Feasibility of Wet and Non-Wet Placement of Seprafilm in Laparoscopic Surgeries: A Randomized Controlled Trial
Abstract
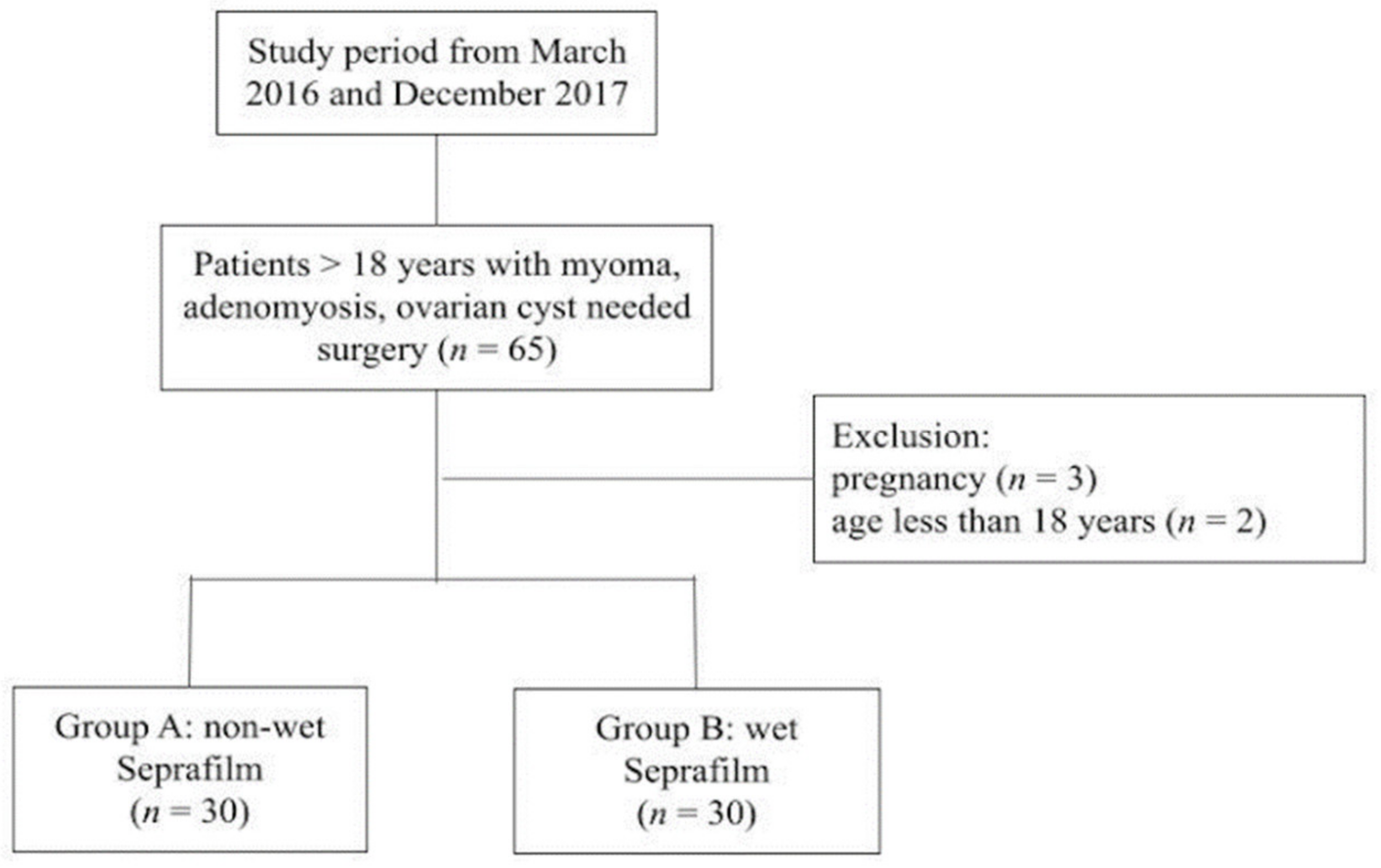
:1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Patients
2.3. Trial Design and Interventions
2.4. Surgical Technique
2.5. Outcomes
2.6. Statistical Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beck, D.E.; Cohen, Z.; Fleshman, J.W.; Kaufman, H.S.; van Goor, H.; Wolff, B.G. A Prospective, Randomized, Multicenter, Controlled Study of the Safety of Seprafilm® Adhesion Barrier in Abdominopelvic Surgery of the Intestine. Dis. Colon Rectum 2003, 46, 1310–1319. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.M.; Dayton, M.T.; Fazio, V.W.; Beck, D.E.; Stryker, S.J.; Wexner, S.D.; Wolff, B.G.; Roberts, P.L.; Smith, L.E.; Sweeney, S.A.; et al. Prevention of Postoperative Abdominal Adhesions by a Sodium Hyaluronate-Based Bioresorbable Membrane: A Prospective, Randomized, Double-Blind Multicenter Study. J. Am. Coll. Surg. 1996, 183, 297–306. [Google Scholar] [PubMed]
- Diamond, M.P.; Seprafilm Adhesion Study Group. Reduction of Adhesions after Uterine Myomectomy by Seprafilm* Membrane (HAL-F): A Blinded, Prospective, Randomized, Multicenter Clinical Study. Fertil. Steril. 1996, 66, 904–910. [Google Scholar] [CrossRef]
- Uchida, K.; Urata, H.; Mohri, Y.; Inoue, M.; Miki, C.; Kusunoki, M. Seprafilm Does Not Aggravate Intraperitoneal Septic Conditions or Evoke Systemic Inflammatory Response. Surg. Today 2005, 35, 1054–1059. [Google Scholar] [CrossRef] [PubMed]
- González-Quintero, V.H.; Cruz-Pachano, F.E. Preventing Adhesions in Obstetric and Gynecologic Surgical Procedures. Rev. Obstet. Gynecol. 2009, 2, 38–45. [Google Scholar] [PubMed]
- Khaitan, E.; Scholz, S.; Richards, W.O. Laparoscopic Adhesiolysis and Placement of Seprafilm: A New Technique and Novel Approach to Patients with Intractable Abdominal Pain. J. Laparoendosc. Adv. Surg. Tech. A 2002, 12, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Shinohara, T.; Kashiwagi, H.; Yanagisawa, S.; Yanaga, K. A Simple and Novel Technique for the Placement of Antiadhesive Membrane in Laparoscopic Surgery. Surg. Laparosc. Endosc. Percutan. Tech. 2008, 18, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Kitade, M.; Kikuchi, I.; Shimanuki, H.; Kinoshita, K. A Novel Instrument and Technique for Using Seprafilm Hyaluronic Acid/carboxymethylcellulose Membrane during Laparoscopic Myomectomy. J. Laparoendosc. Adv. Surg. Tech. A 2006, 16, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Fan, C.-N.; Cho, F.-N.; Kan, Y.-Y.; Chang, Y.-H.; Kang, H.-Y. A Novel Technique to Apply a Seprafilm (hyaluronate-Carboxymethylcellulose) Barrier Following Laparoscopic Surgeries. Fertil. Steril. 2008, 90, 1959–1963. [Google Scholar] [CrossRef] [PubMed]
- Kusuki, I.; Suganuma, I.; Ito, F.; Akiyama, M.; Sasaki, A.; Yamanaka, K.; Tatsumi, H.; Kitawaki, J. Usefulness of Moistening Seprafilm before Use in Laparoscopic Surgery. Surg. Laparosc. Endosc. Percutan. Tech. 2014, 24, e13–e15. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-K.; Ding, D.-C. Seprafilm® Application Method in Laparoscopic Surgery. JSLS 2017, 21, e2016.00097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, K.; Sato, K.; Ogasawara, J.; Takahashi, T.; Mizunuma, H.; Tanaka, M. Safe and Easy Technique for the Laparoscopic Application of Seprafilm® in Gynecologic Surgery. Asian J. Endosc. Surg. 2019, 12, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.-C.; Lu, H.-F.; Peng, F.-S.; Ting, W.-H.S.; Tu, F.-C.; Chen, M.-J.; Kan, Y.-Y. Modified Novel Technique for Improving the Success Rate of Applying Seprafilm by Using Laparoscopy. J. Minim. Invasive Gynecol. 2014, 21, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Weng, C.H.; Chao, A.-S.; Huang, H.-Y.; Huang, Y.-T.; Wu, K.-Y.; Su, Y.-Y.; Yang, L.-Y.; Chao, A.; Wang, C.-J. A Simple Technique for the Placement of Seprafilm, a Sodium Hyaluronate or Carboxymethylcellulose Absorbable Barrier, during Laparoscopic Myomectomy. J. Minim. Invasive Gynecol. 2020, 27, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
| A (Non-Wet) (n = 30) | B (Wet) (n = 30) | p Value | |
|---|---|---|---|
| Age | 44.2 ± 6.8 | 41.1 ± 5.6 | 0.063 |
| Body mass index (kg/m2) | 24.0 ± 4.0 | 24.2 ± 3.9 | 0.804 |
| Parity | 1.5 ± 1.1 | 1.2 ± 1.0 | 0.291 |
| Surgical time (minute) | 161.3 ± 58.3 | 142.3 ± 32.5 | 0.125 |
| Hospital stay (days) | 4.2 ± 1.1 | 4.9 ± 1.2 | 0.04 |
| Operation type | 0.027 | ||
| LAVH | 18 (60%) | 11 (36.7%) | |
| LM | 6 (20%) | 3 (10%) | |
| Adnexa surgery | 6 (20%) | 16 (53.3%) |
| A (Non-Wet) (n = 30) | B (Wet) (n = 30) | p Value | |
|---|---|---|---|
| Preparation time (second) | 32.6 ± 16.6 | 79.5 ± 22.0 | <0.001 |
| Placement time (second) | 599.5 ± 90.1 | 592.5 ± 105.8 | 0.7 |
| Successful placement rate | 95.% (171/180) | 97.70% (176/180) | 0.09 |
| Correct placement rate | 93.80% (169/180) | 96.60% (174/180) | 0.2 |
| Failure rate | 30% (9/30) | 13% (4/30) | 0.2 |
| Operative Time | n | Mean | SD | F Value | p Value |
|---|---|---|---|---|---|
| Failure | 13 | 172.3 | 43.7 | 3.15 | 0.08 |
| Success | 47 | 146.2 | 47.8 |
| Group A (Non-Wet) | Group B (Wet) | p Value | |
|---|---|---|---|
| N | 9 | 4 | |
| Age | 46 ± 6.8 | 43.3 ± 5.7 | 0.5 |
| Parity | 1.7 ± 1.0 | 0.7 ± 0.9 | 0.1 |
| BMI | 24.4 ± 5.0 | 23.6 ± 3.9 | 0.7 |
| Operative time (minute) | 169.4 ± 48.5 | 178.8 ± 35.6 | 0.7 |
| Hospital stay (day) | 4 ± 0.8 | 5.7 ± 0.9 | 0.008 |
| Preparation time (second) | 39.4 ± 26.8 | 86.5 ± 32.9 | 0.02 |
| Placement time (second) | 638.5 ± 88.3 | 691.5 ± 97.8 | 0.3 |
| Operation type | 0.1 | ||
| LAVH | 5 | 0 | |
| LM | 2 | 1 | |
| Adnexa surgery | 2 | 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsu, C.-S.; Ding, D.-C. Feasibility of Wet and Non-Wet Placement of Seprafilm in Laparoscopic Surgeries: A Randomized Controlled Trial. J. Clin. Med. 2021, 10, 3526. https://doi.org/10.3390/jcm10163526
Hsu C-S, Ding D-C. Feasibility of Wet and Non-Wet Placement of Seprafilm in Laparoscopic Surgeries: A Randomized Controlled Trial. Journal of Clinical Medicine. 2021; 10(16):3526. https://doi.org/10.3390/jcm10163526
Chicago/Turabian StyleHsu, Chun-Shuo, and Dah-Ching Ding. 2021. "Feasibility of Wet and Non-Wet Placement of Seprafilm in Laparoscopic Surgeries: A Randomized Controlled Trial" Journal of Clinical Medicine 10, no. 16: 3526. https://doi.org/10.3390/jcm10163526
APA StyleHsu, C.-S., & Ding, D.-C. (2021). Feasibility of Wet and Non-Wet Placement of Seprafilm in Laparoscopic Surgeries: A Randomized Controlled Trial. Journal of Clinical Medicine, 10(16), 3526. https://doi.org/10.3390/jcm10163526







