Effects of Statins on the Incidence and Mortality of Sepsis in Patients with New Cancer Diagnosis
Abstract
:1. Introduction
2. Methods
Statistical Analysis
3. Results
3.1. Effect of Statin Use on Overall Survival
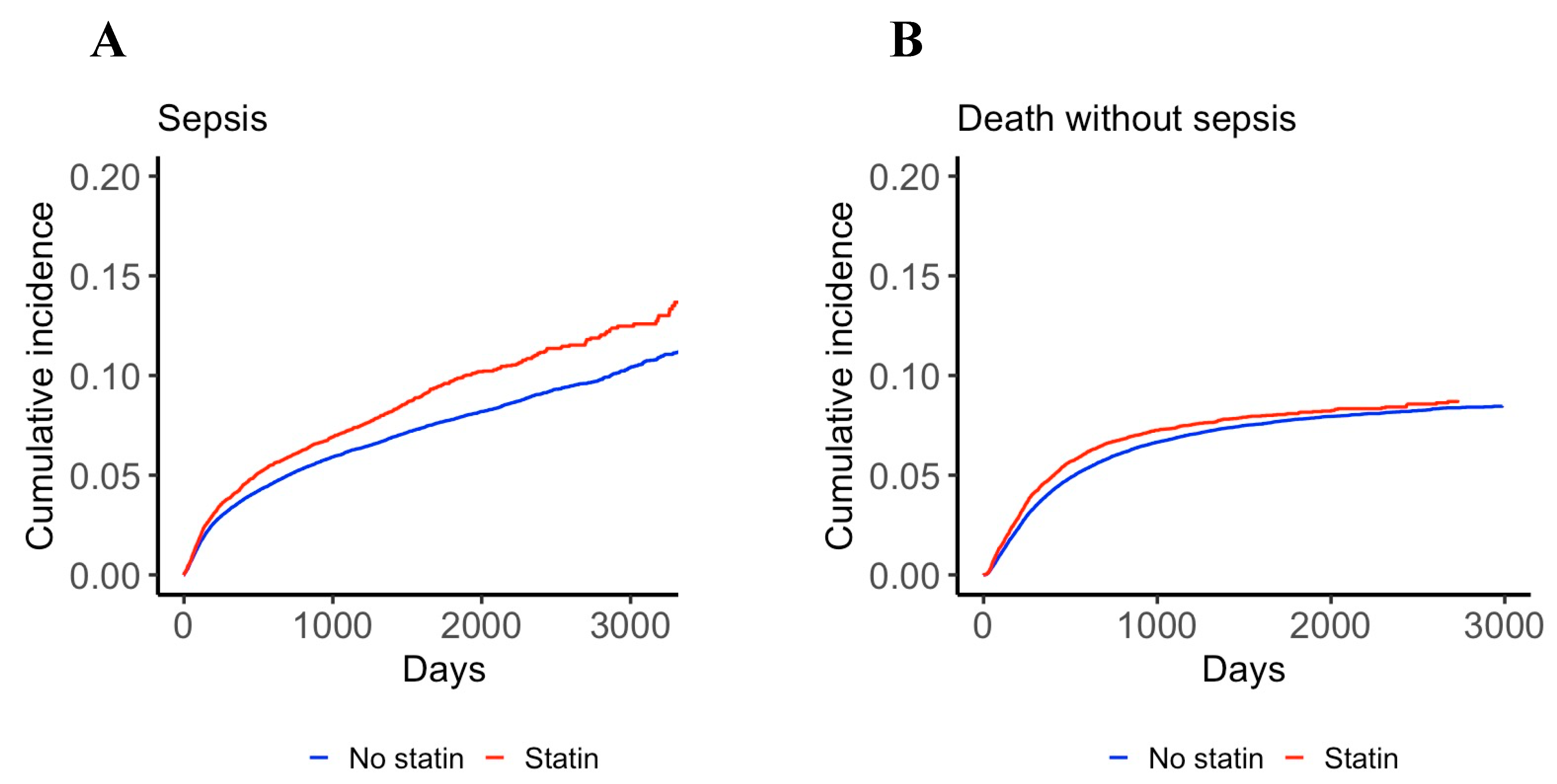
3.2. Effect of Statin Use on the Incidence of Sepsis
3.3. Effect of Statin Use on Sepsis Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez, F.; Maron, D.J.; Knowles, J.W.; Virani, S.S.; Lin, S.; Heidenreich, P.A. Association Between Intensity of Statin Therapy and Mortality in Patients With Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2017, 2, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, S.F.; Nordestgaard, B.G.; Bojesen, S.E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 2012, 367, 1792–1802. [Google Scholar] [CrossRef] [Green Version]
- Mei, Z.; Liang, M.; Li, L.; Zhang, Y.; Wang, Q.; Yang, W. Effects of statins on cancer mortality and progression: A systematic review and meta-analysis of 95 cohorts including 1,111,407 individuals. Int. J. Cancer 2017, 140, 1068–1081. [Google Scholar] [CrossRef]
- Altwairgi, A.K. Statins are potential anticancerous agents (review). Oncol. Rep. 2015, 33, 1019–1039. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.K.; Oza, A.M.; Siu, L.L. The statins as anticancer agents. Clin. Cancer Res. 2003, 9, 10–19. [Google Scholar]
- Inano, H.; Suzuki, K.; Onoda, M.; Wakabayashi, K. Anti-carcinogenic activity of simvastatin during the promotion phase of radiation-induced mammary tumorigenesis of rats. Carcinogenesis 1997, 18, 1723–1727. [Google Scholar] [CrossRef] [Green Version]
- Clutterbuck, R.D.; Millar, B.C.; Powles, R.L.; Newman, A.; Catovsky, D.; Jarman, M.; Millar, J.L. Inhibitory effect of simvastatin on the proliferation of human myeloid leukaemia cells in severe combined immunodeficient (SCID) mice. Br. J. Haematol. 1998, 102, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Reith, C.; Emberson, J.; Armitage, J.; Baigent, C.; Blackwell, L.; Blumenthal, R.; Danesh, J.; Smith, G.D.; DeMets, D.; et al. Interpretation of the evidence for the efficacy and safety of statin therapy. Lancet 2016, 388, 2532–2561. [Google Scholar] [CrossRef] [Green Version]
- Yusuf, S.; Bosch, J.; Dagenais, G.; Zhu, J.; Xavier, D.; Liu, L.; Pais, P.; Lopez-Jaramillo, P.; Leiter, L.A.; Dans, A.; et al. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N. Engl. J. Med. 2016, 374, 2021–2031. [Google Scholar] [CrossRef] [Green Version]
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.Y.; Singh, N. Antimicrobial and immunomodulatory attributes of statins: Relevance in solid-organ transplant recipients. Clin. Infect. Dis. 2009, 48, 745–755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azoulay, E.; Mokart, D.; Pene, F.; Lambert, J.; Kouatchet, A.; Mayaux, J.; Vincent, F.; Nyunga, M.; Bruneel, F.; Laisne, L.M.; et al. Outcomes of critically ill patients with hematologic malignancies: Prospective multicenter data from France and Belgium—A groupe de recherche respiratoire en reanimation onco-hematologique study. J. Clin. Oncol. 2013, 31, 2810–2818. [Google Scholar] [CrossRef]
- Torres, V.B.; Azevedo, L.C.; Silva, U.V.; Caruso, P.; Torelly, A.P.; Silva, E.; Carvalho, F.B.; Vianna, A.; Souza, P.C.; Godoy, M.M.; et al. Sepsis-associated outcomes in critically ill patients with malignancies. Ann. Am. Thorac. Soc. 2015, 12, 1185–1192. [Google Scholar] [CrossRef]
- Hackam, D.G.; Mamdani, M.; Li, P.; Redelmeier, D.A. Statins and sepsis in patients with cardiovascular disease: A population-based cohort analysis. Lancet 2006, 367, 413–418. [Google Scholar] [CrossRef]
- Lee, C.C.; Lee, M.G.; Hsu, T.C.; Porta, L.; Chang, S.S.; Yo, C.H.; Tsai, K.C.; Lee, M. A population-based cohort study on the drug-specific effect of statins on sepsis outcome. Chest 2018, 153, 805–815. [Google Scholar] [CrossRef]
- Deshpande, A.; Pasupuleti, V.; Rothberg, M.B. Statin therapy and mortality from sepsis: A meta-analysis of randomized trials. Am. J. Med. 2015, 128, 410–417 e411. [Google Scholar] [CrossRef] [PubMed]
- Van de Louw, A.; Cohrs, A.; Leslie, D. Clinical features and outcome of thrombotic microangiopathies: Comparison between patients with and without malignancy. Thromb. Haemost. 2021, 121, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.C.M.; Upadhyay, U.D.; Liu, G.; Kerns, J.L.; Ba, D.; Beam, N.; Leslie, D.L. Association of facility type with procedural-related morbidities and adverse events among patients undergoing induced abortions. JAMA 2018, 319, 2497–2506. [Google Scholar] [CrossRef] [Green Version]
- Van de Louw, A.; Cohrs, A.; Leslie, D. Incidence of sepsis and associated mortality within the first year after cancer diagnosis in middle aged adults: A US population based study. PLoS ONE 2020, 15, e0243449. [Google Scholar] [CrossRef]
- Tamburrino, D.; Crippa, S.; Partelli, S.; Archibugi, L.; Arcidiacono, P.G.; Falconi, M.; Capurso, G. Statin use improves survival in patients with pancreatic ductal adenocarcinoma: A meta-analysis. Dig. Liver Dis. 2020, 52, 392–399. [Google Scholar] [CrossRef]
- Van Wyhe, R.D.; Rahal, O.M.; Woodward, W.A. Effect of statins on breast cancer recurrence and mortality: A review. Breast Cancer 2017, 9, 559–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, W.; Ning, L.; Huang, Y.; Liu, Y.; Zhang, W.; Hu, Y.; Lang, J.; Yang, J. Statin use and survival outcomes in endocrine-related gynecologic cancers: A systematic review and meta-analysis. Oncotarget 2017, 8, 41508–41517. [Google Scholar] [CrossRef] [Green Version]
- Nayan, M.; Punjani, N.; Juurlink, D.N.; Finelli, A.; Austin, P.C.; Kulkarni, G.S.; Uleryk, E.; Hamilton, R.J. Statin use and kidney cancer survival outcomes: A systematic review and meta-analysis. Cancer Treat. Rev. 2017, 52, 105–116. [Google Scholar] [CrossRef]
- Gray, R.T.; Coleman, H.G.; Hughes, C.; Murray, L.J.; Cardwell, C.R. Statin use and survival in colorectal cancer: Results from a population-based cohort study and an updated systematic review and meta-analysis. Cancer Epidemiol. 2016, 45, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hindler, K.; Cleeland, C.S.; Rivera, E.; Collard, C.D. The role of statins in cancer therapy. Oncologist 2006, 11, 306–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaleska, M.; Mozenska, O.; Bil, J. Statins use and cancer: An update. Future Oncol. 2018, 14, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Sturgeon, K.M.; Deng, L.; Bluethmann, S.M.; Zhou, S.; Trifiletti, D.M.; Jiang, C.; Kelly, S.P.; Zaorsky, N.G. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur. Heart J. 2019, 40, 3889–3897. [Google Scholar] [CrossRef] [Green Version]
- Merx, M.W.; Liehn, E.A.; Janssens, U.; Lutticken, R.; Schrader, J.; Hanrath, P.; Weber, C. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation 2004, 109, 2560–2565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merx, M.W.; Liehn, E.A.; Graf, J.; van de Sandt, A.; Schaltenbrand, M.; Schrader, J.; Hanrath, P.; Weber, C. Statin treatment after onset of sepsis in a murine model improves survival. Circulation 2005, 112, 117–124. [Google Scholar] [CrossRef] [Green Version]
- Janda, S.; Young, A.; Fitzgerald, J.M.; Etminan, M.; Swiston, J. The effect of statins on mortality from severe infections and sepsis: A systematic review and meta-analysis. J. Crit. Care 2010, 25, 656.e7–656.e22. [Google Scholar] [CrossRef]
- National Heart, L.; Blood Institute, A.C.T.N.; Truwit, J.D.; Bernard, G.R.; Steingrub, J.; Matthay, M.A.; Liu, K.D.; Albertson, T.E.; Brower, R.G.; Shanholtz, C.; et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N. Engl. J. Med. 2014, 370, 2191–2200. [Google Scholar] [CrossRef] [Green Version]
- Papazian, L.; Roch, A.; Charles, P.E.; Penot-Ragon, C.; Perrin, G.; Roulier, P.; Goutorbe, P.; Lefrant, J.Y.; Wiramus, S.; Jung, B.; et al. Effect of statin therapy on mortality in patients with ventilator-associated pneumonia: A randomized clinical trial. JAMA 2013, 310, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Kruger, P.; Bailey, M.; Bellomo, R.; Cooper, D.J.; Harward, M.; Higgins, A.; Howe, B.; Jones, D.; Joyce, C.; Kostner, K.; et al. A multicenter randomized trial of atorvastatin therapy in intensive care patients with severe sepsis. Am. J. Respir. Crit. Care Med. 2013, 187, 743–750. [Google Scholar] [CrossRef]
- Pertzov, B.; Eliakim-Raz, N.; Atamna, H.; Trestioreanu, A.Z.; Yahav, D.; Leibovici, L. Hydroxymethylglutaryl-CoA reductase inhibitors (statins) for the treatment of sepsis in adults—A systematic review and meta-analysis. Clin. Microbiol. Infect. 2019, 25, 280–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almog, Y.; Shefer, A.; Novack, V.; Maimon, N.; Barski, L.; Eizinger, M.; Friger, M.; Zeller, L.; Danon, A. Prior statin therapy is associated with a decreased rate of severe sepsis. Circulation 2004, 110, 880–885. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.G.; Lee, C.C.; Lai, C.C.; Hsu, T.C.; Porta, L.; Lee, M.; Chang, S.S.; Chien, K.L.; Chen, Y.M.; National Taiwan University Hospital Health Economics and Outcome Research Group; et al. Preadmission statin use improves the outcome of less severe sepsis patients—A population-based propensity score matched cohort study. Br. J. Anaesth. 2017, 119, 645–654. [Google Scholar] [CrossRef] [Green Version]
- Chinaeke, E.E.; Love, B.L.; Magagnoli, J.; Yunusa, I.; Reeder, G. The impact of statin use prior to intensive care unit admission on critically ill patients with sepsis. Pharmacotherapy 2021, 41, 162–171. [Google Scholar] [CrossRef]
- Gupta, R.; Plantinga, L.C.; Fink, N.E.; Melamed, M.L.; Coresh, J.; Fox, C.S.; Levin, N.W.; Powe, N.R. Statin use and sepsis events [corrected] in patients with chronic kidney disease. JAMA 2007, 297, 1455–1464. [Google Scholar] [CrossRef] [PubMed]
- Danai, P.A.; Moss, M.; Mannino, D.M.; Martin, G.S. The epidemiology of sepsis in patients with malignancy. Chest 2006, 129, 1432–1440. [Google Scholar] [CrossRef]
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef]
- Te Marvelde, L.; Whitfield, A.; Shepheard, J.; Read, C.; Milne, R.L.; Whitfield, K. Epidemiology of sepsis in cancer patients in Victoria, Australia: A population-based study using linked data. Aust. N. Z. J. Public Health 2020, 44, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.D.; Braun, L.A.; Cooper, L.M.; Johnston, J.; Weiss, R.V.; Qualy, R.L.; Linde-Zwirble, W. Hospitalized cancer patients with severe sepsis: Analysis of incidence, mortality, and associated costs of care. Crit. Care 2004, 8, R291–R298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, R.W. The lesser known effects of statins: Benefits on infectious outcomes may be explained by “healthy user” effect. BMJ 2006, 333, 980–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danaei, G.; Tavakkoli, M.; Hernan, M.A. Bias in observational studies of prevalent users: Lessons for comparative effectiveness research from a meta-analysis of statins. Am. J. Epidemiol. 2012, 175, 250–262. [Google Scholar] [CrossRef]
- Emilsson, L.; Garcia-Albeniz, X.; Logan, R.W.; Caniglia, E.C.; Kalager, M.; Hernan, M.A. Examining bias in studies of statin treatment and survival in patients with cancer. JAMA Oncol. 2018, 4, 63–70. [Google Scholar] [CrossRef]
- Ferro, D.; Parrotto, S.; Basili, S.; Alessandri, C.; Violi, F. Simvastatin inhibits the monocyte expression of proinflammatory cytokines in patients with hypercholesterolemia. J. Am. Coll. Cardiol. 2000, 36, 427–431. [Google Scholar] [CrossRef] [Green Version]
- O’Driscoll, G.; Green, D.; Taylor, R.R. Simvastatin, an HMG-coenzyme A reductase inhibitor, improves endothelial function within 1 month. Circulation 1997, 95, 1126–1131. [Google Scholar] [CrossRef]
- Kempner, W. The nature of leukemic blood cells as determined by their metabolism. J. Clin. Investig. 1939, 18, 291–300. [Google Scholar] [CrossRef] [PubMed]
| No Statin (n = 99,911) | Statin (n = 19,468) | Total (n = 119,379) | p Value | |
|---|---|---|---|---|
| Female, n (%) | 63,174 (63.2%) | 9758 (50.1%) | 72,932 (61.1%) | <0.001 |
| Age, years | 54 (49–59) | 58 (54–61) | 55 (50–60) | <0.001 |
| Comorbidities: | ||||
| Myocardial infarction, n (%) | 478 (0.5%) | 459 (2.4%) | 937 (0.8%) | <0.001 |
| Congestive heart failure, n (%) | 1126 (1.1%) | 539 (2.8%) | 1665 (1.4%) | <0.001 |
| Peripheral vascular disease, n (%) | 2010 (2.0%) | 990 (5.1%) | 3000 (2.5%) | <0.001 |
| Cerebrovascular disease, n (%) | 2212 (2.2%) | 1091 (5.6%) | 3303 (2.8%) | <0.001 |
| Chronic pulmonary disease, n (%) | 10,222 (10.2%) | 2462 (12.6%) | 12,684 (10.6%) | <0.001 |
| Renal disease, n (%) | 1265 (1.3%) | 574 (2.9%) | 1839 (1.5%) | <0.001 |
| Modified Charlson comorbidity index risk, n (%) | <0.001 | |||
| mCCI = 0 | 68,540 (70.5%) | 9454 (48.7%) | 77,994 (66.9%) | |
| mCCI 1–2 | 25,773 (26.5%) | 8605 (44.3%) | 34,378 (29.5%) | |
| mCCI 3–4 | 2162 (2.2%) | 1121 (5.8%) | 3283 (2.8%) | |
| mCCI ≥ 5 | 723 (0.7%) | 226 (1.2%) | 949 (0.8%) | |
| Cancer site: | ||||
| Head and neck, n (%) | 1771 (1.8%) | 407 (2.1%) | 2178 (1.8%) | 0.002 |
| Gastrointestinal, n (%) | 15,728 (15.7%) | 3132 (16.1%) | 18,860 (15.8%) | 0.226 |
| Lung, n (%) | 7504 (7.5%) | 1918 (9.9%) | 9422 (7.9%) | <0.001 |
| Musculoskeletal and breast, n (%) | 32,556 (32.6%) | 4773 (24.5%) | 37,329 (31.3%) | <0.001 |
| Genitourinary, n (%) | 13,404 (13.4%) | 3337 (17.1%) | 16,741 (14.0%) | <0.001 |
| Hematological malignancy, n (%) | 9160 (9.2%) | 1790 (9.2%) | 10,950 (9.2%) | 0.907 |
| Metastases, n (%) | 10,755 (10.8%) | 2027 (10.4%) | 12,782 (10.7%) | 0.145 |
| Covariate | Hazard Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Female gender | 0.857 | 0.822–0.894 | <0.0001 |
| Age | 1.023 | 1.019–1.026 | <0.0001 |
| Modified Charlson comorbidity index group (0 as the reference): | |||
| mCCI 1–2 | 1.392 | 1.334–1.452 | <0.0001 |
| mCCI 3–4 | 1.874 | 1.711–2.052 | <0.0001 |
| mCCI ≥ 5 | 2.233 | 1.918–2.560 | <0.0001 |
| Cancer site (hematological malignancy as the reference): | |||
| Gastrointestinal | 1.160 | 1.082–1.244 | <0.0001 |
| Genitourinary | 0.405 | 0.370–0.443 | <0.0001 |
| Head and neck | 0.522 | 0.435–0.626 | <0.0001 |
| Musculoskeletal and breast | 0.273 | 0.250–0.297 | <0.0001 |
| Lung | 2.262 | 2.105–2.432 | <0.0001 |
| Statin use | 0.897 | 0.851–0.945 | <0.0001 |
| Covariate | Subdistribution Hazard Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Female gender | 0.931 | 0.885–0.979 | 0.005 |
| Age | 1.017 | 1.013–1.021 | <0.0001 |
| Modified Charlson comorbidity index group (0 as the reference): | |||
| mCCI 1–2 | 1.438 | 1.368–1.512 | <0.0001 |
| mCCI 3–4 | 2.243 | 2.028–2.482 | <0.0001 |
| mCCI ≥ 5 | 2.899 | 2.472–3.400 | <0.0001 |
| Cancer site (hematological malignancy as the reference): | |||
| Gastrointestinal | 0.879 | 0.817–0.946 | 0.0006 |
| Genitourinary | 0.445 | 0.407–0.486 | <0.0001 |
| Head and neck | 0.580 | 0.486–0.692 | <0.0001 |
| Musculoskeletal and breast | 0.265 | 0.243–0.290 | <0.0001 |
| Lung | 0.771 | 0.703–0.845 | <0.0001 |
| Statin use | 0.990 | 0.932–1.050 | 0.73 |
| Survivors (n = 5630) | Non Survivors (n = 1704) | p Value | |
|---|---|---|---|
| Female, n (%) | 2926 (52.0%) | 817 (47.9%) | 0.012 |
| Age, years | 56 (50–59) | 57 (52–60) | <0.001 |
| Comorbidities: | |||
| Myocardial infarction, n (%) | 71 (1.3%) | 13 (0.8%) | 0.069 |
| Congestive heart failure, n (%) | 157 (2.8%) | 51 (3.0%) | 0.130 |
| Peripheral vascular disease, n (%) | 213 (3.8%) | 77 (4.5%) | 0.025 |
| Cerebrovascular disease, n (%) | 176 (3.1%) | 83 (4.9%) | <0.001 |
| Chronic pulmonary disease, n (%) | 776 (13.8%) | 260 (15.3%) | 0.017 |
| Renal disease, n (%) | 216 (3.8%) | 61 (3.6%) | 0.056 |
| Modified Charlson comorbidity index risk, n (%) | 0.299 | ||
| mCCI = 0 | 3033 (55.3%) | 881 (53.6%) | |
| mCCI 1–2 | 2034 (37.1%) | 613 (37.3%) | |
| mCCI 3–4 | 301 (5.5%) | 108 (6.6%) | |
| mCCI ≥ 5 | 114 (2.1%) | 42 (2.6%) | |
| Statin use, n (%) | 1053 (18.7%) | 312 (18.3%) | 0.514 |
| Cancer site: | |||
| Head and neck, n (%) | 111 (2.0%) | 28 (1.6%) | 0.420 |
| Gastrointestinal, n (%) | 1306 (23.2%) | 399 (23.4%) | 0.282 |
| Lung, n (%) | 505 (9.0%) | 235 (13.8%) | <0.001 |
| Musculoskeletal and breast, n (%) | 900 (16.0%) | 182 (10.7%) | <0.001 |
| Genitourinary, n (%) | 683 (12.1%) | 152 (8.9%) | <0.001 |
| Hematological malignancy, n (%) | 921 (16.4%) | 301 (17.7%) | 0.436 |
| Metastases, n (%) | 775 (13.8%) | 327 (19.2%) | <0.001 |
| Severe sepsis, n (%) | 943 (16.7%) | 486 (28.5%) | <0.001 |
| Septic shock, n (%) | 1168 (20.7%) | 919 (53.9%) | <0.001 |
| Covariate | Odds Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Statin use | 0.952 | 0.829–1.091 | 0.479 |
| Age | 1.018 | 1.008–1.028 | <0.001 |
| History of cerebrovascular disease | 1.439 | 1.071–1.920 | 0.014 |
| History of myocardial infarction | 0.680 | 0.375–1.165 | 0.179 |
| Cancer site (hematological malignancy as the reference): | |||
| Gastrointestinal | 0.914 | 0.763–1.095 | 0.330 |
| Genitourinary | 0.683 | 0.545–0.852 | <0.001 |
| Head and neck | 0.629 | 0.385–0.993 | 0.055 |
| Musculoskeletal and breast | 0.694 | 0.564–0.853 | <0.001 |
| Lung | 1.176 | 0.944–1.464 | 0.146 |
| Metastases | 1.300 | 1.106–1.526 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van de Louw, A.; Cohrs, A.; Leslie, D. Effects of Statins on the Incidence and Mortality of Sepsis in Patients with New Cancer Diagnosis. J. Clin. Med. 2021, 10, 3427. https://doi.org/10.3390/jcm10153427
Van de Louw A, Cohrs A, Leslie D. Effects of Statins on the Incidence and Mortality of Sepsis in Patients with New Cancer Diagnosis. Journal of Clinical Medicine. 2021; 10(15):3427. https://doi.org/10.3390/jcm10153427
Chicago/Turabian StyleVan de Louw, Andry, Austin Cohrs, and Douglas Leslie. 2021. "Effects of Statins on the Incidence and Mortality of Sepsis in Patients with New Cancer Diagnosis" Journal of Clinical Medicine 10, no. 15: 3427. https://doi.org/10.3390/jcm10153427
APA StyleVan de Louw, A., Cohrs, A., & Leslie, D. (2021). Effects of Statins on the Incidence and Mortality of Sepsis in Patients with New Cancer Diagnosis. Journal of Clinical Medicine, 10(15), 3427. https://doi.org/10.3390/jcm10153427






