Early Measurement of Blood sST2 Is a Good Predictor of Death and Poor Outcomes in Patients Admitted for COVID-19 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Variables and Definitions
2.3. Circulating sST2 Measurements
2.4. Statistical Analysis
3. Results
3.1. Hospitalized COVID-19 Patients Exhibit Elevated Serum sST2 Concentrations
3.2. Serum sST2 Levels Correlate with Clinical and Laboratory Index of Disease Severity
3.3. Serum sST2 Concentrations Associate with Adverse Outcomes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Casas-Rojo, J.M.; Antón-Santos, J.M.; Millán-Núñez-Cortés, J.; Lumbreras-Bermejo, C.; Ramos-Rincón, J.M.; Roy-Vallejo, E.; Artero-Mora, A.; Arnalich-Fernández, F.; García-Bruñén, J.M.; Vargas-Núñez, J.A.; et al. Clinical characteristics of patients hospitalized with COVID-19 in Spain: Results from the SEMI-COVID-19 Registry. Rev. Clin. Esp. 2020, 220, 480–494. [Google Scholar] [CrossRef]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Ackermann, M.; Verleden, S.E.; Kuehnel, M.; Haverich, A.; Welte, T.; Laenger, F.; Vanstapel, A.; Werlein, C.; Stark, H.; Tzankov, A.; et al. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N. Engl. J. Med. 2020, 383, 120–128. [Google Scholar] [CrossRef]
- Kox, M.; Waalders, N.J.B.; Kooistra, E.J.; Gerretsen, J.; Pickkers, P. Cytokine Levels in Critically Ill Patients With COVID-19 and Other Conditions. JAMA 2020, 324, 1565–1567. [Google Scholar] [CrossRef]
- Deng, Q.; Hu, B.; Zhang, Y.; Wang, H.; Zhou, X.; Hu, W.; Cheng, Y.; Yan, J.; Ping, H.; Zhou, Q. Suspected myocardial injury in patients with COVID-19: Evidence from front-line clinical observation in Wuhan, China. Int. J. Cardiol. 2020, 311, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Giustino, G.; Croft, L.B.; Stefanini, G.G.; Bragato, R.; Silbiger, J.J.; Vicenzi, M.; Danilov, T.; Kukar, N.; Shaban, N.; Kini, A.; et al. Characterization of Myocardial Injury in Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 2043–2055. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.-C.; Kim, J.Y.; Kim, H.A.; Han, S. COVID-19-related myocarditis in a 21-year-old female patient. Eur. Heart J. 2020, 41, 1859. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Figal, D.A.; Pérez-Martínez, M.T.; Asensio-Lopez, M.C.; Sanchez-Más, J.; García-García, M.E.; Martinez, C.M.; Lencina, M.; Jara, R.; Januzzi, J.L.; Lax, A. Pulmonary Production of Soluble ST2 in Heart Failure. Circ. Heart Fail. 2018, 11, e005488. [Google Scholar] [CrossRef]
- Villacorta, H.; Maisel, A.S. Soluble ST2 Testing: A Promising Biomarker in the Management of Heart Failure. Arq. Bras. Cardiol. 2016, 106, 145–152. [Google Scholar] [CrossRef]
- Griesenauer, B.; Paczesny, S. The ST2/IL-33 Axis in Immune Cells during Inflammatory Diseases. Front. Immunol. 2017, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Bayés-Genis, A.; González, A.; Lupón, J. ST2 in Heart Failure. Circ. Heart Fail. 2018, 11, e005582. [Google Scholar] [CrossRef]
- Bajwa, E.K.; Mebazaa, A.; Januzzi, J.L. ST2 in Pulmonary Disease. Am. J. Cardiol. 2015, 115, 44B–47B. [Google Scholar] [CrossRef] [PubMed]
- Bajwa, E.K.; Volk, J.A.; Christiani, D.C.; Harris, R.S.; Matthay, M.A.; Thompson, B.T.; Januzzi, J.L.; National Heart, L.A.B.I.A.R.D.S.N. Prognostic and diagnostic value of plasma soluble suppression of tumorigenicity-2 concentrations in acute respiratory distress syndrome. Crit. Care Med. 2013, 41, 2521–2531. [Google Scholar] [CrossRef]
- Gupta, R.K.; Harrison, E.M.; Ho, A.; Docherty, A.B.; Knight, S.R.; van Smeden, M.; Abubakar, I.; Lipman, M.; Quartagno, M.; Pius, R.; et al. Development and validation of the ISARIC 4C Deterioration model for adults hospitalised with COVID-19: A prospective cohort study. Lancet Respir. Med. 2021, 9, 349–359. [Google Scholar] [CrossRef]
- Gupta, R.K.; Marks, M.; Samuels, T.H.A.; Luintel, A.; Rampling, T.; Chowdhury, H.; Quartagno, M.; Nair, A.; Lipman, M.; Abubakar, I.; et al. Systematic evaluation and external validation of 22 prognostic models among hospitalised adults with COVID-19: An observational cohort study. Eur. Respir. J. 2020, 56, 2003498. [Google Scholar] [CrossRef]
- Gutiérrez-Gutiérrez, B.; Del Toro, M.D.; Borobia, A.M.; Carcas, A.; Jarrín, I.; Yllescas, M.; Ryan, P.; Pachón, J.; Carratalà, J.; Berenguer, J.; et al. Identification and validation of clinical phenotypes with prognostic implications in patients admitted to hospital with COVID-19: A multicentre cohort study. Lancet Infect. Dis. 2021, 21, 783–792. [Google Scholar] [CrossRef]
- Chua, F.; Vancheeswaran, R.; Draper, A.; Vaghela, T.; Knight, M.; Mogal, R.; Singh, J.; Spencer, L.G.; Thwaite, E.; Mitchell, H.; et al. Early prognostication of COVID-19 to guide hospitalisation versus outpatient monitoring using a point-of-test risk prediction score. Thorax 2021, 76, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Torres-Macho, J.; Ryan, P.; Valencia, J.; Pérez-Butragueño, M.; Jiménez, E.; Fontán-Vela, M.; Izquierdo-García, E.; Fernandez-Jimenez, I.; Álvaro-Alonso, E.; Lazaro, A.; et al. The PANDEMYC Score. An Easily Applicable and Interpretable Model for Predicting Mortality Associated With COVID-19. J. Clin. Med. 2020, 9, 3066. [Google Scholar] [CrossRef]
- Heldt, F.S.; Vizcaychipi, M.P.; Peacock, S.; Cinelli, M.; McLachlan, L.; Andreotti, F.; Jovanović, S.; Dürichen, R.; Lipunova, N.; Fletcher, R.A.; et al. Early risk assessment for COVID-19 patients from emergency department data using machine learning. Sci. Rep. 2021, 11, 4200. [Google Scholar] [CrossRef]
- Estiri, H.; Strasser, Z.H.; Murphy, S.N. Individualized prediction of COVID-19 adverse outcomes with MLHO. Sci. Rep. 2021, 11, 5322. [Google Scholar] [CrossRef]
- Tersalvi, G.; Vicenzi, M.; Calabretta, D.; Biasco, L.; Pedrazzini, G.; Winterton, D. Elevated Troponin in Patients with Coronavirus Disease 2019: Possible Mechanisms. J. Card. Fail. 2020, 26, 470–475. [Google Scholar] [CrossRef]
- Malik, P.; Patel, U.; Mehta, D.; Patel, N.; Kelkar, R.; Akrmah, M.; Gabrilove, J.L.; Sacks, H. Biomarkers and outcomes of COVID-19 hospitalisations: Systematic review and meta-analysis. BMJ Evid. Based Med. 2021, 26, 107–108. [Google Scholar] [CrossRef]
- Rubio-Gracia, J.; Giménez-López, I.; Garcés-Horna, V.; López-Delgado, D.; Sierra-Monzón, J.L.; Martínez-Lostao, L.; Josa-Laorden, C.; Ruiz-Laiglesia, F.; Pérez-Calvo, J.I.; Crespo-Aznarez, S.; et al. Point-of-care lung ultrasound assessment for risk stratification and therapy guiding in COVID-19 patients. A prospective non-interventional study. Eur. Respir. J. 2021, 2004283. [Google Scholar] [CrossRef]
- Sánchez-Marteles, M.; Rubio-Gracia, J.; Peña-Fresneda, N.; Garcés-Horna, V.; Gracia-Tello, B.; Martínez-Lostao, L.; Crespo-Aznárez, S.; Pérez-Calvo, J.I.; Giménez-López, I. Early measurement of blood sST2 is a good predictor of death and poor outcomes in patients admitted for COVID-19 infection. medRxiv 2021. [Google Scholar] [CrossRef]
- Zeng, Z.; Hong, X.Y.; Li, Y.; Chen, W.; Ye, G.; Li, Y.; Luo, Y. Serum-soluble ST2 as a novel biomarker reflecting inflammatory status and illness severity in patients with COVID-19. Biomark. Med. 2020, 14, 1619–1629. [Google Scholar] [CrossRef]
- Burke, H.; Freeman, A.; Cellura, D.C.; Stuart, B.L.; Brendish, N.J.; Poole, S.; Borca, F.; Phan, H.T.T.; Sheard, N.; Williams, S.; et al. Inflammatory phenotyping predicts clinical outcome in COVID-19. Respir. Res. 2020, 21, 245. [Google Scholar] [CrossRef]
- Abers, M.S.; Delmonte, O.M.; Ricotta, E.E.; Fintzi, J.; Fink, D.L.; de Jesus, A.A.A.; Zarember, K.A.; Alehashemi, S.; Oikonomou, V.; Desai, J.V.; et al. An immune-based biomarker signature is associated with mortality in COVID-19 patients. JCI Insight 2021, 6, 144455. [Google Scholar] [CrossRef] [PubMed]
- Luft, T.; Wendtner, C.M.; Kosely, F.; Radujkovic, A.; Benner, A.; Korell, F.; Kihm, L.; Bauer, M.F.; Dreger, P.; Merle, U. EASIX for Prediction of Outcome in Hospitalized SARS-CoV-2 Infected Patients. Front. Immunol. 2021, 12, 634416. [Google Scholar] [CrossRef] [PubMed]
- Alladina, J.W.; Giacona, F.L.; White, E.B.; Brait, K.L.; Abe, E.A.; Michelhaugh, S.A.; Hibbert, K.A.; Januzzi, J.L.; Thompson, B.T.; Cho, J.L.; et al. Soluble Suppression of Tumorigenicity-2 Associates with Ventilator Dependence in Coronavirus Disease 2019 Respiratory Failure. Crit. Care Explor. 2021, 3, e0480. [Google Scholar] [CrossRef] [PubMed]
- Huang, J. Comparing biomarkers for COVID-19 disease with commonly associated preexisting conditions and complications. medRxiv 2020. [Google Scholar] [CrossRef]
- Zizzo, G.; Cohen, P.L. Imperfect storm: Is interleukin-33 the Achilles heel of COVID-19 Lancet Rheumatol. Lancet Rheumatol. 2020, 2, e779–e790. [Google Scholar] [CrossRef]
- Liang, Y.; Ge, Y.; Sun, J. IL-33 in COVID-19: Friend or foe? Cell. Mol. Immunol. 2021, 18, 1602–1604. [Google Scholar] [CrossRef]
- Miftode, R.S.; Petriș, A.O.; Onofrei Aursulesei, V.; Cianga, C.; Costache, I.I.; Mitu, O.; Miftode, I.L.; Șerban, I.L. The Novel Perspectives Opened by ST2 in the Pandemic: A Review of Its Role in the Diagnosis and Prognosis of Patients with Heart Failure and COVID-19. Diagnostics 2021, 11, 175. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, R.; Basta, G.; Del Turco, S.; Caselli, C. A possible role for ST2 as prognostic biomarker for COVID-19. Vascul. Pharmacol. 2021, 138, 106857. [Google Scholar] [CrossRef]
- Magat, J.M.; Thomas, J.L.; Dumouchel, J.P.; Murray, F.; Li, W.X.; Li, J. Endogenous IL-33 and Its Autoamplification of IL-33/ST2 Pathway Play an Important Role in Asthma. J. Immunol. 2020, 204, 1592–1597. [Google Scholar] [CrossRef] [Green Version]
- Le Goffic, R.; Arshad, M.I.; Rauch, M.; L’Helgoualc’h, A.; Delmas, B.; Piquet-Pellorce, C.; Samson, M. Infection with influenza virus induces IL-33 in murine lungs. Am. J. Respir Cell Mol. Biol. 2011, 45, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Lynch, J.P.; Werder, R.B.; Simpson, J.; Loh, Z.; Zhang, V.; Haque, A.; Spann, K.; Sly, P.D.; Mazzone, S.B.; Upham, J.W.; et al. Aeroallergen-induced IL-33 predisposes to respiratory virus-induced asthma by dampening antiviral immunity. J. Allergy Clin. Immunol. 2016, 138, 1326–1337. [Google Scholar] [CrossRef] [Green Version]
- Toubiana, J.; Poirault, C.; Corsia, A.; Bajolle, F.; Fourgeaud, J.; Angoulvant, F.; Debray, A.; Basmaci, R.; Salvador, E.; Biscardi, S.; et al. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: Prospective observational study. BMJ 2020, 369, m2094. [Google Scholar] [CrossRef]
- Sato, Y.Z.; Molkara, D.P.; Daniels, L.B.; Tremoulet, A.H.; Shimizu, C.; Kanegaye, J.T.; Best, B.M.; Snider, J.V.; Frazer, J.R.; Maisel, A.; et al. Cardiovascular biomarkers in acute Kawasaki disease. Int. J. Cardiol. 2013, 164, 58–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Tang, K.; Levin, M.; Irfan, O.; Morris, S.K.; Wilson, K.; Klein, J.D.; Bhutta, Z.A. COVID-19 and multisystem inflammatory syndrome in children and adolescents. Lancet Infect. Dis. 2020, 20, e276–e288. [Google Scholar] [CrossRef]
- Clerkin, K.J.; Fried, J.A.; Raikhelkar, J.; Sayer, G.; Griffin, J.M.; Masoumi, A.; Jain, S.S.; Burkhoff, D.; Kumaraiah, D.; Rabbani, L.; et al. COVID-19 and Cardiovascular Disease. Circulation 2020, 141, 1648–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayes-Genis, A.; de Antonio, M.; Galán, A.; Sanz, H.; Urrutia, A.; Cabanes, R.; Cano, L.; González, B.; Díez, C.; Pascual, T.; et al. Combined use of high-sensitivity ST2 and NTproBNP to improve the prediction of death in heart failure. Eur. J. Heart Fail. 2012, 14, 32–38. [Google Scholar] [CrossRef] [PubMed]


| Variable | Total | p < 25 | P25 to 75 | p > 75 | p-Value |
|---|---|---|---|---|---|
| Total size (n) | 144 | 36 | 73 | 35 | |
| Age (years) | 57.5 ± 12.8 | 58.5 ± 12.3 | 57.2 ± 13.6 | 57.1 ± 12.0 | 0.878 |
| Gender-Male (n(%)) | 87 (60.4) | 17 (47.2) | 44 (60.3) | 26 (74.3) | 0.066 |
| Duration of symptom (days) | 6.5 ± 3.3 | 7.0 ± 3.6 | 6.3 ± 3.3 | 6.3 ± 2.7 | 0.486 |
| Time until COVID confirmation (Days) | 3 (7) | 3 (7) | 3 (6) | 3 (6) | 0.492 |
| Comorbidities (n(%)): | |||||
| - Hypertension | 54 (37.5) | 15 (41.7) | 26 (35.6) | 13 (37.1) | 0.827 |
| - Heart failure | 4 (2.8) | 1 (2.8) | 2 (2.8) | 1 (2.9) | 1.000 |
| - Dyslipidemia | 42 (29.2) | 9 (25.0) | 19 (26.0) | 14 (40.0) | 0.267 |
| - Coronary artery disease | 5 (3.5) | 1 (2.8) | 3 (4.1) | 1 (2.9) | 0.914 |
| - Diabetes | 25 (17.4) | 6 (16.7) | 9 (12.3) | 10 (28.6) | 0.113 |
| - History of smoking | 48 (33.6) | 10 (27.8) | 22 (30.6) | 16 (45.7) | 0.207 |
| - COPD/Asthma | 16 (11.1) | 3 (8.3) | 10 (13.7) | 3 (8.6) | 0.605 |
| - Atrial/flutter fibrillation | 5 (3.6) | 1 (2.9) | 3 (4.3) | 1 (2.9) | 0.901 |
| - CKD | 7 (4.9) | 3 (8.3) | 2 (2.7) | 2 (5.7) | 0.427 |
| Clinical variables | |||||
| - BMI (Kgs/m2) | 28.9 (6.4) | 27.5 (0.5) | 28.7 (5.7) | 30.0 (6.0) | 0.434 |
| - SBP (mmHg) | 126.9 ± 16.7 | 132.5 ± 14.4 | 125.2 ± 15.7 | 124.6 ± 17.0 | 0.066 |
| - DBP (mmHg) | 77.2 ± 10.9 | 80.7 ± 11.6 | 77.0 ± 10.3 | 74.2 ± 10.6 | 0.051 |
| - HR (bpm) | 80.9 ± 12.8 | 80.9 ± 12.1 | 81.0 ± 12.8 | 80.5 ± 13.7 | 0.980 |
| - Estimated PAFI (mmHg) | 367 (92) | 429 (101) | 403 (99) | 341 (108) | <0.001 |
| - Borg scale for dyspnea (points) | 4 (6) | 3 (6) | 5 (5) | 4 (6) | 0.486 |
| Laboratory: | |||||
| - Urea (mg/dL) | 33 (19) | 38 (16) | 32 (18) | 32 (20) | 0.069 |
| - Creatinine (mg/dL) | 0.94 (0.29) | 0.91 (0.27) | 0.88 (0.29) | 0.92 (0.36) | 0.318 |
| Laboratory: | |||||
| - Aspartate aminotransferase (U/L) | 37 (27) | 30 (16) | 38 (31) | 41 (24) | <0.001 |
| - Alanine aminotransferase (U/L) | 31 (28) | 23 (23) | 32 (43) | 33 (18) | 0.006 |
| - Creatin phosphokinase (U/L) | 94 (92) | 71 (80) | 98 (92) | 116 (143) | 0.007 |
| - Lactate dehydrogenase (U/L) | 306 (145) | 267 (70) | 310 (106) | 398 (208) | <0.001 |
| - C-Reactive Protein (mg/L) | 63 (81) | 46 (63) | 59 (68) | 112 (137) | 0.005 |
| - Ferritin (ng/mL) | 707 (908) | 619 (838) | 676 (813) | 1338 (1061) | 0.007 |
| - Hemoglobin (g/dL) | 14.2 ± 1.5 | 14.2 ± 1.3 | 14.3 ± 1.6 | 14.1 ± 1.4 | 0.234 |
| - Total leucocytes (×1000) | 5.6 (3.1) | 5.2 (2.1) | 5.4 (3.4) | 6.1 (3.4) | 0.251 |
| - Total lymphocytes (×1000) | 0.9 (0.7) | 1.0 (0.5) | 0.9 (0.6) | 0.6 (0.6) | 0.021 |
| - D-Dimer (ng/mL) | 688 (633) | 719 (856) | 625 (502) | 976 (830) | 0.030 |
| - Fibrinogen (mg/dL) | 775 (208) | 739 (257) | 761 (200) | 811 (252) | 0.011 |
| - Interleukine-6 (pg/mL) | 40 (30) | 26.8 (32.4) | 42.3 (26.2) | 50.0 (24.3) | 0.008 |
| Chest X-Ray (n(%)): | 0.222 | ||||
| - Normal | 25 (17.9) | 8 (23.5) | 13 (18.3) | 4 (11.4) | |
| - Unilateral pneumoniae | 35 (25.0) | 9 (26.5) | 13 (18.3) | 13 (37.1) | |
| - Bilateral pneumoniae | 80 (57.1) | 17 (50.0) | 45 (63.4) | 18 (51.4) | |
| Baseline therapies (n(%)) | |||||
| - Colchicine | 10 (6.9) | 2 (5.6) | 5 (6.8) | 3 (8.6) | 0.882 |
| - Plasma | 1 (0.7) | 0 (0.0) | 1 (1.4) | 0 (0.0) | 0.613 |
| - Remdesivir | 46 (31.9) | 9 (25.0) | 23 (31.5) | 14 (40.0) | 0.397 |
| - Systemic corticosteroids | 113 (78.5) | 26 (72.2) | 56 (76.8) | 31 (88.6) | 0.214 |
| - Medium dose of corticosteroids (Dexamethasone (mg)) | 6 (3) | 6 (3) | 6 (3) | 6 (3) | 1.000 |
| - Low molecular weight heparin | 138 (95.8) | 34 (94.4) | 70 (95.9) | 34 (97.2) | 0.488 |
| Variable | Total | sST2 < P25 | sST2 P25–P75 | sST2 > P75 | p-Value |
|---|---|---|---|---|---|
| Primary outcome (n[%]): | |||||
| • ICU admission and/or death | 15 (10.4) | 0 (0) | 6 (8.2) | 9 (25.7) | <0.001 |
| Secondary outcomes: | |||||
| • Length of stay (days) | 8 (6) | 8 (6) | 7 (5) | 8 (7) | 0.328 |
| • Need for HOF at 48/72 h (n[%]) | 47 (34.1) | 10 (28.6) | 20 (28.6) | 17 (51.5) | 0.053 |
| • Need to increase COVID-19 treatment at 48/72 h (n[%]) | 53 (37.9) | 11 (30.6) | 25 (35.7) | 17 (50.0) | 0.214 |
| • Necessity of HOF or increase COVID-19 treatment at 48/72 h (n[%]) | 66 (48.5) | 14 (40.0) | 32 (47.1) | 20 (60.6) | 0.223 |
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Variable | HR (CI 95%) | p-Value | HR (CI 95%) | p-Value |
| Age | 1.04 (0.99–1.09) | 0.073 | ||
| Gender-male | 1.38 (0.47–4.05) | 0.555 | ||
| BMI | 1.08 (0.98–1.16) | 0.089 | ||
| Diabetes | 2.73 (0.84–8.82) | 0.094 | ||
| Dyslipidemia | 3.19 (1.08–9.47) | 0.036 | ||
| SBP | 1.00 (0.97–1.03) | 0.937 | ||
| DBP | 0.97 (0.93–1.03) | 0.366 | ||
| Estimated PAFI * | 0.98 (0.97–0.99) | 0.001 | ||
| Urea * | 1.45 (0.55–3.83) | 0.455 | ||
| Aspartate transaminase * | 1.31 (0.51–3.41) | 0.576 | ||
| Alanine transaminase * | 0.94 (0.41–2.15) | 0.941 | ||
| Creatin phosphokinase * | 2.06 (1.01–4.20) | 0.047 | ||
| Lactate dehydrogenase * | 5.13 (0.93–28.2) | 0.060 | ||
| C-Reactive Protein * | 1.33 (0.73–2.44) | 0.357 | ||
| Ferritin * | 1.00 (0.56–1.80) | 0.999 | ||
| Total lymphocytes * | 1.13 (0.54–2.36) | 0.747 | ||
| D-Dimer * | 1.23 (0.68–2.22) | 0.501 | ||
| Fibrinogen * | 0.57 (0.06–5.51) | 0.629 | ||
| Interleukin-6 * | 1.28 (0.63–2.58) | 0.491 | ||
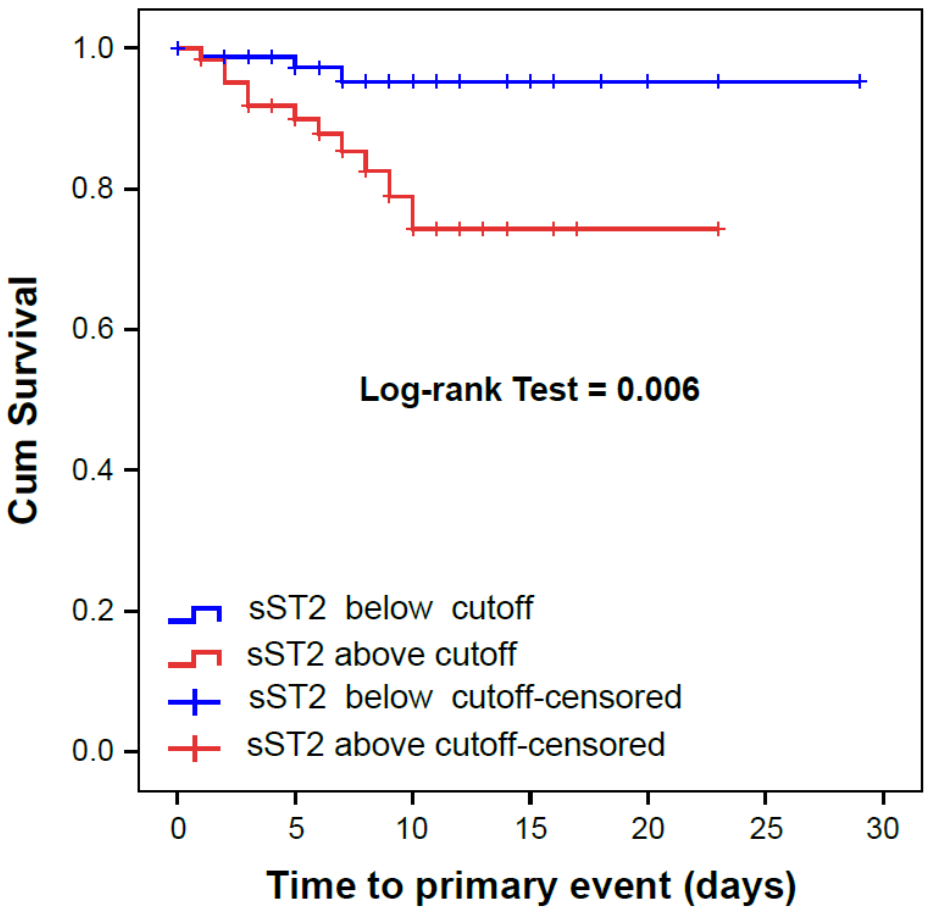
| sST2 (cut-off > 58.9 ng/mL †) | 6.32 (1.70–23.5) | 0.006 | 9.73 (2.12–44.8) | 0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Marteles, M.; Rubio-Gracia, J.; Peña-Fresneda, N.; Garcés-Horna, V.; Gracia-Tello, B.; Martínez-Lostao, L.; Crespo-Aznárez, S.; Pérez-Calvo, J.I.; Giménez-López, I. Early Measurement of Blood sST2 Is a Good Predictor of Death and Poor Outcomes in Patients Admitted for COVID-19 Infection. J. Clin. Med. 2021, 10, 3534. https://doi.org/10.3390/jcm10163534
Sánchez-Marteles M, Rubio-Gracia J, Peña-Fresneda N, Garcés-Horna V, Gracia-Tello B, Martínez-Lostao L, Crespo-Aznárez S, Pérez-Calvo JI, Giménez-López I. Early Measurement of Blood sST2 Is a Good Predictor of Death and Poor Outcomes in Patients Admitted for COVID-19 Infection. Journal of Clinical Medicine. 2021; 10(16):3534. https://doi.org/10.3390/jcm10163534
Chicago/Turabian StyleSánchez-Marteles, Marta, Jorge Rubio-Gracia, Natacha Peña-Fresneda, Vanesa Garcés-Horna, Borja Gracia-Tello, Luis Martínez-Lostao, Silvia Crespo-Aznárez, Juan Ignacio Pérez-Calvo, and Ignacio Giménez-López. 2021. "Early Measurement of Blood sST2 Is a Good Predictor of Death and Poor Outcomes in Patients Admitted for COVID-19 Infection" Journal of Clinical Medicine 10, no. 16: 3534. https://doi.org/10.3390/jcm10163534
APA StyleSánchez-Marteles, M., Rubio-Gracia, J., Peña-Fresneda, N., Garcés-Horna, V., Gracia-Tello, B., Martínez-Lostao, L., Crespo-Aznárez, S., Pérez-Calvo, J. I., & Giménez-López, I. (2021). Early Measurement of Blood sST2 Is a Good Predictor of Death and Poor Outcomes in Patients Admitted for COVID-19 Infection. Journal of Clinical Medicine, 10(16), 3534. https://doi.org/10.3390/jcm10163534






