, 96. [Google Scholar] [CrossRef] [Green Version]
- Orban, J.C.; Novain, M.; Cattet, F.; Plattier, R.; Nefzaoui, M.; Hyvernat, H.; Raguin, O.; Kaidomar, M.; Kerever, S.; Ichai, C. Association of serum lactate with outcome after out-of-hospital cardiac arrest treated with therapeutic hypothermia. PLoS ONE 2017, 12, e0173239. [Google Scholar] [CrossRef] [Green Version]
- Schiller, P.; Hellgren, L.; Vikholm, P. Survival after refractory cardiogenic shock is comparable in patients with Impella and veno-arterial extracorporeal membrane oxygenation when adjusted for SAVE score. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 329–337. [Google Scholar] [CrossRef]
- Mourad, M.; Gaudard, P.; Arena, P.D.L.; Eliet, J.; Zeroual, N.; Rouviere, P.; Roubille, F.; Albat, B.; Colson, P.H. Circulatory Support with Extracorporeal Membrane Oxygenation and/or Impella for Cardiogenic Shock during Myocardial Infarction. ASAIO J. 2018, 64, 708–714. [Google Scholar] [CrossRef]
- Lemor, A.; Dehkordi, S.H.H.; Basir, M.B.; Villablanca, P.A.; Jain, T.; Koenig, G.C.; Alaswad, K.; Moses, J.W.; Kapur, N.K.; O’Neill, W. Impella Versus Extracorporeal Membrane Oxygenation for Acute Myocardial Infarction Cardiogenic Shock. Cardiovasc. Revasc. Med. 2020, 21, 1465–1471. [Google Scholar] [CrossRef]
- Harjola, V.P.; Lassus, J.; Sionis, A.; Kober, L.; Tarvasmaki, T.; Spinar, J.; Parissis, J.; Banaszewski, M.; Silva-Cardoso, J.; Carubelli, V.; et al. Clinical picture and risk prediction of short-term mortality in cardiogenic shock. Eur. J. Heart Fail. 2015, 17, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Karami, M.; den Uil, C.A.; Ouweneel, D.M.; Scholte, N.T.; Engstrom, A.E.; Akin, S.; Lagrand, W.K.; Vlaar, A.P.; Jewbali, L.S.; Henriques, J.P. Mechanical circulatory support in cardiogenic shock from acute myocardial infarction: Impella CP/5.0 versus ECMO. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Ouweneel, D.M.; Engstrom, A.E.; Sjauw, K.D.; Hirsch, A.; Hill, J.M.; Gockel, B.; Tuseth, V.; van der Schaaf, R.J.; Henriques, J.P. Experience from a randomized controlled trial with Impella 2.5 versus IABP in STEMI patients with cardiogenic pre-shock. Lessons learned from the IMPRESS in STEMI trial. Int. J. Cardiol. 2016, 202, 894–896. [Google Scholar] [CrossRef] [PubMed]
- Holmberg, M.; Holmberg, S.; Herlitz, J. Incidence, duration and survival of ventricular fibrillation in out-of-hospital cardiac arrest patients in sweden. Resuscitation 2000, 44, 7–17. [Google Scholar] [CrossRef]
- Barbone, A.; Malvindi, P.G.; Ferrara, P.; Tarelli, G. Left ventricle unloading by percutaneous pigtail during extracorporeal membrane oxygenation. Interact. Cardiovasc. Thorac. Surg. 2011, 13, 293–295. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, D.; Gojo, S.; Nishimura, T.; Itoda, Y.; Kitahori, K.; Motomura, N.; Morota, T.; Murakami, A.; Takamoto, S.; Kyo, S.; et al. Left ventricular mechanical support with Impella provides more ventricular unloading in heart failure than extracorporeal membrane oxygenation. ASAIO J. 2011, 57, 169–176. [Google Scholar] [CrossRef]
- Hlavacek, A.M.; Atz, A.M.; Bradley, S.M.; Bandisode, V.M. Left atrial decompression by percutaneous cannula placement while on extracorporeal membrane oxygenation. J. Thorac. Cardiovasc. Surg. 2005, 130, 595–596. [Google Scholar] [CrossRef] [Green Version]
- Aiyagari, R.M.; Rocchini, A.P.; Remenapp, R.T.; Graziano, J.N. Decompression of the left atrium during extracorporeal membrane oxygenation using a transseptal cannula incorporated into the circuit. Crit. Care Med. 2006, 34, 2603–2606. [Google Scholar] [CrossRef]
- Pappalardo, F.; Schulte, C.; Pieri, M.; Schrage, B.; Contri, R.; Soeffker, G.; Greco, T.; Lembo, R.; Mullerleile, K.; Colombo, A.; et al. Concomitant implantation of Impella((R)) on top of veno-arterial extracorporeal membrane oxygenation may improve survival of patients with cardiogenic shock. Eur. J. Heart Fail. 2017, 19, 404–412. [Google Scholar] [CrossRef]
- Watanabe, S.; Fish, K.; Kovacic, J.C.; Bikou, O.; Leonardson, L.; Nomoto, K.; Aguero, J.; Kapur, N.K.; Hajjar, R.J.; Ishikawa, K. Left Ventricular Unloading Using an Impella CP Improves Coronary Flow and Infarct Zone Perfusion in Ischemic Heart Failure. J. Am. Heart Assoc. 2018, 7, e006462. [Google Scholar] [CrossRef] [Green Version]
- Remmelink, M.; Sjauw, K.D.; Henriques, J.P.; de Winter, R.J.; Koch, K.T.; van der Schaaf, R.J.; Vis, M.M.; Tijssen, J.G.; Piek, J.J.; Baan, J., Jr. Effects of left ventricular unloading by Impella recover LP2.5 on coronary hemodynamics. Catheter. Cardiovasc. Interv. 2007, 70, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Meyns, B.; Stolinski, J.; Leunens, V.; Verbeken, E.; Flameng, W. Left ventricular support by catheter-mounted axial flow pump reduces infarct size. J. Am. Coll. Cardiol. 2003, 41, 1087–1095. [Google Scholar] [CrossRef] [Green Version]
- Ouweneel, D.M.; de Brabander, J.; Karami, M.; Sjauw, K.D.; Engstrom, A.E.; Vis, M.M.; Wykrzykowska, J.J.; Beijk, M.A.; Koch, K.T.; Baan, J.; et al. Real-life use of left ventricular circulatory support with Impella in cardiogenic shock after acute myocardial infarction: 12 years AMC experience. Eur. Heart J. Acute Cardiovasc. Care 2019, 8, 338–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrage, B.; Ibrahim, K.; Loehn, T.; Werner, N.; Sinning, J.M.; Pappalardo, F.; Pieri, M.; Skurk, C.; Lauten, A.; Landmesser, U.; et al. Impella Support for Acute Myocardial Infarction Complicated by Cardiogenic Shock. Circulation 2019, 139, 1249–1258. [Google Scholar] [CrossRef] [PubMed]
- Combes, A.; Leprince, P.; Luyt, C.E.; Bonnet, N.; Trouillet, J.L.; Leger, P.; Pavie, A.; Chastre, J. Outcomes and long-term quality-of-life of patients supported by extracorporeal membrane oxygenation for refractory cardiogenic shock. Crit. Care Med. 2008, 36, 1404–1411. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Hachamovitch, R.; Kittleson, M.; Patel, J.; Arabia, F.; Moriguchi, J.; Esmailian, F.; Azarbal, B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1866 adult patients. Ann. Thorac. Surg. 2014, 97, 610–616. [Google Scholar] [CrossRef] [PubMed]

| Variable | All Patients (n = 159) | Impella (n = 105) | ECLS (n = 54) | p-Value | Impella (n = 40) | ECLS (n = 40) | p-Value |
|---|---|---|---|---|---|---|---|
| Age (years) | 66.91 ± 11.8 | 67.56 ± 13.65 | 61.76 ± 10.38 | <0.001 | 67.68 ± 12.1 | 62.05 ± 10.83 | 0.03 |
| Gender (male/female) | 125/34 | 81/24 | 44/10 | 0.68 | 30/10 | 31/9 | 1 |
| BMI (kg/m2) | 27.76 ± 4.09 | 27.63 ± 3.73 | 28 ± 4.75 | 0.6 | 28.38 ± 3.88 | 27.61 ± 4.59 | 0.42 |
| Baseline LVEF (%) | 32.32 ± 6.89 | 32.32 ± 6.89 | 34.45 ± 7.21 | 0.07 | 32.91 ± 6.74 | 34.89 ± 7.61 | 0.2 |
| STEMI on presentation, n (%) | 77 (48.4) | 49 (46.7) | 28 (51.9) | 0.62 | 16 (40) | 19 (47.5) | 0.66 |
| Medical comorbidities | |||||||
| Hypertension, n (%) | 118 (74.2) | 79 (75.5) | 39 (72.2) | 0.7 | 26 (65) | 30 (75) | 0.46 |
| Diabetes, n (%) | 58 (36.4) | 38 (36.2) | 20 (37) | 1 | 13 (32.5) | 12 (30) | 1 |
| PAD, n (%) | 56 (35.2) | 33 (31.4) | 23 (42.6) | 0.22 | 14 (35) | 15 (37.5) | 1 |
| Stroke, n (%) | 14 (8.8) | 8 (7.6) | 6 (11.1) | 0.56 | 4 (10) | 5 (12.5) | 1 |
| PCI, n (%) | 50 (31.4) | 32 (30.5) | 18 (33.3) | 0.72 | 12 (30) | 13 (32.5) | 1 |
| CABG, n(%) | 20 (12.6) | 12 (11.5) | 8 (14.8) | 0.62 | 5 (12.5) | 6 (15) | 1 |
| Myocardial infarction, n (%) | 58 (36.5) | 36 (34.3) | 22 (40.7 | 0.49 | 14 35) | 16 (40) | 0.82 |
| CAD | 65 (40.9) | 40 (38.1) | 25 (46.3) | 0.39 | 14 (35) | 18 (45) | 0.5 |
| Charlson Comorbidity Index | 4 (3–6) | 4 (2.5–6) | 5 (4–7) | 0.02 | 4.13 ± 2.69 | 4.78 ± 2.09 | 0.23 |
| Cardiac arrest variables | |||||||
| Witnessed arrest, n (%) | 128 (80.5) | 84 (80) | 44 (81.5) | 1 | 29 (72.5) | 31 (77.5) | 0.8 |
| Bystander CPR, n (%) | 120 (75.5) | 80 (76.2) | 40 (74.1) | 0.85 | 29 (72.5) | 29 (72.5) | 1 |
| First rhythm VT or VF, n (%) | 96 (60.4) | 68 (64.8) | 28 (51.9) | 0.13 | 27 (67.5) | 23 (57.5) | 0.49 |
| No flow time (min) | 4 (2–8) | 4 (1.5–7) | 5 (2–9) | 0.22 | 4 (2–6.75) | 4.5 (2.25–8.75) | 0.32 |
| Low flow time (min) | 25.80 ± 14.29 | 24.45 ± 13.99 | 28.43 ± 14.64 | 0.08 | 23–75 ± 12–2 | 23–28 ± 11–92 | 0.86 |
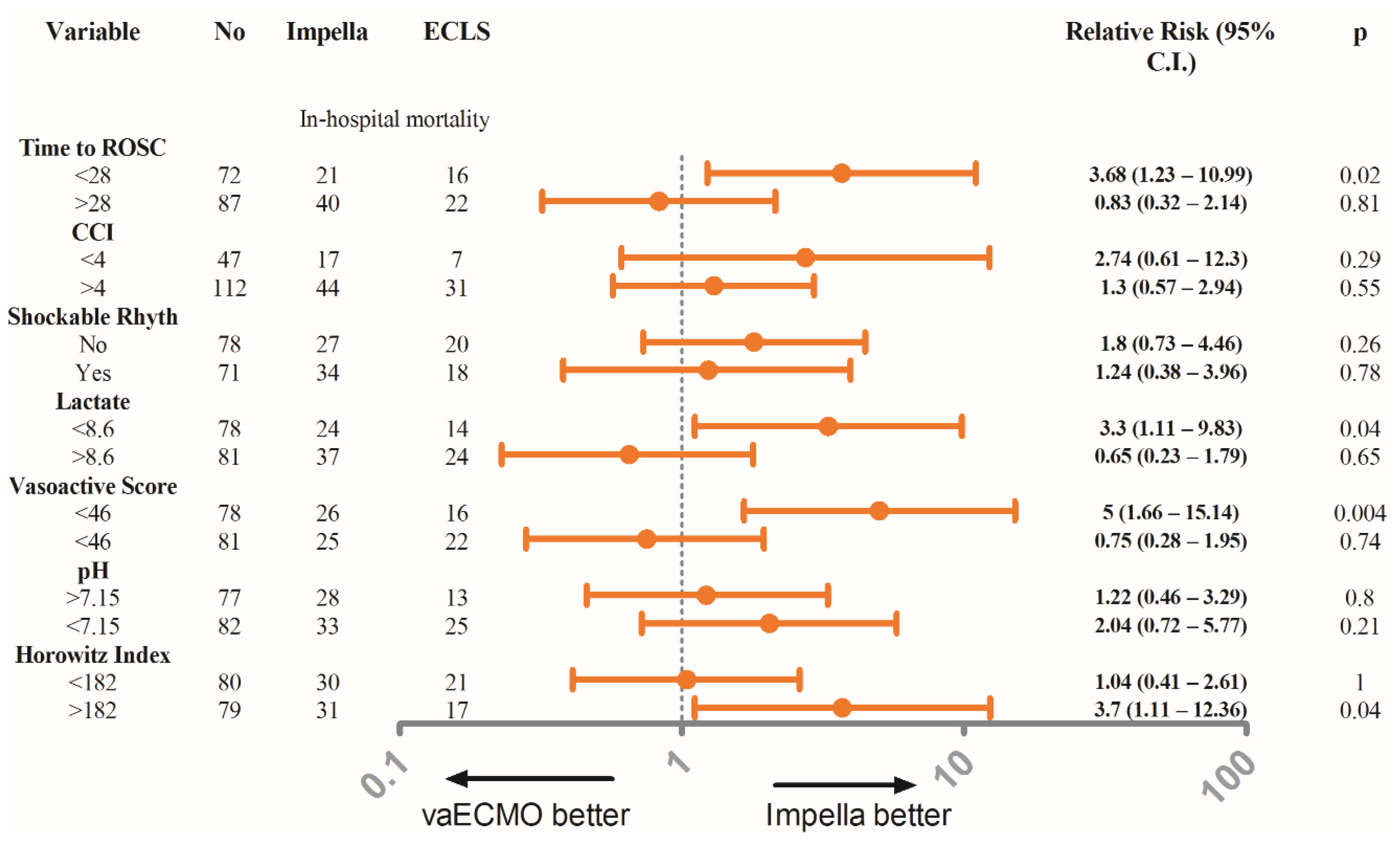
| Time till ROSC (min) | 30.61 ± 14.87 | 28.9 ± 14.92 | 33.94 ± 14.32 | 0.04 | 28–13 ± 14–2 | 28–45 ± 10–62 | 0.91 |
| Epinephrine during CPR, n (%) | 152 (95.6) | 98 (93.3) | 54 (96.4) | 0.1 | 39 (97.5) | 40 (100) | 1 |
| Total epinephrine during CPR (mg) | 5 (3–8) | 4 (2–6.5) | 7 (4.75–9) | 0.005 | 4 (2–6) | 6–7 ± 2–95 | 0.003 |
| Catecholamines | |||||||
| Dobutamine, n (%) | 93 (58.5) | 61 (58.1) | 32 (59.3) | 1 | 21 (52.5) | 25 (62.5) | 0.5 |
| Dobutamine (µg/kg/min) | 5.88 (4.28–7.49) | 5.55 (3.7–7.27) | 6.05 (5.33–8.38) | 0.04 | 5.57 ± 2–29 | 6.34 ± 2–38 | 0.27 |
| Norepinephrine, n (%) | 159 (100) | 105 (100) | 54 (100) | 1 | 40 (100) | 40 (100) | 1 |
| Norepinephrine (µg/kg/min) | 0.47 ± 0.32 | 0.4 ± 0.24 | 0.6 ± 0.41 | 0.002 | 0.44 ± 0.27 | 0.43 ± 0.25 | 0.87 |
| Epinephrine, n (%) | 36 (22.6) | 23 (14.7) | 13 (24.1) | 0.84 | 7 (17.5) | 7 (17.5) | 1 |
| Epinephrine (µg/kg/min) * | 0.35 (0.17–0.67) | 0.47 (0.32–0.65) | 0.35 ± 0.29 | 0.25 | 0.27 (0.14–0.97) | 0.47 (0.32–0.65) | 0.4 |
| Vasoactive Score ** | 61.30 ± 43.47 | 55.04 ± 42.25 | 73.46 ± 43.61 | 0.011 | 60.33 ± 36.34 | 56.32 ± 30.46 | 0.59 |
| Mechanical ventilation | 159 (100) | 105 (100) | 54 (100) | 1 | 40 (100) | 40 (100) | 1 |
| Hemodynamic variables on admission | |||||||
| Heart rate (bpm) | 88.77 ± 24.02 | 88.31 ± 22.21 | 89.67 ± 27.42 | 0.74 | 91.38 ± 22.76 | 91.35 ± 27.72 | 1 |
| Systolic Blood Pressure (mmHg) | 97.30 ± 22.20 | 98.53 ± 25.54 | 95.57 ± 20.41 | 0.63 | 98.53 ± 25.54 | 94.28 ±20.83 | 0.42 |
| Diastolic Blood Pressure (mmHg) | 56.06 ± 14.33 | 57.25 ± 15.56 | 53.80 ± 11.56 | 0.32 | 57.25 ± 15.56 | 53.68 ± 10.46 | 0.23 |
| Mean blood pressure (mmHg) | 69.81 ± 15.56 | 71.01 ± 17.66 | 67.98 ± 10.93 | 0.44 | 71.01 ± 17.66 | 67.58 ± 10.58 | 0.29 |
| Blood values on admission | |||||||
| Lactate (mmol/L) | 9.05 ± 3.95 | 8.59 ± 3.93 | 9.01 ± 3.56 | 0.038 | 9.94 ± 3.86 | 8.95 ± 3.37 | 0.94 |
| GFR (mL/min) | 49.78 ± 20.4 | 49.02 ± 20.51 | 50.67 ± 20.43 | 0.67 | 51.73 ± 19.48 | 53.95 ± 22.31 | 0.68 |
| Creatinine (mg/dl) | 1.52 ± 0.7 | 1.52 ± 0.52 | 1.52 ± 0.87 | 0.97 | 1.4 ± 0.37 | 1.4 ± 0.84 | 0.99 |
| Arterial pH | 7.16 ± 0.16 | 7.18 ± 0.16 | 7.13 ± 0.16 | 0.04 | 7.18 ± 0.14 | 7.16 ± 0.15 | 0.43 |
| PaO2/FiO2 | 194 ± 104.9 | 210.1 ± 115.3 | 162.5 ± 72.07 | 0.006 | 175.6 ± 103.3 | 179 ± 68.98 | 0.86 |
| Variable | All Patients (n = 159) | Impella (n = 105) | ECLS (n = 54) | p-Value | Impella Matched (n = 40) | ECLS Matched (n = 40) | p-Value |
|---|---|---|---|---|---|---|---|
| Door to MCS (min) | 108.6 ± 55.79 | 109 ± 55.69 | 108.1 ± 56.47 | 0.93 | 107.4 ± 52.58 | 100.9 ± 53.91 | 0.61 |
| Duration of support (hours) | 72 (18.5–130.5) | 65.5 (14–127.5) | 88.5 (23.75–141.8) | 0.19 | 48 (9.5–147) | 105 (24–146.3) | 0.15 |
| Door to Balloon (min) | 87.71 ± 44.95 | 84.96 ± 40.69 | 92.24 ± 51.28 | 0.35 | 85.88 ± 35.8 | 85.4 ± 50.72 | 0.96 |
| Time from ROSC to hospital admission (min) | 74.21 ± 37.57 | 73.61 ± 35.88 | 75.17 ± 40.46 | 0.81 | 73.03 ± 36.89 | 76.35 ± 43.85 | 0.73 |
| Culprit vessel, (n %) | NS for all comparisons | NS for all comparisons | |||||
| Left main | 7 (4.4) | 5 (4.8) | 2 (3.7) | 1(2.5) | 2 (5) | ||
| LAD | 86 (54.1) | 58 (55.2) | 28 (51.9) | 19 (47.5) | 20 (50) | ||
| LCx | 30 (18.9) | 19 (18.1) | 11 (20.4) | 9 (22.5) | 8 (20) | ||
| RCA | 28 (17.6) | 18 (17.1) | 10 (18.5) | 7 (17.5) | 8 (20) | ||
| Bypass-graft | 8 (5) | 5 (4.8) | 3 (5.5) | 4 (10) | 2 (5) | ||
| Multivessel disease * | 107 (67.3) | 78 (74.3) | 39 (72.2) | 0.85 | 28 (70) | 21 (52.5) | 0.17 |
| Multivessel Intervention | 41 (25.8) | 24 (22.9) | 17(31.5) | 0.26 | 9 (22.5) | 10 (25) | 1 |
| Successful PCI | 156 (98.1) | 103(98.1) | 53 (98.1) | 1 | 40 (100) | 40 (100) | 1 |
| Contrast Agent (mL) | 276.6 ± 123.7 | 287.5 ± 121.9 | 259.3 ± 125.8 | 0.19 | 292.5 ± 127.0 | 244.2 ± 119.1 | 0.11 |
| Outcome | All Patients (n = 159) | Impella (n = 105) | ECLS (n = 54) | p-Value | Impella Matched (n = 40) | ECLS Matched (n = 40) | p-Value |
|---|---|---|---|---|---|---|---|
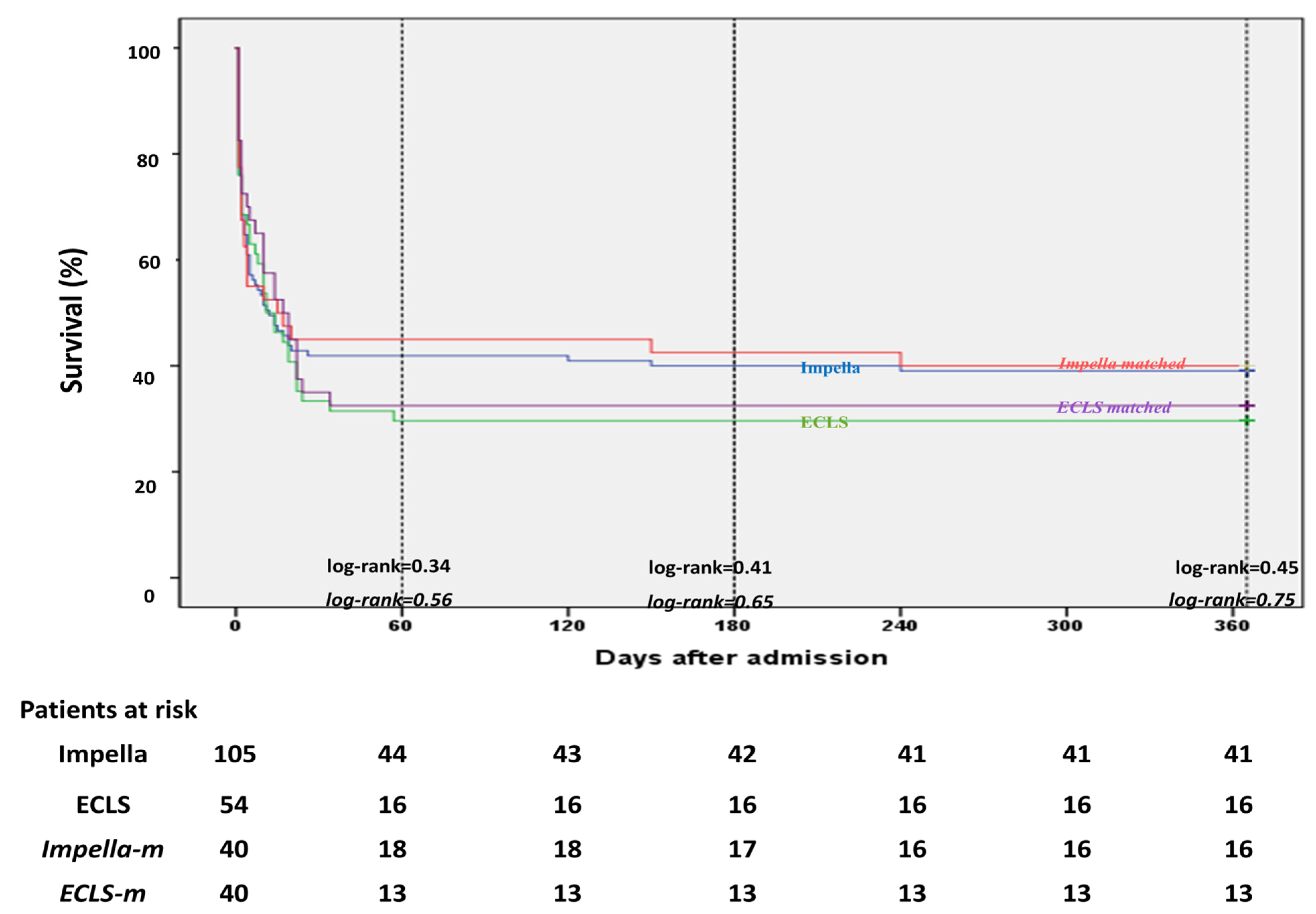
| Survival to hospital discharge, n (%) | 60 (37.7) | 44 (41.9) | 16 (29.6) | 0.17 | 18 (45) | 13 (32.5) | 0.36 |
| • CPC 1–2, n (%) | 41 (68.3) | 30 (68.1) | 11 (68.8) | 1 | 14 (77.8) | 11 (84.6) | 1 |
| • CPC 3–4, n (%) | 19 (31.7) | 14 (31.9) | 5 (31.2) | 1 | 4 (22.2) | 2 (15.4) | 1 |
| Survival at 12 months, n (%) | 57 (35.8) | 41 (39) | 16 (29.9) | 0.3 | 16 (40) | 13 (32.5) | 0.64 |
| Mortality to discharge, n (%) | 99 (62.3) | 61 (58.1) | 38 (71.4) | 0.83 | 22 (55) | 27 (67.5) | 0.36 |
| Causes of death | |||||||
| • Cardiogenic shock/MOF | 88 (88.9) | 55 (90.1) | 33 (86.4) | 0.74 | 20 (90.1) | 25 (92.6) | 1 |
| • Brain death | 11 (11.1) | 6 (9.9) | 5 (13.6) | 2 (9.1) | 2 (7.4) | ||
| Complications | |||||||
| Access site bleeding requiring transfusion, n (%) | 35 (22) | 16 (15.2) | 19 (35.1) | <0.01 | 4 (10) | 13 (32.5) | <0.01 |
| Limb ischemia requiring intervention, n (%) | 19 (14.7) | 8 (7.6) | 11 (20.4) | 0.04 | 1 (2.5) | 8 (20) | 0.03 |
| Myocardial Reinfarction, n (%) | 1 (0.6) | 0 (0) | 1 (1.9) | 1 | 0 (0) | 0 (0) | 1 |
| Pericardial effusion needing paracentesis, n (%) | 2 (1.3) | 1 (1) | 1 (1.9) | 1 | 0 (0) | 0 (0) | 1 |
| Stroke, n (%) | 3 (1.9) | 1 (1) | 2 (3.7) | 0.29 | 0 (0) | 0 (0) | 1 |
| Non-device related bleeding, n (%) | 8 (5) | 5 (4.8) | 3 (5.6) | 1 | 2 (5) | 2 (5) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syntila, S.; Chatzis, G.; Markus, B.; Ahrens, H.; Waechter, C.; Luesebrink, U.; Divchev, D.; Schuett, H.; Tsalouchidou, P.-E.; Jerrentrup, A.; et al. Comparison of Mechanical Support with Impella or Extracorporeal Life Support in Post-Cardiac Arrest Cardiogenic Shock: A Propensity Scoring Matching Analysis. J. Clin. Med. 2021, 10, 3583. https://doi.org/10.3390/jcm10163583
Syntila S, Chatzis G, Markus B, Ahrens H, Waechter C, Luesebrink U, Divchev D, Schuett H, Tsalouchidou P-E, Jerrentrup A, et al. Comparison of Mechanical Support with Impella or Extracorporeal Life Support in Post-Cardiac Arrest Cardiogenic Shock: A Propensity Scoring Matching Analysis. Journal of Clinical Medicine. 2021; 10(16):3583. https://doi.org/10.3390/jcm10163583
Chicago/Turabian StyleSyntila, Styliani, Georgios Chatzis, Birgit Markus, Holger Ahrens, Christian Waechter, Ulrich Luesebrink, Dimitar Divchev, Harald Schuett, Panagiota-Eleni Tsalouchidou, Andreas Jerrentrup, and et al. 2021. "Comparison of Mechanical Support with Impella or Extracorporeal Life Support in Post-Cardiac Arrest Cardiogenic Shock: A Propensity Scoring Matching Analysis" Journal of Clinical Medicine 10, no. 16: 3583. https://doi.org/10.3390/jcm10163583
APA StyleSyntila, S., Chatzis, G., Markus, B., Ahrens, H., Waechter, C., Luesebrink, U., Divchev, D., Schuett, H., Tsalouchidou, P.-E., Jerrentrup, A., Parahuleva, M., Schieffer, B., & Karatolios, K. (2021). Comparison of Mechanical Support with Impella or Extracorporeal Life Support in Post-Cardiac Arrest Cardiogenic Shock: A Propensity Scoring Matching Analysis. Journal of Clinical Medicine, 10(16), 3583. https://doi.org/10.3390/jcm10163583







