Effect of an Acute Resistance Training Bout and Long-Term Resistance Training Program on Arterial Stiffness: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Types of Studies
2.3. Types of Outcomes
2.4. Data Extraction and Evaluation
2.5. Statistical Analysis and Calculation of Effect Size
3. Results
3.1. Study Characteristics
3.2. Changes in Arterial Stiffness and Pulse Wave Velocity as a Result of an Acute Resistance Training Bout
3.3. Changes in Arterial Stiffness and Pulse Wave Velocity as a Result of a Long-Term RT
4. Discussion
Practical Implications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Avolio, A.; Deng, F.-Q.; Li, W.-Q.; Luo, Y.-F.; Huang, Z.-D.; Xing, L.; O’rourke, M. Effects of aging on arterial distensibility in populations with high and low prevalence of hypertension: Comparison between urban and rural communities in China. Circulation 1985, 71, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Alan, S.; Ulgen, M.S.; Ozturk, O.; Alan, B.; Ozdemir, L.; Toprak, N. Relation between coronary artery disease, risk factors and intima-media thickness of carotid artery, arterial distensibility, and stiffness index. Angiology 2003, 54, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Rhee, M.-Y.; Lee, H.-Y.; Park, J.B. Measurements of arterial stiffness: Methodological aspects. Korean Circ. J. 2008, 38, 343–350. [Google Scholar] [CrossRef]
- Han, S.H.; Park, C.G.; Park, S.W.; Shin, S.H.; Ahn, J.C.; Seo, H.S.; Oh, D.J.; Shin, E.K.; Ro, Y.M. High aortic stiffness assessed by pulse wave velocity is an independent predictor of coronary artery calcification and stenosis in suspected coronary artery disease patients. Korean Circ. J. 2004, 34, 468–476. [Google Scholar] [CrossRef]
- Kaess, B.M.; Rong, J.; Larson, M.G.; Hamburg, N.M.; Vita, J.A.; Levy, D.; Benjamin, E.J.; Vasan, R.S.; Mitchell, G.F. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA 2012, 308, 875–881. [Google Scholar] [CrossRef]
- Choi, C.U.; Kim, E.J.; Kim, S.H.; Shin, S.Y.; Choi, U.-J.; Kim, J.W.; Lim, H.E.; Rha, S.-W.; Park, C.G.; Seo, H.S. Differing effects of aging on central and peripheral blood pressures and pulse wave velocity: A direct intraarterial study. J. Hypertens. 2010, 28, 1252–1260. [Google Scholar] [CrossRef]
- Yu, S.; McEniery, C.M. Central versus peripheral artery stiffening and cardiovascular risk. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1028–1033. [Google Scholar] [CrossRef]
- Evans, W.; Willey, Q.; Hanson, E.D.; Stoner, L. Effects of resistance training on arterial stiffness in persons at risk for cardiovascular disease: A meta-analysis. Sports Med. 2018, 48, 2785–2795. [Google Scholar] [CrossRef]
- Seals, D.R.; DeSouza, C.A.; Donato, A.J.; Tanaka, H. Habitual exercise and arterial aging. J. Appl. Physiol. 2008, 105, 1323–1332. [Google Scholar] [CrossRef]
- Thompson, P.D.; Buchner, D.; Piña, I.L.; Balady, G.J.; Williams, M.A.; Marcus, B.H.; Berra, K.; Blair, S.N.; Costa, F.; Franklin, B. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: A statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation 2003, 107, 3109–3116. [Google Scholar] [PubMed]
- Otsuki, T.; Maeda, S.; Iemitsu, M.; Saito, Y.; Tanimura, Y.; Ajisaka, R.; Goto, K.; Miyauchi, T. Effects of athletic strength and endurance exercise training in young humans on plasma endothelin-1 concentration and arterial distensibility. Exp. Biol. Med. 2006, 231, 789–793. [Google Scholar]
- Tanaka, H.; Dinenno, F.A.; Monahan, K.D.; Clevenger, C.M.; DeSouza, C.A.; Seals, D.R. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000, 102, 1270–1275. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Smart, N.A. Exercise training for blood pressure: A systematic review and meta-analysis. J. Am. Heart Assoc. 2013, 2, e004473. [Google Scholar] [CrossRef] [PubMed]
- Jurik, R.; Stastny, P. Role of Nutrition and Exercise Programs in Reducing Blood Pressure: A Systematic Review. J. Clin. Med. 2019, 8, 1393. [Google Scholar] [CrossRef] [PubMed]
- Pescatello, L.S.; Buchner, D.M.; Jakicic, J.M.; Powell, K.E.; Kraus, W.E.; Bloodgood, B.; Campbell, W.W.; Dietz, S.; DiPietro, L.; George, S.M. Physical activity to prevent and treat hypertension: A systematic review. Med. Sci. Sports Exerc. 2019, 51, 1314–1323. [Google Scholar] [CrossRef]
- Ashor, A.W.; Lara, J.; Siervo, M.; Celis-Morales, C.; Mathers, J.C. Effects of exercise modalities on arterial stiffness and wave reflection: A systematic review and meta-analysis of randomized controlled trials. PLoS ONE 2014, 9, e110034. [Google Scholar] [CrossRef]
- Lopes, S.; Afreixo, V.; Teixeira, M.; Garcia, C.; Leitão, C.; Gouveia, M.; Figueiredo, D.; Alves, A.J.; Polonia, J.; Oliveira, J. Exercise training reduces arterial stiffness in adults with hypertension: A systematic review and meta-analysis. J. Hypertens. 2021, 39, 214–222. [Google Scholar] [CrossRef]
- García-Mateo, P.; García-de-Alcaraz, A.; Rodríguez-Peréz, M.A.; Alcaraz-Ibáñez, M. Effects of resistance training on arterial stiffness in healthy people: A systematic review. J. Sports Sci. Med. 2020, 19, 444. [Google Scholar]
- Miyachi, M. Effects of resistance training on arterial stiffness: A meta-analysis. Br. J. Sports Med. 2013, 47, 393–396. [Google Scholar] [CrossRef]
- Ceciliato, J.; Costa, E.C.; Azevêdo, L.; Sousa, J.C.; Fecchio, R.Y.; Brito, L.C. Effect of Resistance Training on Arterial Stiffness in Healthy Subjects: A Systematic Review and Meta-Analysis. Curr. Hypertens. Rep. 2020, 22, 1–8. [Google Scholar] [CrossRef]
- Jacobs, P.L. NSCA’s Essentials of Training Special Populations; Human Kinetics: Champaign, IL, USA, 2017. [Google Scholar]
- Ratamess, N.; Alvar, B.; Evetoch, T.; Housh, T.; Kibler, W.; Kraemer, W. Progression models in resistance training for healthy adults [ACSM position stand]. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar]
- Bertovic, D.A.; Waddell, T.K.; Gatzka, C.D.; Cameron, J.D.; Dart, A.M.; Kingwell, B.A. Muscular strength training is associated with low arterial compliance and high pulse pressure. Hypertension 1999, 33, 1385–1391. [Google Scholar] [CrossRef]
- Miyachi, M.; Donato, A.J.; Yamamoto, K.; Takahashi, K.; Gates, P.E.; Moreau, K.L.; Tanaka, H. Greater age-related reductions in central arterial compliance in resistance-trained men. Hypertension 2003, 41, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Tanaka, H.; Miyachi, M. Resistance training and arterial compliance: Keeping the benefits while minimizing the stiffening. J. Hypertens. 2006, 24, 1753–1759. [Google Scholar] [CrossRef]
- Peters, P.G.; Alessio, H.M.; Hagerman, A.E.; Ashton, T.; Nagy, S.; Wiley, R.L. Short-term isometric exercise reduces systolic blood pressure in hypertensive adults: Possible role of reactive oxygen species. Int. J. Cardiol. 2006, 110, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Mattace-Raso, F.; Hofman, A.; Verwoert, G.; Wittemana, J.; Wilkinson, I.; Cockcroft, J.; McEniery, C.; Laurent, S.; Boutouyrie, P.; Bozec, E. The Reference Values for Arterial Stifness? Collaboration. Determinants of Pulse wave velocity in healthy people amd im the presence of cardiovascular rsik factors. Eur. Heart J. 2010, 31, 2338–2350. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; for the PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Moseley, A.M.; Herbert, R.D.; Sherrington, C.; Maher, C.G. Evidence for physiotherapy practice: A survey of the Physiotherapy Evidence Database (PEDro). Aust. J. Physiother. 2002, 48, 43–49. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Verhagen, A.; De Vet, H.; Bie, R.; Kessels, A.G.; Boers, M.; Bouter, L.; Knipschild, P.G. The Delphi List: A Criteria List for Quality Assessment of Randomized Clinical Trials for Conducting Systematic Reviews Developed by Delphi Consensus. J. Clin. Epidemiol. 1999, 51, 1235–1241. [Google Scholar] [CrossRef]
- Wilson, D.B.; Lipsey, M.W. Practical Meta-Analysis; Sage: Thousand Oaks, CA, USA, 2001. [Google Scholar]
- DeVan, A.E.; Anton, M.M.; Cook, J.N.; Neidre, D.B.; Cortez-Cooper, M.Y.; Tanaka, H. Acute effects of resistance exercise on arterial compliance. J. Appl. Physiol. 2005, 98, 2287–2291. [Google Scholar] [CrossRef]
- Barnes, J.N.; Trombold, J.R.; Dhindsa, M.; Lin, H.-F.; Tanaka, H. Arterial stiffening following eccentric exercise-induced muscle damage. J. Appl. Physiol. 2010, 109, 1102–1108. [Google Scholar] [CrossRef]
- Yoon, E.S.; Jung, S.J.; Cheun, S.K.; Oh, Y.S.; Kim, S.H.; Jae, S.Y. Effects of acute resistance exercise on arterial stiffness in young men. Korean Circ. J. 2010, 40, 16–22. [Google Scholar] [CrossRef]
- Tomschi, F.; Köster, P.; Predel, H.G.; Lay, D.; Bloch, W.; Grau, M. Acute effects of lower and upper body-resistance training on arterial stiffness, peripheral, and central blood pressure in young normotensive women. Sport Sci. Health 2018, 14, 357–363. [Google Scholar] [CrossRef]
- Okamoto, T.; Min, S.; Sakamaki-Sunaga, M. Arterial compliance and stiffness following low-intensity resistance exercise. Eur. J. Appl. Physiol. 2014, 114, 235–241. [Google Scholar] [CrossRef]
- Kingsley, J.D.; Tai, Y.L.; Mayo, X.; Glasgow, A.; Marshall, E. Free-weight resistance exercise on pulse wave reflection and arterial stiffness between sexes in young, resistance-trained adults. Eur. J. Sport Sci. 2017, 17, 1056–1064. [Google Scholar] [CrossRef]
- Nitzsche, N.; Weigert, M.; Baumgärtel, L.; Auerbach, T.; Schuffenhauer, D.; Nitzsche, R.; Schulz, H. Acute effects of different strength training protocols on arterial stiffness in healthy subjects. Group 2016, 6, 197–202. [Google Scholar]
- Lefferts, W.; Hughes, W.; Heffernan, K.S. Effect of acute high-intensity resistance exercise on optic nerve sheath diameter and ophthalmic artery blood flow pulsatility. J. Hum. Hypertens. 2015, 29, 744–748. [Google Scholar] [CrossRef]
- Rodríguez-Perez, M.A.; Alcaraz-Ibáñez, M.; Lorente-Camacho, D.; García-Ramos, A. Does the level of effort during resistance training influence arterial stiffness and blood pressure in young healthy adults? Isokinet. Exerc. Sci. 2020, 28, 375–382. [Google Scholar] [CrossRef]
- Parks, J.C.; Marshall, E.M.; Tai, Y.L.; Kingsley, J.D. Free-weight versus weight machine resistance exercise on pulse wave reflection and aortic stiffness in resistance-trained individuals. Eur. J. Sport Sci. 2020, 20, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Cooper, M.Y.; DeVan, A.E.; Anton, M.M.; Farrar, R.P.; Beckwith, K.A.; Todd, J.S.; Tanaka, H. Effects of high intensity resistance training on arterial stiffness and wave reflection in women. Am. J. Hypertens. 2005, 18, 930–934. [Google Scholar] [CrossRef] [PubMed]
- Croymans, D.; Krell, S.; Oh, C.; Katiraie, M.; Lam, C.; Harris, R.A.; Roberts, C. Effects of resistance training on central blood pressure in obese young men. J. Hum. Hypertens. 2014, 28, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Au, J.S.; Oikawa, S.Y.; Morton, R.W.; MacDonald, M.J.; Phillips, S.M. Arterial Stiffness Is Reduced Regardless of Resistance Training Load in Young Men. Med. Sci. Sports Exerc. 2017, 49, 342–348. [Google Scholar] [CrossRef]
- Casey, D.P.; Beck, D.T.; Braith, R.W. Progressive resistance training without volume increases does not alter arterial stiffness and aortic wave reflection. Exp. Biol. Med. 2007, 232, 1228–1235. [Google Scholar] [CrossRef]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Upper but not lower limb resistance training increases arterial stiffness in humans. Eur. J. Appl. Physiol. 2009, 107, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Effects of muscle contraction timing during resistance training on vascular function. J. Hum. Hypertens. 2009, 23, 470–478. [Google Scholar] [CrossRef]
- Yoshizawa, M.; Maeda, S.; Miyaki, A.; Misono, M.; Saito, Y.; Tanabe, K.; Kuno, S.; Ajisaka, R. Effect of 12 weeks of moderate–intensity resistance training on arterial stiffness: A randomised controlled trial in women aged 32–59 years. Br. J. Sports Med. 2009, 43, 615–618. [Google Scholar] [CrossRef] [PubMed]
- Beck, D.T.; Martin, J.S.; Casey, D.P.; Braith, R.W. Exercise training reduces peripheral arterial stiffness and myocardial oxygen demand in young prehypertensive subjects. Am. J. Hypertens. 2013, 26, 1093–1102. [Google Scholar] [CrossRef]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Effect of low-intensity resistance training on arterial function. Eur. J. Appl. Physiol. 2011, 111, 743–748. [Google Scholar] [CrossRef]
- Figueroa, A.; Gil, R.; Wong, A.; Hooshmand, S.; Park, S.Y.; Vicil, F.; Sanchez-Gonzalez, M.A. Whole-body vibration training reduces arterial stiffness, blood pressure and sympathovagal balance in young overweight/obese women. Hypertens. Res. 2012, 35, 667–672. [Google Scholar] [CrossRef]
- Lai, C.-L.; Chen, H.-Y.; Tseng, S.-Y.; Liao, W.-C.; Liu, B.-T.; Lee, M.-C.; Chen, H.-S. Effect of whole-body vibration for 3 months on arterial stiffness in the middle-aged and elderly. Clin. Interv. Aging 2014, 9, 821. [Google Scholar] [PubMed]
- Miura, H.; Takahashi, Y.; Maki, Y.; Sugino, M. Effects of exercise training on arterial stiffness in older hypertensive females. Eur. J. Appl. Physiol. 2015, 115, 1847–1854. [Google Scholar] [CrossRef]
- Guimaraes, G.V.; Ciolac, E.G.; Carvalho, V.O.; D’Avila, V.M.; Bortolotto, L.A.; Bocchi, E.A. Effects of continuous vs. interval exercise training on blood pressure and arterial stiffness in treated hypertension. Hypertens. Res. Off. J. Jpn. Soc. Hypertens. 2010, 33, 627–632. [Google Scholar] [CrossRef]
- Edwards, D.G.; Schofield, R.S.; Magyari, P.M.; Nichols, W.W.; Braith, R.W. Effect of exercise training on central aortic pressure wave reflection in coronary artery disease. Am. J. Hypertens. 2004, 17, 540–543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Na, S.-H.; Kim, Y.-S.; Bae, J.-H.; Nah, D.-Y.; Kim, Y.-K.; Lee, M.-M.; Kim, H.-Y.; Rhee, M.-Y. Effects of physical activity and aerobic exercise capacity on aortic stiffness in patients with untreated hypertension. Korean Circ. J. 2009, 39, 52–56. [Google Scholar] [CrossRef]
- Figueroa, A.; Kalfon, R.; Madzima, T.A.; Wong, A. Whole-body vibration exercise training reduces arterial stiffness in postmenopausal women with prehypertension and hypertension. Menopause 2014, 21, 131–136. [Google Scholar] [CrossRef]
- Weinstein, Y.; Kamerman, T.; Berry, E.; Falk, B. Mechanical efficiency of normal-weight prepubertal boys predisposed to obesity. Med. Sci. Sports Exerc. 2004, 36, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Wing, R.; Lang, W.; Wadden, T.; Safford, M.; Knowler, W.; Bertoni, A.; Hill, J.; Brankati, F.L.; Peters, A.; WagenKnecht, L. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care 2011, 34, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Paul, P.; Giles Thomas, D.; Bray George, A.; Yuling, H.; Stern Judith, S.; Xavier, P.-S.F.; Eckel Robert, H. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 968–976. [Google Scholar]
- American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef] [PubMed]
- Feigenbaum, M.S.; Pollock, M.L. Prescription of resistance training for health and disease. Med. Sci. Sports Exerc. 1999, 31, 38–45. [Google Scholar] [CrossRef]
- Figueiredo, T.; de Salles, B.F.; Dias, I.; Reis, V.M.; Fleck, S.J.; Simão, R. Acute hypotensive effects after a strength training session: A review. Int. Sport Med. J. 2014, 15, 308–329. [Google Scholar]
- Saz-Lara, A.; Cavero-Redondo, I.; Álvarez-Bueno, C.; Notario-Pacheco, B.; Ruiz-Grao, M.C.; Martínez-Vizcaíno, V. The acute effect of exercise on arterial stiffness in healthy subjects: A meta-analysis. J. Clin. Med. 2021, 10, 291. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Laurent, S. Mechanisms of arterial stiffening: From mechanotransduction to epigenetics. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 1055–1062. [Google Scholar] [CrossRef]
- MacDougall, J.D.; Tuxen, D.; Sale, D.G.; Moroz, J.R.; Sutton, J.R. Arterial blood pressure response to heavy resistance exercise. J. Appl. Physiol. 1985, 58, 785–790. [Google Scholar] [CrossRef]
- Baum, K.; Rüther, T.; Essfeld, D. Reduction of blood pressure response during strength training through intermittent muscle relaxations. Int. J. Sports Med. 2003, 24, 441–445. [Google Scholar] [CrossRef]
- Gjovaag, T.; Hjelmeland, A.K.; Øygard, J.B.; Vikne, H.; Mirtaheri, P. Acute hemodynamic and cardiovascular responses following resistance exercise to voluntary exhaustion. Effects of different loadings and exercise durations. J. Sports Med. Phys. Fit. 2016, 56, 616–623. [Google Scholar]
- Okamoto, T.; Masuhara, M.; Ikuta, K. Effects of eccentric and concentric resistance training on arterial stiffness. J. Hum. Hypertens. 2006, 20, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Joyner, M.; Nauss, L.; Warner, M.; Warner, D. Sympathetic modulation of blood flow and O2 uptake in rhythmically contracting human forearm muscles. Am. J. Physiol. Heart Circ. Physiol. 1992, 263, H1078–H1083. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S. Progressive resistance exercise and resting blood pressure: A meta-analysis of randomized controlled trials. Hypertension 2000, 35, 838–843. [Google Scholar] [CrossRef]
- Stenberg, J.; Astrand, P.; Ekblom, B.; Royce, J.; Saltin, B. Hemodynamic response to work with different muscle groups, sitting and supine. J. Appl. Physiol. 1967, 22, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Doederlein Polito, M.; Simão, R.; Weber Senna, G.; de Tarso Veras Farinatti, P. Hypotensive effects of resistance exercises performed at different intensities and same work volumes. Rev. Bras. Med. Esporte 2003, 9, 74–77. [Google Scholar] [CrossRef]
- Lamotte, M.; Fleury, F.; Pirard, M.; Jamon, A.; van de Borne, P. Acute cardiovascular response to resistance training during cardiac rehabilitation: Effect of repetition speed and rest periods. Eur. J. Prev. Cardiol. 2010, 17, 329–336. [Google Scholar] [CrossRef]
- Chilibeck, P.D.; Calder, A.W.; Sale, D.G.; Webber, C.E. A comparison of strength and muscle mass increases during resistance training in young women. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 77, 170–175. [Google Scholar] [CrossRef]
- Seguin, R.; Nelson, M.E. The benefits of strength training for older adults. Am. J. Prev. Med. 2003, 25, 141–149. [Google Scholar] [CrossRef]
- DeVallance, E.; Fournier, S.; Lemaster, K.; Moore, C.; Asano, S.; Bonner, D.; Donley, D.; Olfert, I.; Chantler, P. The effects of resistance exercise training on arterial stiffness in metabolic syndrome. Eur. J. Appl. Physiol. 2016, 116, 899–910. [Google Scholar] [CrossRef]
- Jones, L.M.; Stoner, L.; Baldi, J.C.; McLaren, B. Circuit resistance training and cardiovascular health in breast cancer survivors. Eur. J. Cancer Care 2020, 29, e13231. [Google Scholar] [CrossRef]
- Collier, S.R.; Kanaley, J.A.; Carhart, R., Jr.; Frechette, V.; Tobin, M.M.; Hall, A.K.; Luckenbaugh, A.N.; Fernhall, B. Effect of 4 weeks of aerobic or resistance exercise training on arterial stiffness, blood flow and blood pressure in pre- and stage-1 hypertensives. J. Hum. Hypertens. 2008, 22, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.G.; Lang, J.T. Augmentation index and systolic load are lower in competitive endurance athletes. Am. J. Hypertens. 2005, 18, 679–683. [Google Scholar]
- Figueroa, A.; Park, S.Y.; Seo, D.Y.; Sanchez-Gonzalez, M.A.; Baek, Y.H. Combined resistance and endurance exercise training improves arterial stiffness, blood pressure, and muscle strength in postmenopausal women. Menopause 2011, 18, 980–984. [Google Scholar] [CrossRef]
- de Zepetnek, J.O.T.; Giangregorio, L.M.; Craven, B.C. Whole-body vibration as potential intervention for people with low bone mineral density and osteoporosis: A review. J. Rehabil. Res. Dev. 2009, 46, 529–542. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Kalfon, R.; Wong, A. Whole-body vibration training decreases ankle systolic blood pressure and leg arterial stiffness in obese postmenopausal women with high blood pressure. Menopause 2015, 22, 423–427. [Google Scholar] [CrossRef]
- Figueroa, A.; Going, S.B.; Milliken, L.A.; Blew, R.M.; Sharp, S.; Teixeira, P.J.; Lohman, T.G. Effects of exercise training and hormone replacement therapy on lean and fat mass in postmenopausal women. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2003, 58, M266–M270. [Google Scholar] [CrossRef] [PubMed]
- Patten, R.K.; Boyle, R.A.; Moholdt, T.; Kiel, I.; Hopkins, W.G.; Harrison, C.L.; Stepto, N.K. Exercise interventions in polycystic ovary syndrome: A systematic review and meta-analysis. Front. Physiol. 2020, 11, 606. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Vicil, F.; Sanchez-Gonzalez, M.A.; Wong, A.; Ormsbee, M.J.; Hooshmand, S.; Daggy, B. Effects of diet and/or low-intensity resistance exercise training on arterial stiffness, adiposity, and lean mass in obese postmenopausal women. Am. J. Hypertens. 2013, 26, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Collier, S.R.; Frechette, V.; Sandberg, K.; Schafer, P.; Ji, H.; Smulyan, H.; Fernhall, B. Sex differences in resting hemodynamics and arterial stiffness following 4 weeks of resistance versus aerobic exercise training in individuals with pre-hypertension to stage 1 hypertension. Biol. Sex Differ. 2011, 2, 9. [Google Scholar] [CrossRef]
- Casey, D.P.; Nichols, W.W.; Braith, R.W. Impact of aging on central pressure wave reflection characteristics during exercise. Am. J. Hypertens. 2008, 21, 419–424. [Google Scholar] [CrossRef]
- Otsuki, T.; Maeda, S.; Iemitsu, M.; Saito, Y.; Tanimura, Y.; Ajisaka, R.; Miyauchi, T. Relationship between arterial stiffness and athletic training programs in young adult men. Am. J. Hypertens. 2007, 20, 967–973. [Google Scholar] [CrossRef]
- London, G.M.; Guerin, A.P. Influence of arterial pulse and reflected waves on blood pressure and cardiac function. Am. heart J. 1999, 138, S220–S224. [Google Scholar] [CrossRef]
- MacKay-Lyons, M.J.; Macko, R.; Howlett, J. Cardiovascular fitness and adaptations to aerobic training after stroke. Physiother. Can. 2006, 58, 103–113. [Google Scholar] [CrossRef]
- Miyachi, M.; Kawano, H.; Sugawara, J.; Takahashi, K.; Hayashi, K.; Yamazaki, K.; Tabata, I.; Tanaka, H. Unfavorable effects of resistance training on central arterial compliance: A randomized intervention study. Circulation 2004, 110, 2858–2863. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.P.; Chiappa, G.R.; Neder, J.A.; Frankenstein, L. Respiratory muscle function and exercise intolerance in heart failure. Curr. Heart Fail. Rep. 2009, 6, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Burini, D.; Farabollini, B.; Iacucci, S.; Rimatori, C.; Riccardi, G.; Capecci, M.; Provinciali, L.; Ceravolo, M. A randomised controlled cross-over trial of aerobic training versus Qigong in advanced Parkinson’s disease. Eur. Med. 2006, 42, 231. [Google Scholar]
- Kato, M.; Nihei Green, F.; Hotta, K.; Tsukamoto, T.; Kurita, Y.; Kubo, A.; Takagi, H. The efficacy of stretching exercises on arterial stiffness in middle-aged and older adults: A meta-analysis of randomized and non-randomized controlled trials. Int. J. Environ. Res. Public Health 2020, 17, 5643. [Google Scholar] [CrossRef]
- Tanaka, H.; Munakata, M.; Kawano, Y.; Ohishi, M.; Shoji, T.; Sugawara, J.; Tomiyama, H.; Yamashina, A.; Yasuda, H.; Sawayama, T. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J. Hypertens. 2009, 27, 2022–2027. [Google Scholar] [CrossRef]

| Authors | Subjects | PWV Measurement | Acute Resistance Training Bout and Duration | Results |
|---|---|---|---|---|
| High Intensity RT Bout (>80% 1RM) | ||||
| Barnes et al. (2010) [34] | N = 27 men, healthy, young | cfPWV | Eccentric RT bout: 1. Leg press (bilateral): 6 sets of 10 repetitions at 110% 2. Elbow flexion (unilateral): 2 sets of 20 unilateral eccentric elbow flexion contractions | 48 h after leg RT (p < 0.05) and arm RT ↑cfPWV (p < 0.05). |
| Lefferts et al. (2015) [40] | N = 20, healthy, recreationally active | cfPWV | 5 sets, 5 repetition of maximum bench press; 5 sets of 10 repetition maximum biceps curls | An acute high intensity RT had ↑AS and ↑extracranial pressure pulsatility. |
| Moderate Intensity RT_Bout (60–80% 1RM) | ||||
| DeVan et al. (2005) [33] | N = 16, mix, healthy, young | Beta stiffness index | 9 full body exercises, 75% 1RM | RT bout immediately and 30 min after had ↓carotid arterial compliance and ↑stiffness index. These values returned to baseline by 60 min. |
| Yoon et al. (2010) [35] | N = 13 healthy men | cfPWV | 8 full body exercises, 60% 1RM | An acute RT bout had ↑AS. These values returned to baseline by 20 min. |
| Nitzsche et al. (2016) [39] | N = 41, mix healthy, physically active | cfPWV | RT bout: Group 70% 1RM | An acute moderate RT bout had ↓AS and ↓central and systolic BP. |
| Kingsley et al. (2017) [38] | N = 13, mix, healthy, recreationally resistance trained | cfPWV | Free-weight RT: 75% 1RM, 3 sets, 10 repetitions | An acute RT bout had ↑augmentation index and ↑AS without significantly altering aortic BP. |
| Tomschi et al. (2018) [36] | N = 20 healthy women | cfPWV | A. Upper body B. Lower body 12 repetitions with 70% 1RM | The adaptation pattern of the measured PWV as a measure of AS parameters in upper body RT bout compared to lower body RT bout is similar, and all parameters regulate to their baseline values within 60 min. |
| Parks et al. (2020) [42] | N = 32, young, healthy individuals | cfPWV | Free-weight RT bout VS weight machines RT bout: 3 sets of 10 repetitions at 75% 1RM | An acute free-weight and weight-machine RT bout are associated with transient ↑pulse wave reflection and ↑AS. |
| Rodríguez-Perez et al. (2020) [41] | N = 32, physically active, normotensive, and experienced with RT | cfPWV | 3 sets at 75% 1RM, bench press and squat Group 1: high-effort Group 2: low-effort | Both training groups immediately after acute RT bout reported ↑AS and ↑BP while BP and AS returned to baseline levels 5 min and 24 h after completing the RT bout. |
| Low Intensity RT Bout (<60% 1RM) | ||||
| Okamoto et al. (2014) [37] | N = 10, mix, healthy | Beta stiffness index | bench press, 40% of 1RM, 3 sets | Carotid arterial compliance and the β-stiffness index significantly ↑ and ↓, respectively (both p < 0.05), at 30 and 60 min after the acute low intensity RT bout. |
| Nitzsche et al. (2016) [39] | N = 41, mix healthy, physically active | cfPWV | Squats, bench press, rowing with the barbell, biceps curl with the SZ curl bar, lying triceps extensions with the SZ curl bar 1. Group—3 sets at 30% 1RM, 30 repetitions 2. Group—3 sets at 50% 1RM, 20 repetitions 3. Group—4 sets, 70% 1RM, 10 repetitions | An acute moderate RT bout had ↑AS and ↓central systolic BP. |
| Authors | Subjects | PWV Measurement | Frequency, Load and Duration of RT | Results |
|---|---|---|---|---|
| High-Intensity RT (>80% 1RM) | ||||
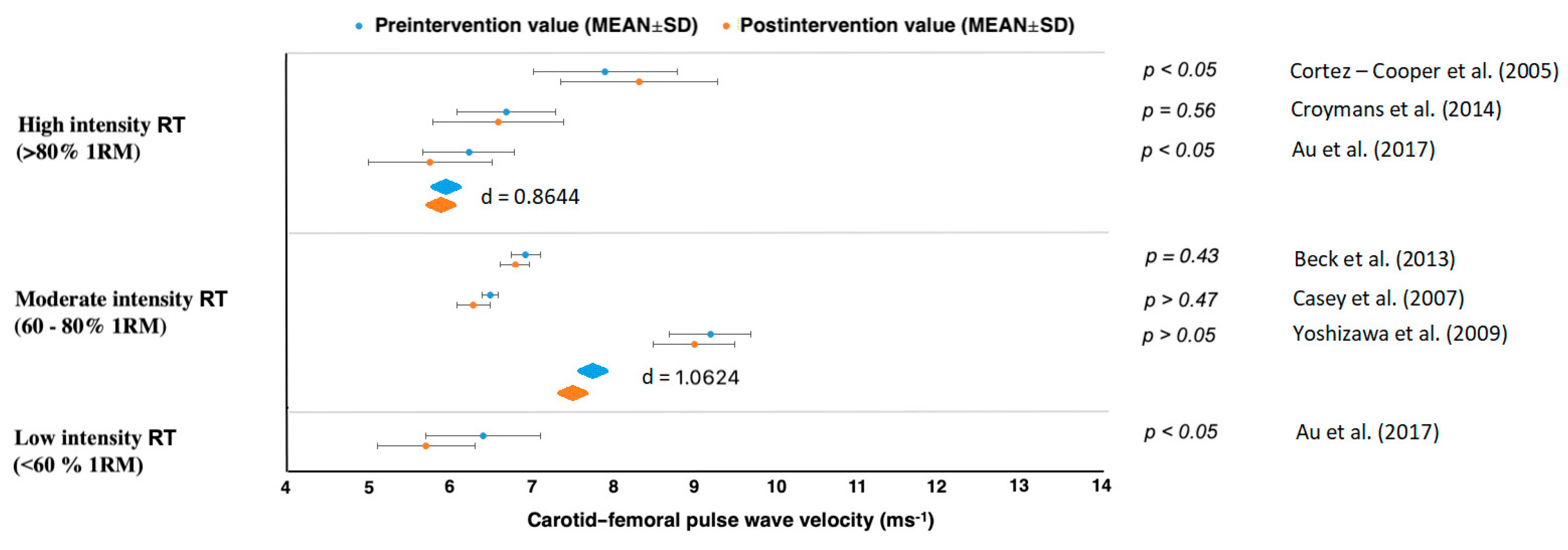
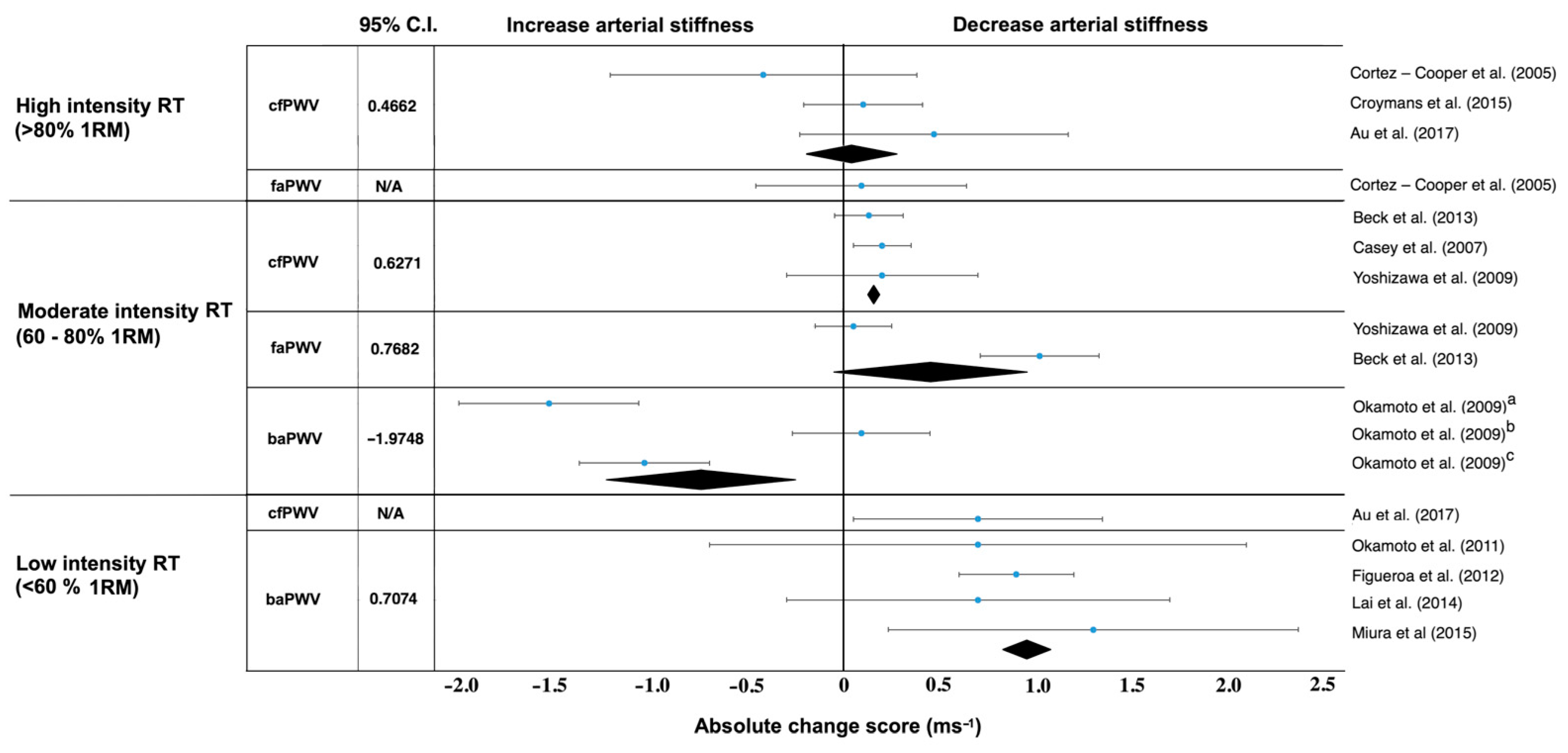
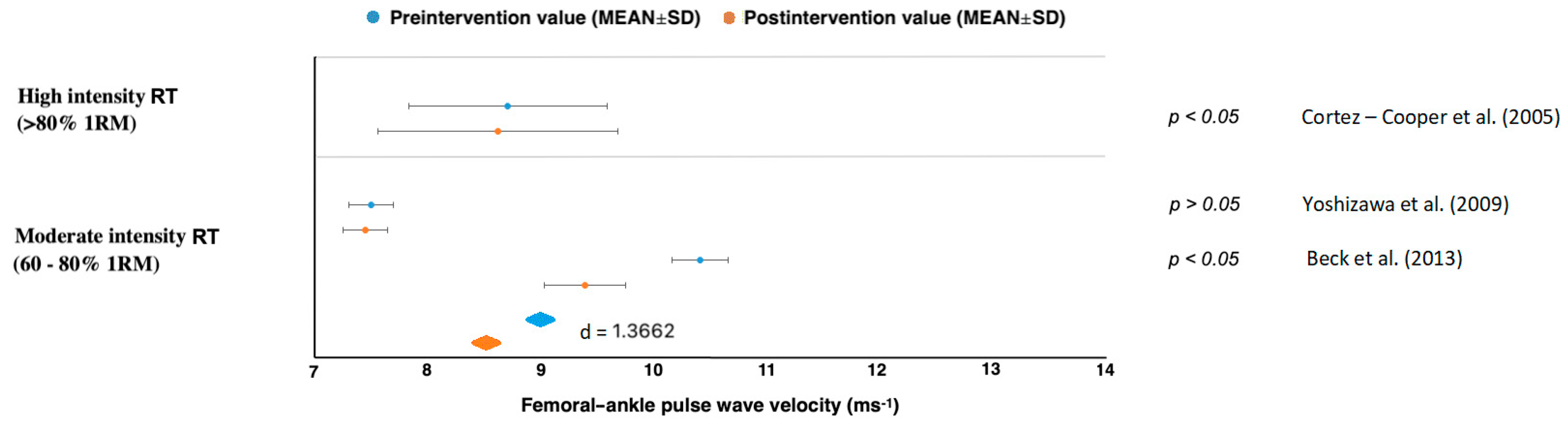
| Cortez-Cooper et al. [43] | n = 23 healthy women, young | cfPWV, faPWV | 11 weeks, 4× per week | Long-term high-intensity RT had ↑AS and ↑wave reflection. |
| Croymans et al. [44] | n = 36, overweight and obese men | cfPWV | 12 weeks, 3× per week | Long-term high-intensity RT had no effect on augmentation index (p = 0.34) and cfPWV (p = 0.43). |
| Au et al. [45] | n = 16, healthy, active males | cfPWV | (heavier-load, lower-repetition), 12 weeks, 4× per week | ↓Central arterial stiffness after RT, regardless of the load lifted. |
| Moderate-Intensity RT (60–80% 1RM) | ||||
| Casey et al. [46] | n = 30, healthy young adults | cfPWV | Progressive RT: 12 weeks, 3× per week | RT consisting of progressively higher intensity without concurrent increases in training volume does not increase central or peripheral AS or alter aortic pressure wave characteristics. |
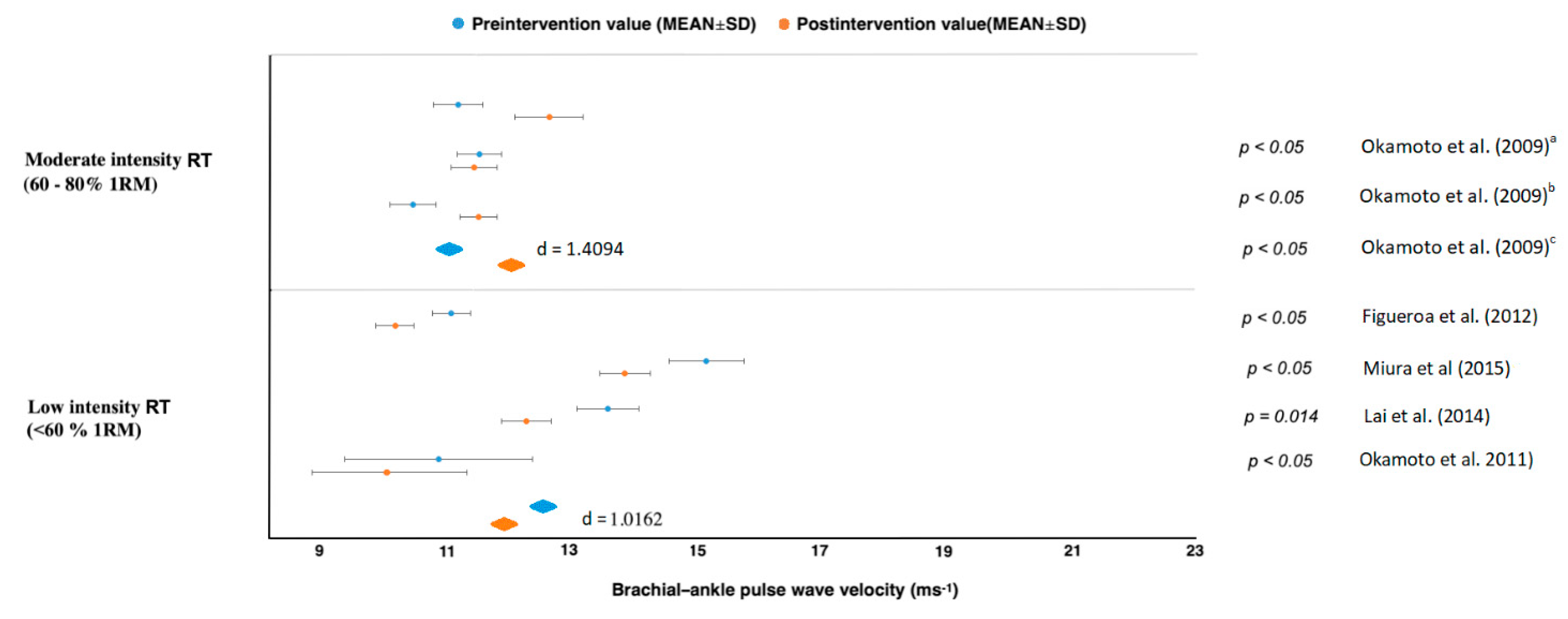
| Okamoto et al. [47] | n = 30, mix, healthy, young | baPWV | Upper and lower limb RT: 10 weeks, 2× per week | upper limb RT had ↑baPWV (p < 0.05). In contrast, baPWV in the lower limb RT had not changed from baseline. |
| Okamoto et al. (2009) [48] | n = 30, healthy men | baPWV | 10 weeks, 2× per week | RT with prolonged eccentric phase did not change from baseline baPWV. In contrast, RT with prolonged concentric phase had ↑baPWV. |
| Yoshizawa et al. [49] | n = 11 healthy, sedentary middle-aged women | cfPWV, faPWV | 12 weeks, 2× per week | Long-term moderate-intensity RT did not increase AS. |
| Beck et al. [50] | n = 43 mix, prehypertension, without medication | cfPWV, faPWV | 8 weeks, 3× per week | Long-term RT and AE alone effectively had ↓peripheral AS, ↓augmentation index. |
| Low-Intensity RT (<60% 1RM) | ||||
| Okamoto et al. [51] | n = 13, mix, young healthy adults | baPWV | Low-intensity RT with short inter-set rest period: 10 weeks, 2× per week | Long-term low-intensity RT with short inter-set rest period had ↓AS and ↓baPWV. |
| Figueroa et al. [52] | n = 10 young, overweight or obesity, women | baPWV | Whole-body vibration RT: 6 weeks, 3× per week | Long-term WBV-RT had ↓systemic AS via improvements in wave reflection and sympathovagal balance. |
| Lai et al. [53] | n = 38, mix, adult and older adults | baPWV | Whole-body vibration training, 3 months, 3× per week | Long-term WBV-RT had ↓AS. |
| Miura et al. [54] | n = 100 women, normotension | baPWV | circuit RT, 12 weeks, 2× per week | Long-term RT had fewer↓ASs. |
| Au et al. [45] | n = 16, healthy, active males | cfPWV | lighter-load, higher-repetition, 12 weeks, 4× per week | Long-term RT had ↓central AS, regardless of the load lifted. |
| Type of RE | Intensity | Sets | Reps | Rest | Duration | Frequency | Exercises Selection | Exercises |
|---|---|---|---|---|---|---|---|---|
| Low intensity RE | 30–60% 1RM | 3–5 | >10 and higher repetition) | 30–60 s | 6–12 week | 2–4× per week | 5–8 exercises, weight-machine RE, free-weight RE, circuit RE, WBV RE | chest press, shoulder press, biceps curl, triceps extensions, seated row, lateral pull down, leg press, leg extension, leg curl, front-plank and sit-ups |
| Moderate intensity RE | 60–80% 1RM | 2–5 | 8–12 | 90–180 s | 8–12 week | 2–3× per week | 5–7 exercises, weight-machine RE, free-weight RE, upper body exercises not in consecutive order. | chest press, shoulder press, biceps curl, seated row, lateral pull down, squat, leg press, leg extension, leg curl, hip adduction, calf raises and sit-ups |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurik, R.; Żebrowska, A.; Stastny, P. Effect of an Acute Resistance Training Bout and Long-Term Resistance Training Program on Arterial Stiffness: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3492. https://doi.org/10.3390/jcm10163492
Jurik R, Żebrowska A, Stastny P. Effect of an Acute Resistance Training Bout and Long-Term Resistance Training Program on Arterial Stiffness: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(16):3492. https://doi.org/10.3390/jcm10163492
Chicago/Turabian StyleJurik, Roman, Aleksandra Żebrowska, and Petr Stastny. 2021. "Effect of an Acute Resistance Training Bout and Long-Term Resistance Training Program on Arterial Stiffness: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 16: 3492. https://doi.org/10.3390/jcm10163492
APA StyleJurik, R., Żebrowska, A., & Stastny, P. (2021). Effect of an Acute Resistance Training Bout and Long-Term Resistance Training Program on Arterial Stiffness: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(16), 3492. https://doi.org/10.3390/jcm10163492








