Impact of a Breathing Intervention on Engagement of Abdominal, Thoracic, and Subclavian Musculature during Exercise, a Randomized Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Study Design
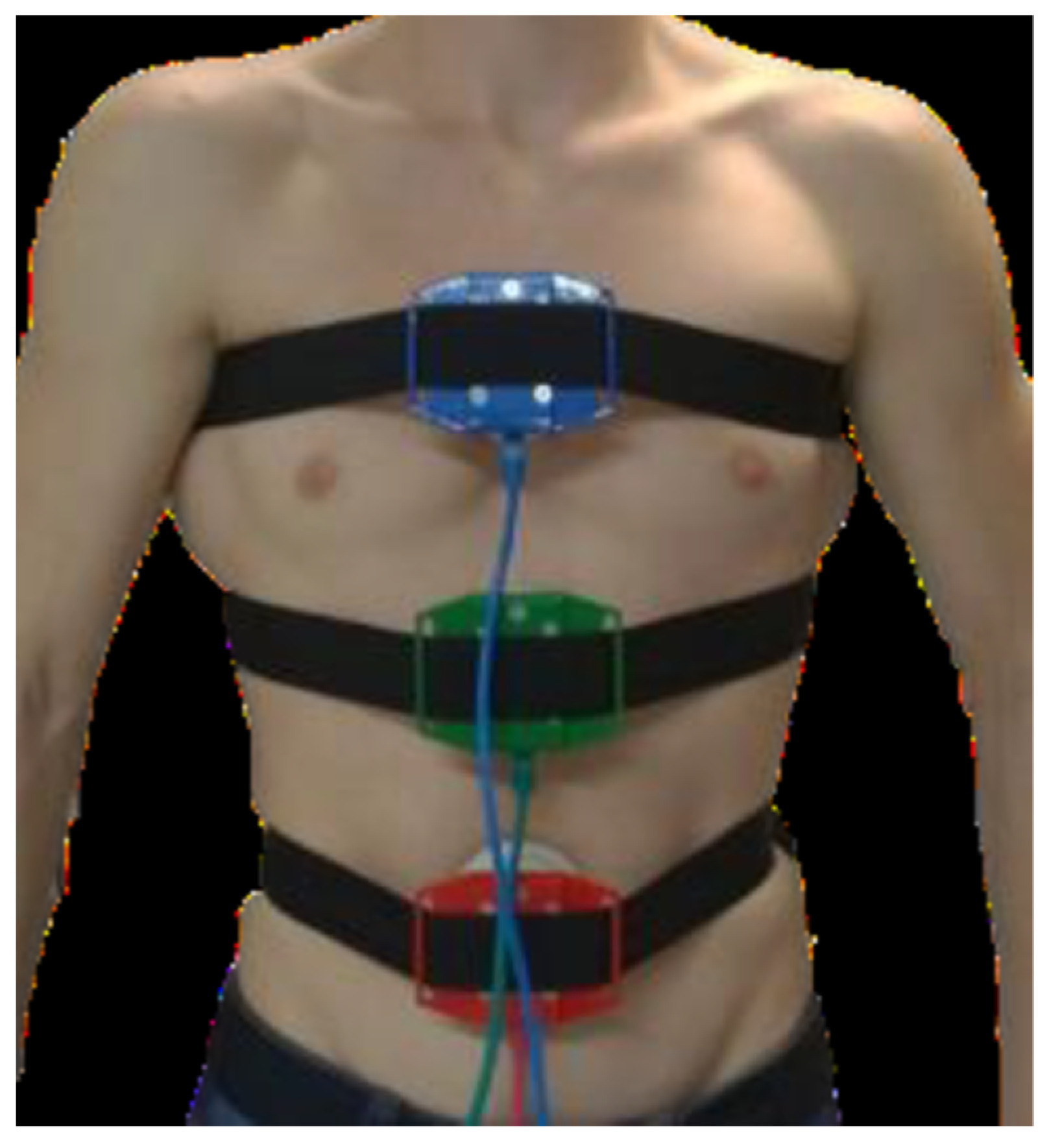
2.2.1. Measurement of Sectors Engagement
2.2.2. Measurement of Ventilatory Parameters
2.2.3. Breathing Exercise Program
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bravo-Sánchez, A.; Morán-García, J.; Abián, P.; Abián-Vicén, J. Association of the Use of the Mobile Phone with Physical Fitness and Academic Performance: A Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 1042. [Google Scholar] [CrossRef] [PubMed]
- Eitivipart, A.C.; Viriyarojanakul, S.; Redhead, L. Musculoskeletal disorder and pain associated with smartphone use: A systematic review of biomechanical evidence. Hong Kong Physiother. J. 2018, 38, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Depiazzi, J.; Everard, M.L. Dysfunctional breathing and reaching one’s physiological limit as causes of exercise-induced dyspnoea. Breathe 2016, 12, 120–129. [Google Scholar] [CrossRef]
- Chaitow, L.; Bradley, D.; Gilbert, C. Multidisciplinary Approaches to Breathing Pattern Disorders; Churchill Livingston: London, UK, 2002. [Google Scholar]
- Kaminoff, L. What yoga therapists should know about the anatomy of breathing. Int. J. Yoga Therap. 2006, 16, 67–77. [Google Scholar] [CrossRef]
- Pryor, J.A.; Prasad, S.A. Physiotherapy for Respiratory and Cardiac Problems; Churchill Livingstone: Edinburgh, UK, 2002. [Google Scholar]
- Yuan, G.; Drost, N.A.; McIvor, R.A. Respiratory Rate and Breathing Pattern. McMaster Univ. Med J. 2013, 10, 23–25. [Google Scholar]
- Aaron, E.A.; Johnson, B.D.; Seow, C.K.; Dempsey, J.A. Oxygen cost of exercise hyperpnea: Measurement. J. Appl. Physiol. 1992, 72, 1810–1817. [Google Scholar] [CrossRef] [PubMed]
- Guenette, J.A.; Sheel, A.W. Physiological consequences of a high work of breathing during heavy exercise in humans. J. Sci. Med. Sport 2007, 10, 341–350. [Google Scholar] [CrossRef]
- McArdle, W.D.; Katch, F.I.; Katch, V.L. Essentials of Exercise Physiology; Lippincott Williams and Wilkins: Baltimore, MD, USA, 2016. [Google Scholar]
- Malátová, R.; Bahenský, P.; Mareš, M.; Rost, M. Breathing pattern of restful and deep breathing. In Proceedings of the 11th International Conference on Kinanthropology, Brno, Czech Republic, 29 Novermber–1 December 2017; Zvonař, M., Sajdlová, Z., Eds.; Masarykova Univerzita: Brno, Czech Republic, 2017. [Google Scholar]
- Benchetrit, G. Breathing pattern in humans: Diversity and individuality. Respir. Physiol. 2000, 122, 123–129. [Google Scholar] [CrossRef]
- Clifton-Smith, T. Breathing pattern disorders and the athlete. In Recognizing and Treating Breathing Disorders E-Book: A Multidisciplinary Approach; Churchill Livingstone: London, UK, 2014. [Google Scholar] [CrossRef]
- Chaitow, L.; Bradley, D.; Gilbert, C. Recognizing and Treating Breathing Disorders. A Multidisciplinary Approach, 2nd ed.; Churchill Livingston: London, UK, 2014. [Google Scholar]
- Hodges, P.W.; Heijnen, I.; Gandevia, S.C. Postural activity of the diaphragm is reduced in humans when respiratory demand increases. J. Physiol. 2001, 537, 999–1008. [Google Scholar] [CrossRef]
- Weavil, J.C.; Amann, M. Neuromuscular fatigue during whole body exercise. Curr. Opin. Physiol. 2019, 10, 128–136. [Google Scholar] [CrossRef]
- Hruzevych, I.; Boguslavska, V.; Kropta, R.; Galan, Y.; Nakonechnyi, I.; Pityn, M. The effectiveness of the endogenous-hypoxic breathing in the physical training of skilled swimmers. J. Phys. Educ. Sport 2017, 17, 1009–1016. [Google Scholar] [CrossRef]
- Kisner, C.; Colby, L.A. Management of pulmonary conditions. In Therapeutic Exercise: Foundations and Techniques, 5th ed.; FA Davis Company: Philadelphia, PA, USA, 2007; pp. 851–882. [Google Scholar]
- Szczepan, S.; Danek, N.; Michalik, K.; Wróblewska, Z.; Zatoń, K. Influence of a Six-Week Swimming Training with Added Respiratory Dead Space on Respiratory Muscle Strength and Pulmonary Function in Recreational Swimmers. Int. J. Environ. Res. Public Health 2020, 17, 5743. [Google Scholar] [CrossRef] [PubMed]
- Verges, S.; Lenherr, O.; Haner, A.C.; Schulz, C.; Spengler, C.M. Increased fatigue resistance of respiratory muscles during exercise after respiratory muscle endurance training. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 292, R1246–R1253. [Google Scholar] [CrossRef] [PubMed]
- Aliverti, A. The respiratory muscles during exercise. Breathe 2016, 12, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Faghy, M.A.; Brown, P.I. Functional training of the inspiratory muscles improves load carriage performance. Ergonomics 2019, 62, 1439–1449. [Google Scholar] [CrossRef] [PubMed]
- Hinde, K.L.; Low, C.; Lloyd, R.; Cooke, C.B. Inspiratory muscle training at sea level improves the strength of inspiratory muscles during load carriage in cold-hypoxia. Ergonomics 2020, 63, 1584–1598. [Google Scholar] [CrossRef]
- Göhl, O.; Walker, D.J.; Walterspacher, S.; Langer, D.; Spengler, C.M.; Wanke, T.; Petrovic, M.; Zwick, R.H.; Stieglitz, S.; Glöckl, R.; et al. Atemmuskeltraining: State-of-the-Art [Respiratory Muscle Training: State of the Art]. Pneumologie 2016, 70, 37–48. [Google Scholar] [CrossRef]
- Moodie, L.; Reeve, J.; Elkins, M. Inspiratory muscle training in mechanically ventilated patients. J. Physiother. 2011, 57, 213–221. [Google Scholar] [CrossRef]
- Sclauser Pessoa, I.M.; Franco Parreira, V.; Fregonezi, G.A.; Sheel, A.W.; Chung, F.; Reid, W.D. Reference values for maximal inspiratory pressure: A systematic review. Can. Respir. J. 2014, 21, 43–50. [Google Scholar] [CrossRef]
- Bockenhauer, S.E.; Chen, H.; Julliard, K.N.; Weedon, J. Measuring thoracic excursion: Reliability of the cloth tape measure technique. J. Am. Osteopath. Assoc. 2007, 107, 191–196. [Google Scholar]
- Cahalin, L.P. Pulmonary evaluation. In Cardiovaskular and Pulmonary Physical Therapy; DeTurkW, E., Cahalin, L.P., Eds.; McGraw-Hill: New York, NY, USA, 2004. [Google Scholar]
- Kaneko, H.; Horie, J. Breathing movements of the chest and abdominal wall in healthy subjects. Respir. Care 2012, 57, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Cala, S.J.; Kenyon, C.M.; Ferrigno, G.; Carnevali, P.; Aliverti, A.; Pedotti, A.; Macklem, P.T.; Rochester, D.F. Chest wall and lung volume estimation by optical reflectance motion analysis. J. Appl. Physiol. 1996, 81, 2680–2689. [Google Scholar] [CrossRef]
- Aliverti, A.; Cala, S.J.; Duranti, R.; Ferrigno, G.; Kenyon, C.M.; Pedotti, A.; Scano, G.; Sliwinski, P.; Macklem, P.T.; Yan, S. Human respiratory muscle actions and control during exercise. J. Appl. Physiol. 1997, 83, 1256–1269. [Google Scholar] [CrossRef]
- Ferrigno, G.; Carnevali, P.; Aliverti, A.; Molteni, F.; Beulcke, G.; Pedotti, A. Three-dimensional optical analysis of chest wall motion. J. Appl. Physiol. 1994, 77, 1224–1231. [Google Scholar] [CrossRef]
- Hostettler, S.; Illi, S.K.; Mohler, E.; Aliverti, A.; Spengler, C.M. Chest wall volume changes during inspiratory loaded breathing. Respir. Physiol. Neurobiol. 2011, 175, 130–139. [Google Scholar] [CrossRef]
- Romagnoli, I.; Gorini, M.; Gigliotti, F.; Bianchi, R.; Lanini, B.; Grazzini, M.; Stendardi, L.; Scano, G. Chest wall kinematics, respiratory muscle action and dyspnoea during arm vs. leg exercise in humans. Acta Physiol. 2006, 188, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Malátová, R.; Pučelík, J.; Rokytová, J.; Kolář, P. The objectification of therapeutical methods used for improvement of the deep stabilizing spinal system. Neuroendocrinol. Lett. 2007, 28, 315–320. [Google Scholar]
- Malátová, R.; Pučelík, J.; Rokytová, J.; Kolář, P. Technical means for objectification of medical treatments in the area of the deep stabilisation spinal system. Neuroendocrinol. Lett. 2008, 29, 125–130. [Google Scholar]
- Malátová, R. The Importance of Breathing Stereotype and Intervention Possibilities. Post Doctoral Thesis, Brno, Czech Republic, 2021. [Google Scholar]
- Bahenský, P.; Bunc, V.; Marko, D.; Malátová, R. Dynamics of ventilation parameters at different load intensities and the options to influence it by a breathing exercise. J. Sports Med. Phys. Fit. 2020, 60, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Bahenský, P.; Marko, D.; Grosicki, G.J.; Malátová, R. Warm-up breathing exercises accelerate VO2 kinetics and reduce subjective strain during incremental cycling exercise in adolescents. J. Phys. Educ. Sport 2020, 20, 3361–3367. [Google Scholar] [CrossRef]
- Gandevia, S.C.; Butler, J.E.; Hodges, P.W.; Taylor, J.L. Balancing acts: Respiratory sensations, motor control and human posture. Clin. Exp. Pharmacol. Phys. 2002, 29, 118–121. [Google Scholar] [CrossRef]
- Cipriano, G.F.; Cipriano, G., Jr.; Santos, F.V.; Güntzel Chiappa, A.M.; Pires, L.; Cahalin, L.P.; Chiappa, G.R. Current insights of inspiratory muscle training on the cardiovascular system: A systematic review with meta-analysis. Integr. Blood Press. Control 2019, 12, 1–11. [Google Scholar] [CrossRef]
- Taylor, B.J.; How, S.C.; Romer, L.M. Expiratory Muscle Fatigue Does Not Regulate Operating Lung Volumes during High-Intensity Exercise in Healthy Humans. J. Appl. Physiol. 2013, 114, 1569–1576. [Google Scholar] [CrossRef][Green Version]
- de Abreu, R.M.; Rehder-Santos, P.; Minatel, V.; Dos Santos, G.L.; Catai, A.M. Effects of inspiratory muscle training on cardiovascular autonomic control: A systematic review. Auton. Neurosci. 2017, 208, 29–35. [Google Scholar] [CrossRef]
- Billman, G.E. The LF/HF ratio does not accurately measure cardiac sympathovagal balance. Front. Physiol. 2013, 4, 26. [Google Scholar] [CrossRef] [PubMed]
- Hodges, P.W.; Gandevia, S.C. Activation of the human diaphragm during a repetitive postural task. J. Physiol. 2000, 522, 165–175. [Google Scholar] [CrossRef]
- Bahenský, P.; Malátová, R.; Bunc, V. Changed dynamic ventilation parameters as a result of a breathing exercise intervention programme. J. Sports Med. Phys. Fit. 2019, 59, 1369–1375. [Google Scholar] [CrossRef]
- Kenney, W.L.; Wilmore, J.H.; Costill, D.L. Physiology of Sport and Exercise; Human Kinetics: Champaign, IL, USA, 2015. [Google Scholar]
- Hamdouni, H.; Kliszczewicz, B.; Zouhal, H.; Rhibi, F.; Ben Salah, F.Z.; Ben Abderrahmann, A. Effect of three fitness programs on strength, speed, flexibility and muscle power on sedentary subjects. J. Sports Med. Phys. Fit. 2021. [Google Scholar] [CrossRef]
- Karthik, P.S.; Chandrasekhar, M.; Ambareesha, K.; Nikhil, C. Effect of pranayama and suryanamaskar on pulmonary functions in medical students. J. Clin. Diagn. Res. 2014, 8, 4–6. [Google Scholar] [CrossRef]
- Langer, D.; Ciavaglia, C.; Faisal, A.; Webb, K.A.; Neder, J.A.; Gosselink, R.; Dacha, S.; Topalovic, M.; Ivanova, A.; O’Donnel, D.E.; et al. Inspiratory muscle training reduces diaphragm activation and dyspnea during exercise in COPD. J. Appl. Physiol. 2018, 125, 381–392. [Google Scholar] [CrossRef]
- Radhakrishnan, K.; Sharma, V.K.; Subramanian, S.K. Does treadmill running performance, heart rate and breathing rate response during maximal graded exercise improve after volitional respiratory muscle training? Br. J. Sports Med. 2017. [Google Scholar] [CrossRef] [PubMed][Green Version]

| Breathing | Time | Rest | Deep–Rest | 2 W·kg−1 | 3 W·kg−1 | 4 W·kg−1 |
|---|---|---|---|---|---|---|
| Sector | [n·cm−2] | [n·cm−2] | [n·cm−2] | [n·cm−2] | [n·cm−2] | [n·cm−2] |
| abdominal | pre | 0.54 ± 0.33 | 0.93 ± 0.36 | 1.38 ± 0.74 | 1.43 ± 0.66 | 1.54 ± 0.62 |
| EG | post | 0.94 ± 0.37 l | 1.79 ± 0.76 l,** | 2.01 ± 0.90 m,* | 2.04 ± 1.01 m,* | 1.91 ± 0.75 m |
| chest | pre | 0.46 ± 0.35 | 0.82 ± 0.56 | 1.32 ± 0.88 | 1.31 ± 0.75 | 1.59 ± 0.89 |
| EG | post | 0.65 ± 0.38 m | 1.34 ± 0.91 m | 1.79 ± 0.75 m | 2.19 ± 1.31 m,** | 2.16 ± 1.24 m |
| subclavian | pre | 0.65 ± 0.49 | 1.43 ± 0.79 | 2.92 ± 1.99 | 3.00 ± 1.39 | 3.59 ± 1.94 |
| EG | post | 0.83 ± 0.42 s | 1.66 ± 0.97 s | 2.70 ± 0.99 | 2.83 ± 0.88 * | 3.08 ± 1.26 s |
| abdominal | pre | 0.56 ± 0.36 | 0.91 ± 0.40 | 1.33 ± 0.67 | 1.39 ± 0.55 | 1.55 ± 0.60 |
| CG | post | 0.57 ± 0.39 | 0.89 ± 0.42 | 1.35 ± 0.70 | 1.42 ± 0.52 | 1.54 ± 0.62 |
| chest | pre | 0.48 ± 0.34 | 0.85 ± 0.57 | 1.35 ± 0.90 | 1.36 ± 0.69 | 1.58 ± 0.90 |
| CG | post | 0.46 ± 0.37 | 0.88 ± 0.59 | 1.35 ± 0.85 | 1.37 ± 0.72 | 1.56 ± 0.88 |
| subclavian | pre | 0.60 ± 0.45 | 1.45 ± 0.82 | 2.95 ± 2.01 | 3.03 ± 1.43 | 3.49 ± 1.45 |
| CG | post | 0.63 ± 0.42 | 1.49 ± 0.88 | 2.91 ± 1.95 | 2.98 ± 1.35 | 3.40 ± 1.42 |
| Rest | Deep-Rest | 2 W·kg−1 | 3 W·kg−1 | 4 W·kg−1 | ||
|---|---|---|---|---|---|---|
| EG | RR | −3.12 s | −3.97 s | −5.85 s | −7.18 s,* | −8.36 m,** |
| VT | - | - | 10.60 s,* | 7.33 s | 6.00 s | |
| VE | - | - | 2.48 | −0.60 | −2.89 | |
| VO2 | - | - | −0.27 | −0.15 | −0.10 | |
| CG | RR | −1.32 | −1.40 | 0.05 | −0.07 | 0.45 |
| VT | - | - | −0.60 | −0.15 | 0.07 | |
| VE | - | - | 1.72 | −0.65 | −0.31 | |
| VO2 | - | - | 0.39 | 0.55 | 0.30 |
| Correlation | 2 W·kg−1 | 3 W·kg−1 | 4 W·kg−1 |
|---|---|---|---|
| VT and abdominal sector engagement | 0.452 * | 0.584 * | 0.531 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bahenský, P.; Bunc, V.; Malátová, R.; Marko, D.; Grosicki, G.J.; Schuster, J. Impact of a Breathing Intervention on Engagement of Abdominal, Thoracic, and Subclavian Musculature during Exercise, a Randomized Trial. J. Clin. Med. 2021, 10, 3514. https://doi.org/10.3390/jcm10163514
Bahenský P, Bunc V, Malátová R, Marko D, Grosicki GJ, Schuster J. Impact of a Breathing Intervention on Engagement of Abdominal, Thoracic, and Subclavian Musculature during Exercise, a Randomized Trial. Journal of Clinical Medicine. 2021; 10(16):3514. https://doi.org/10.3390/jcm10163514
Chicago/Turabian StyleBahenský, Petr, Václav Bunc, Renata Malátová, David Marko, Gregory J. Grosicki, and Jan Schuster. 2021. "Impact of a Breathing Intervention on Engagement of Abdominal, Thoracic, and Subclavian Musculature during Exercise, a Randomized Trial" Journal of Clinical Medicine 10, no. 16: 3514. https://doi.org/10.3390/jcm10163514
APA StyleBahenský, P., Bunc, V., Malátová, R., Marko, D., Grosicki, G. J., & Schuster, J. (2021). Impact of a Breathing Intervention on Engagement of Abdominal, Thoracic, and Subclavian Musculature during Exercise, a Randomized Trial. Journal of Clinical Medicine, 10(16), 3514. https://doi.org/10.3390/jcm10163514








