Questionnaire and Portable Sleep Test Screening of Sleep Disordered Breathing in Acute Stroke and TIA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Sample
2.2. Inclusion and Exclusion Criteria
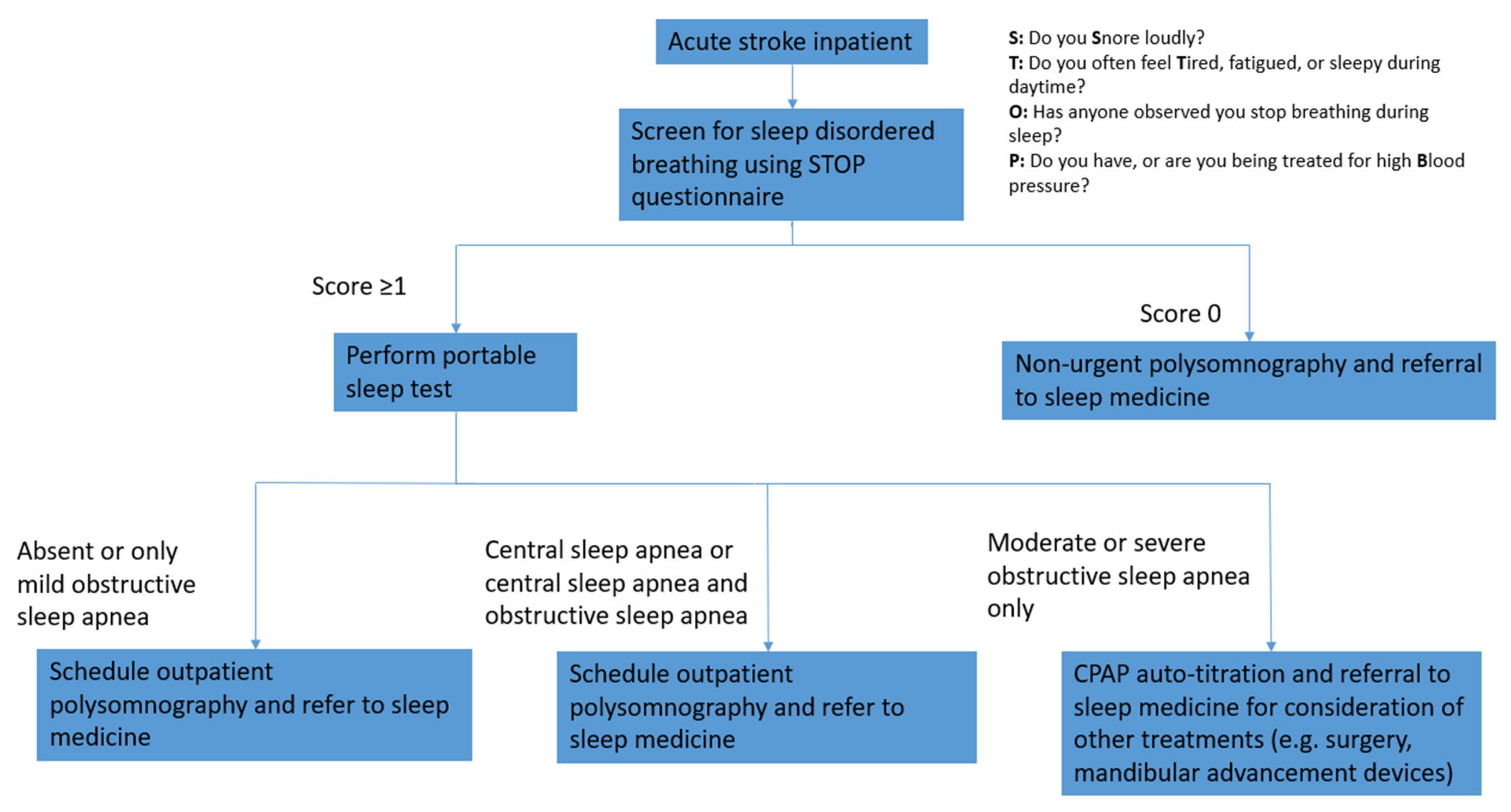
2.3. Questionnaires and Sleep Testing
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Seiler, A.; Camilo, M.; Korostovtseva, L.; Haynes, A.G.; Brill, A.-K.; Horvath, T.; Egger, M.; Bassetti, C.L. Prevalence of sleep-disordered breathing after stroke and TIA. Neurology 2019, 92, e648–e654. [Google Scholar] [CrossRef]
- Hermann, D.M.; Bassetti, C.L. Role of sleep-disordered breathing and sleep-wake disturbances for stroke and stroke recovery. Neurology 2016, 87, 1407–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisabeth, L.D.; Sánchez, B.N.; Lim, D.; Chervin, R.D.; Case, E.; Morgenstern, L.B.; Tower, S.; Brown, D. Sleep-disordered breathing and poststroke outcomes. Ann. Neurol. 2019, 86, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Kernan, W.N.; Ovbiagele, B.; Black, H.R.; Bravata, D.M.; Chimowitz, M.I.; Ezekowitz, M.D.; Fang, M.C.; Fisher, M.; Furie, K.L.; Heck, D.V.; et al. Guidelines for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2014, 45, 2160–2236. [Google Scholar] [CrossRef]
- Brill, A.-K.; Horvath, T.; Seiler, A.; Camilo, M.; Haynes, A.G.; Ott, S.R.; Egger, M.; Bassetti, C.L. CPAP as treatment of sleep apnea after stroke: A meta-analysis of randomized trials. Neurology 2018, 90, e1222–e1230. [Google Scholar] [CrossRef]
- Haba-Rubio, J.; Vujica, J.; Michel, P.; Heinzer, R. Effect of CPAP treatment of sleep apnea on prognosis after ischemic stroke: An observational study. Sleep Med. 2017, 40, e124. [Google Scholar] [CrossRef]
- Brown, D.L.; Jiang, X.; Li, C.; Case, E.; Sozener, C.B.; Chervin, R.D.; Lisabeth, L.D. Sleep apnea screening is uncommon after stroke. Sleep Med. 2019, 59, 90–93. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, F.; Yegneswaran, B.; Liao, P.; Chung, S.A.; Vairavanathan, S.; Islam, S.; Khajehdehi, A.; Shapiro, C.M. STOP Questionnaire. Anesthesiologists 2008, 108, 812–821. [Google Scholar] [CrossRef] [Green Version]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire To Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef]
- Chung, F.; Abdullah, H.R.; Liao, P. STOP-Bang Questionnaire. Chest 2016, 149, 631–638. [Google Scholar] [CrossRef] [Green Version]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The Occurrence of Sleep-Disordered Breathing among Middle-Aged Adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef] [Green Version]
- Ramos, A.R.; Seixas, A.; Dib, S.I. Obstructive sleep apnea and stroke: Links to health disparities. Sleep Heal. 2015, 1, 244–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olafiranye, O.; Akinboboye, O.; Mitchell, J.E.; Ogedegbe, G.; Jean-Louis, G. Obstructive sleep apnea and cardiovascular disease in blacks: A call to action from the Association of Black Cardiologists. Am. Hear. J. 2013, 165, 468–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, S.M.; Yaggi, H.K.; Taylor, S.; Qin, L.; Ivan, C.S.; Austin, C.; Ferguson, J.; Radulescu, R.; Tobias, L.; Sico, J.; et al. Infarct location and sleep apnea: Evaluating the potential association in acute ischemic stroke. Sleep Med. 2015, 16, 1198–1203. [Google Scholar] [CrossRef] [Green Version]
- Nagappa, M.; Liao, P.; Wong, J.; Auckley, D.; Ramachandran, S.K.; Memtsoudis, S.G.; Mokhlesi, B.; Chung, F. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0143697. [Google Scholar] [CrossRef]
- Kuna, S.T.; Gurubhagavatula, I.; Maislin, G.; Hin, S.; Hartwig, K.C.; McCloskey, S.; Hachadoorian, R.; Hurley, S.; Gupta, R.; Staley, B.; et al. Noninferiority of Functional Outcome in Ambulatory Management of Obstructive Sleep Apnea. Am. J. Respir. Crit. Care Med. 2011, 183, 1238–1244. [Google Scholar] [CrossRef]
- Brown, D.L.; Durkalski, V.; Durmer, J.S.; Broderick, J.P.; Zahuranec, D.B.; A Levine, D.; Anderson, C.S.; Bravata, D.M.; Yaggi, H.K.; Morgenstern, L.B.; et al. Sleep for Stroke Management and Recovery Trial (Sleep SMART): Rationale and methods. Int. J. Stroke 2020, 15, 923–929. Available online: https://clinicaltrials.gov/ct2/show/study/NCT03812653 (accessed on 18 February 2020). [CrossRef] [PubMed]
- McEvoy, R.D.; Antic, N.A.; Heeley, E.; Luo, Y.; Ou, Q.; Zhang, X.; Mediano, O.; Chen, R.; Drager, L.F.; Liu, Z.; et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. N. Engl. J. Med. 2016, 375, 919–931. [Google Scholar] [CrossRef] [PubMed]
- Marti-Soler, H.; Hirotsu, C.; Marques-Vidal, P.; Vollenweider, P.; Waeber, G.; Preisig, M.; Tafti, M.; Tufik, S.B.; Bittencourt, L.; Haba-Rubio, J.; et al. The NoSAS score for screening of sleep-disordered breathing: A derivation and validation study. Lancet Respir. Med. 2016, 4, 742–748. [Google Scholar] [CrossRef] [Green Version]
| Clinical Characteristics | Patient Groups | ||
|---|---|---|---|
| Group 1 (n = 33) | Group 2 (n = 28) | Group 3 (n = 49) | |
| Age, years, mean ± SD | 57.9 ± 9.9 | 59.0 ± 11.8 | 58.4 ± 11.4 |
| Male | 59.4 | 50.0 | 61.2 |
| Race, white | 17.2 | 22.2 | 17.0 |
| Race, Black | 62.1 | 59.3 | 66.0 |
| Ethnicity, Hispanic | 13.8 | 14.8 | 12.8 |
| Ethnicity, Asian | 6.9 | 3.7 | 4.3 |
| Body mass index, kg/m2 ± SD | 30.2 ± 6.8 | 30.0 ± 6.0 | 28.7 ± 5.8 |
| Neck circumference, cm ± SD * | 38.5 ± 4.3 | 37.8 ± 5.8 | 38 ± 5.1 |
| Hypertension | 93.9 | 77.8 | 85.4 |
| Diabetes mellitus | 51.5 | 51.9 | 45.8 |
| Ischemic stroke | 78.8 | 74.1 | 93.8 |
| Intracerebral hemorrhage | 3.0 | 3.7 | 0 |
| Transient ischemic attack | 15.2 | 18.5 | 4.2 |
| Subarachnoid hemorrhage | 3.0 | 3.7 | 2.1 |
| Previous diagnosis of SDB | 0 | 0 | 2.0 |
| Questionnaires | ESS/BQ/SB | n/a | SB |
| Epworth Sleepiness Scale ≥10 | 36.4 | - | - |
| Berlin Questionnaire ≥2 | 60.6 | - | - |
| STOP-BANG ≥3 | 81.8 | - | 77.6 |
| Sleep study results, n (%) | PST | PSG | PST |
| Mild or greater SDB (AHI > 5) | 25/33 (75.8) | 26/28 (92.9) | 37/49 (75.5) |
| Moderate or greater SDB (AHI > 15) | 14/33 (42.4) | 16/28 (57.1) | 21/49 (42.9) |
| OSA only, no CSA | 17/25 (68.0) | 17/26 (65.4) | 21/37 (56.8) |
| CSA only, no OSA | 0 | 0 | 1/37 (2.7) |
| Both OSA and CSA | 8/25 (32.0) | 9/26 (34.6) | 15/37 (40.5) |
| Parameter | Administered Questionnaires | ||
|---|---|---|---|
| BQ (n = 33) | ESS (n = 33) | SB (n = 49) | |
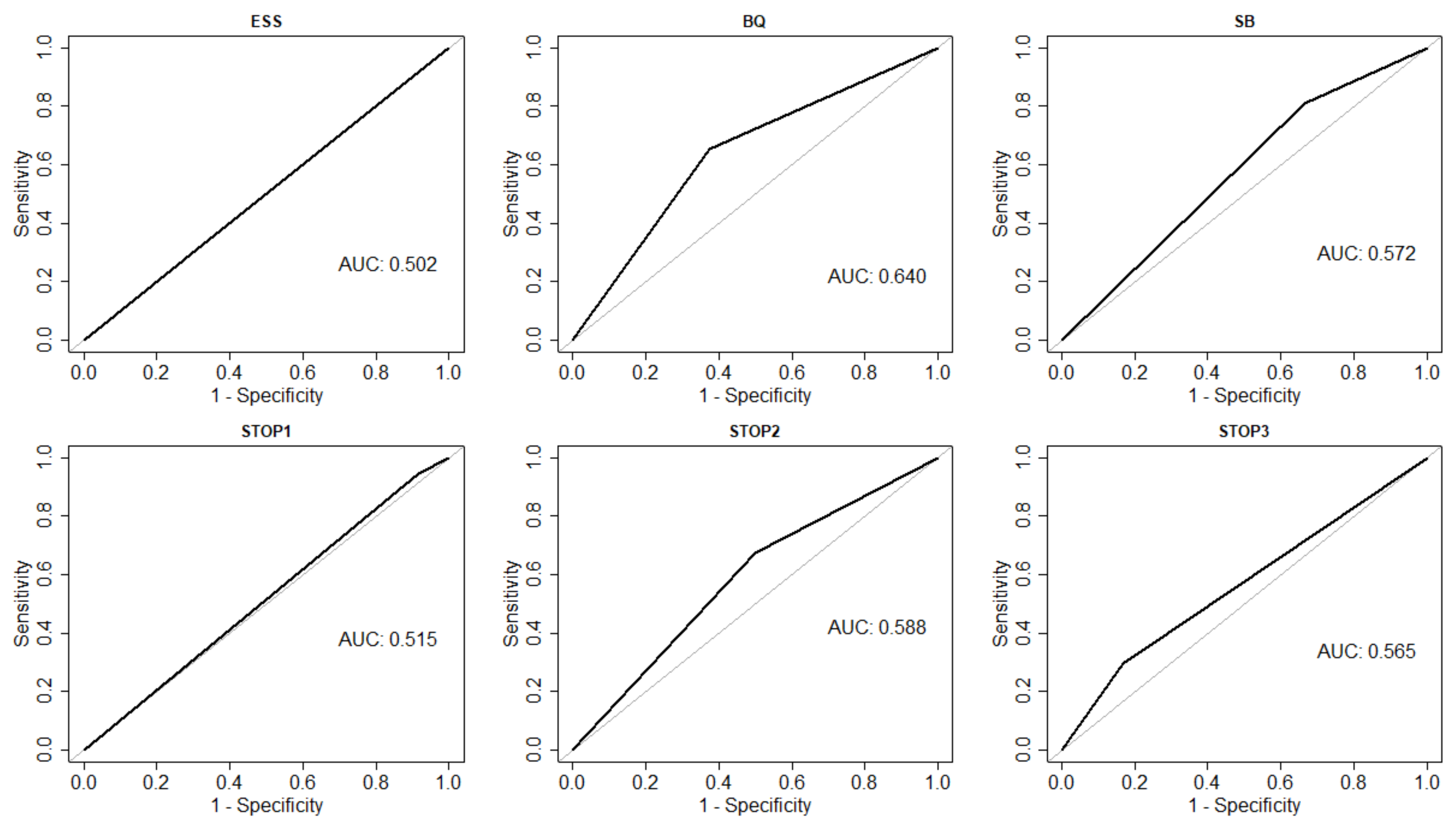
| Sensitivity (95% CI) | 0.68 (0.46,0.85) | 0.36 (0.18,0.57) | 0.81 (0.65,0.92) |
| Specificity (95% CI) | 0.62 (0.24,0.91) | 0.62 (0.24,0.91) | 0.33 (0.10,0.65) |
| Parameter | STOP Questionnaire | ||
|---|---|---|---|
| STOP ≥ 1 (n = 49) | STOP ≥ 2 (n = 49) | STOP ≥ 3 (n = 49) | |
| Sensitivity (95% CI) | 0.95 (0.82, 0.99) | 0.68 (0.50, 0.82) | 0.30 (0.16, 0.47) |
| Specificity (95% CI) | 0.08 (0.00, 0.38) | 0.50 (0.21, 0.79) | 0.83 (0.52, 0.98) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrie, B.K.; Sturzoiu, T.; Shulman, J.; Abbas, S.; Masoud, H.; Romero, J.R.; Filina, T.; Nguyen, T.N.; Lau, H.; Clark, J.; et al. Questionnaire and Portable Sleep Test Screening of Sleep Disordered Breathing in Acute Stroke and TIA. J. Clin. Med. 2021, 10, 3568. https://doi.org/10.3390/jcm10163568
Petrie BK, Sturzoiu T, Shulman J, Abbas S, Masoud H, Romero JR, Filina T, Nguyen TN, Lau H, Clark J, et al. Questionnaire and Portable Sleep Test Screening of Sleep Disordered Breathing in Acute Stroke and TIA. Journal of Clinical Medicine. 2021; 10(16):3568. https://doi.org/10.3390/jcm10163568
Chicago/Turabian StylePetrie, Benjamin K., Tudor Sturzoiu, Julie Shulman, Saleh Abbas, Hesham Masoud, Jose Rafael Romero, Tatiana Filina, Thanh N. Nguyen, Helena Lau, Judith Clark, and et al. 2021. "Questionnaire and Portable Sleep Test Screening of Sleep Disordered Breathing in Acute Stroke and TIA" Journal of Clinical Medicine 10, no. 16: 3568. https://doi.org/10.3390/jcm10163568
APA StylePetrie, B. K., Sturzoiu, T., Shulman, J., Abbas, S., Masoud, H., Romero, J. R., Filina, T., Nguyen, T. N., Lau, H., Clark, J., Auerbach, S., Pyatkevich, Y. G., & Aparicio, H. J. (2021). Questionnaire and Portable Sleep Test Screening of Sleep Disordered Breathing in Acute Stroke and TIA. Journal of Clinical Medicine, 10(16), 3568. https://doi.org/10.3390/jcm10163568







