Change in Shoulder Function in the Early Recovery Phase after Breast Cancer Surgery: A Prospective Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Designs
2.3. Outcome Measures
2.3.1. Range of Motion
2.3.2. Shoulder Strength
2.3.3. Shoulder Function Score
2.4. Statistical Analysis
3. Results
3.1. Participants’ Characteristics
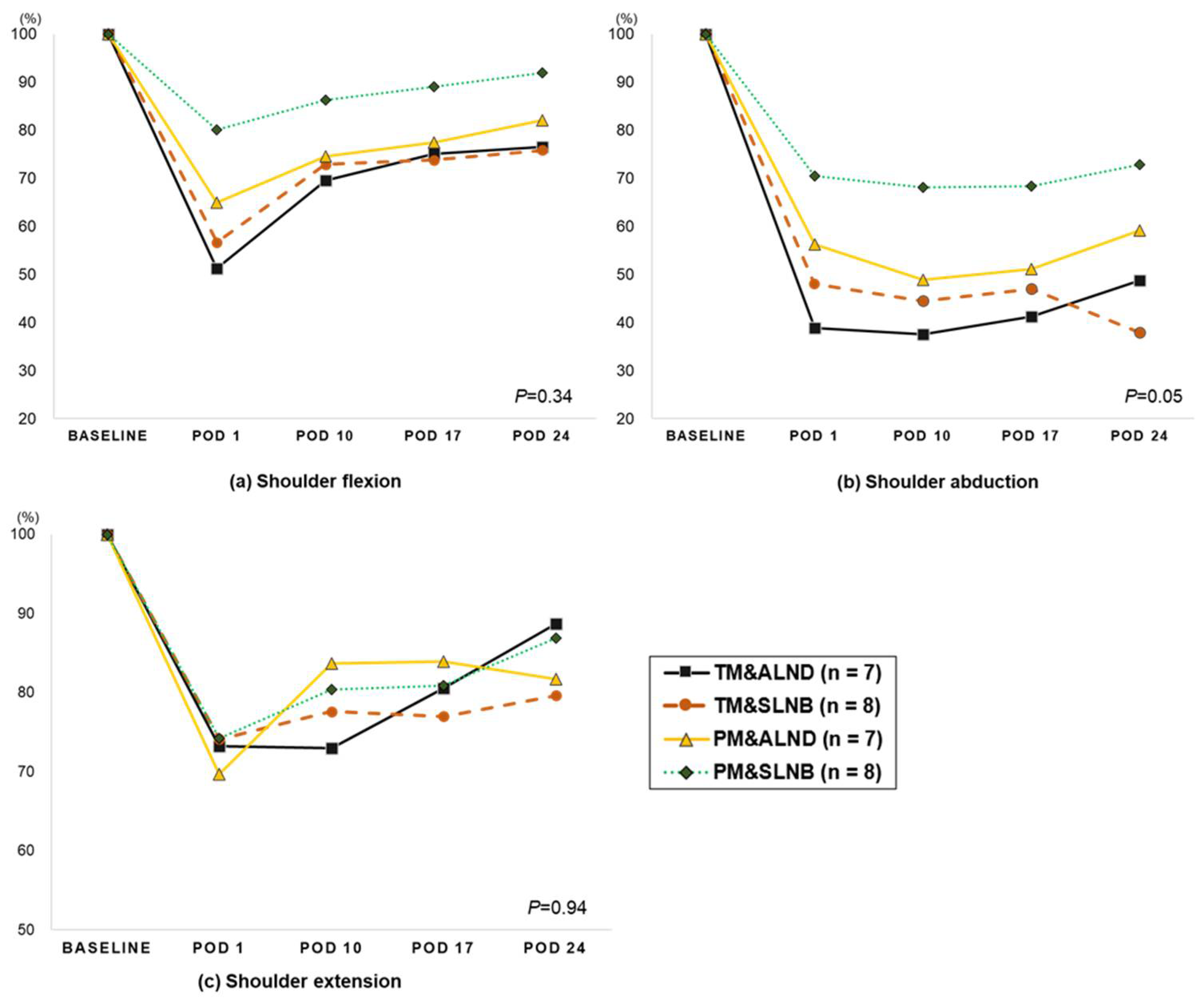
3.2. Change in Shoulder Range of Motion (from Pre-Surgery to 4-Week Post-Surgery)
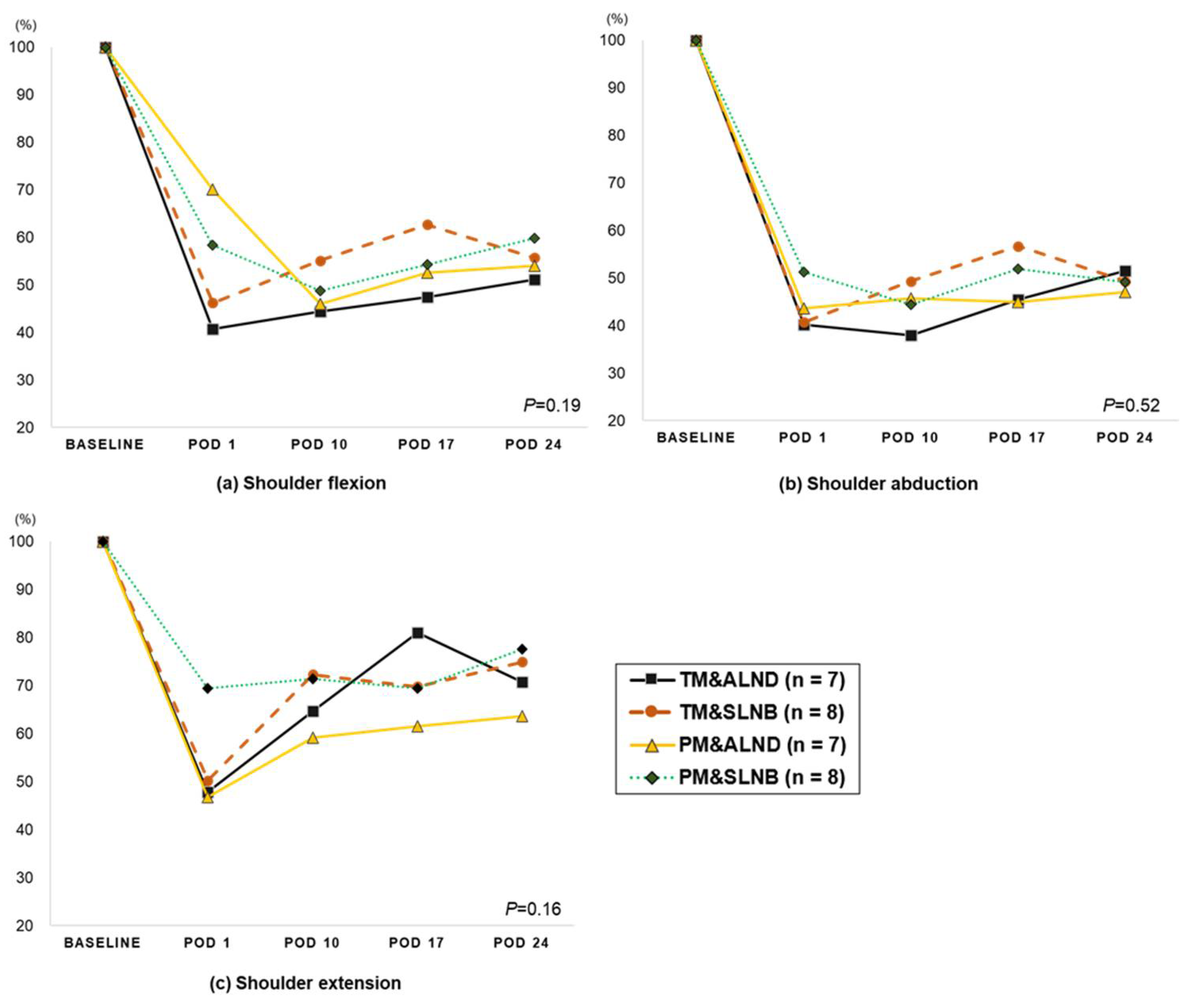
3.3. Change in Shoulder Strength (from Pre-Surgery to 4-Week Post-Surgery)
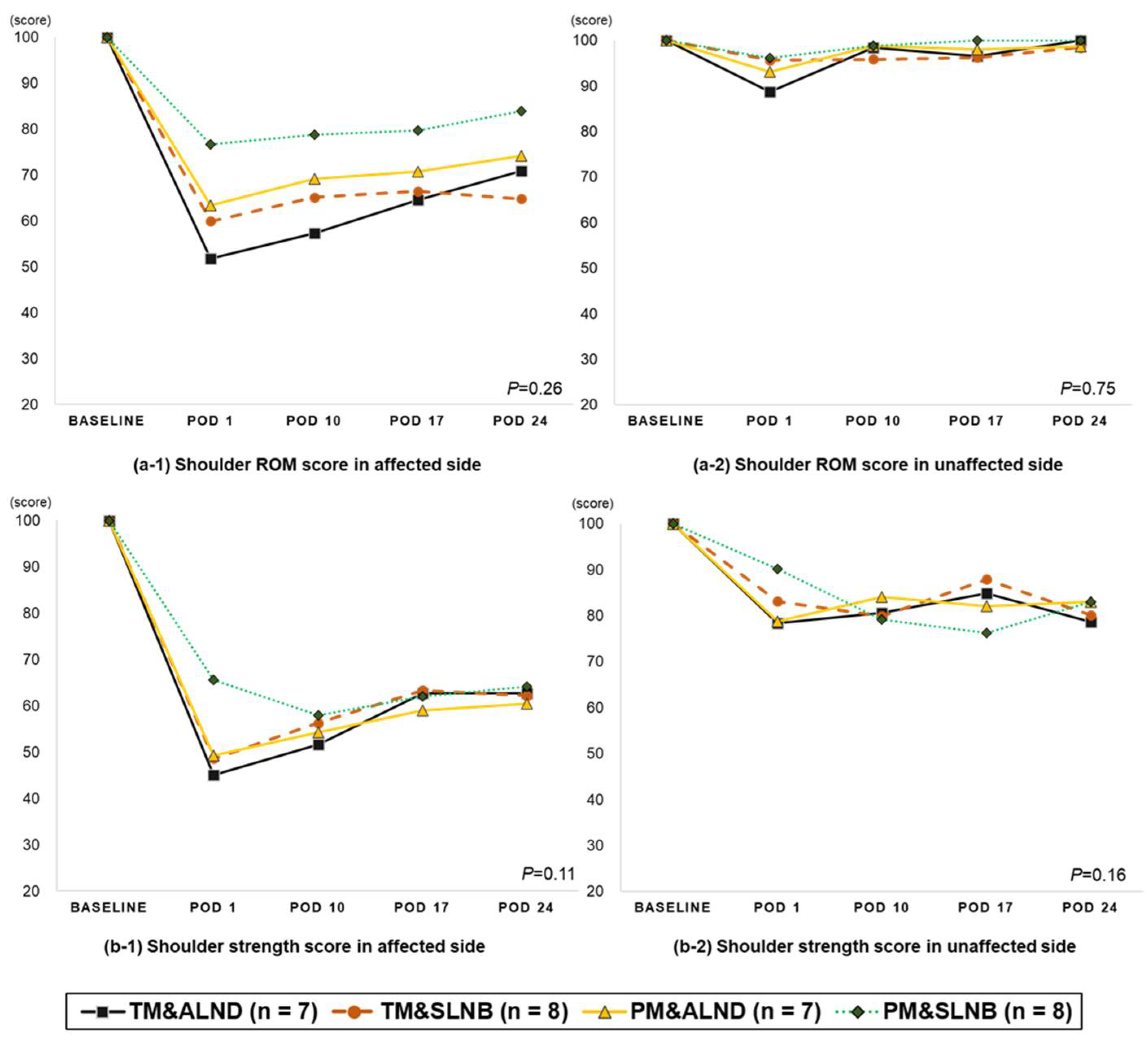
3.4. Change in Shoulder Function (from Pre-Surgery to 4-Week Post-Surgery)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loh, S.Y.; Musa, A.N. Methods to improve rehabilitation of patients following breast cancer surgery: A review of systematic reviews. Breast Cancer 2015, 7, 81–98. [Google Scholar] [CrossRef]
- Verbelen, H.; Tjalma, W.; Meirte, J.; Gebruers, N. Long-term morbidity after a negative sentinel node in breast cancer patients. Eur. J. Cancer Care 2019, 28, e13077. [Google Scholar] [CrossRef]
- Rietman, J.S.; Dijkstra, P.U.; Debreczeni, R.; Geertzen, J.H.B.; Robinson, D.P.H.; De Vries, J. Impairments, disabilities and health related quality of life after treatment for breast cancer: A follow-up study 2.7 years after surgery. Disabil. Rehabil. 2004, 26, 78–84. [Google Scholar] [CrossRef]
- Andersen, K.G.; Kehlet, H. Persistent Pain After Breast Cancer Treatment: A Critical Review of Risk Factors and Strategies for Prevention. J. Pain 2011, 12, 725–746. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Kilbreath, S.L.; Refshauge, K.M.; Herbert, R.D.; Beith, J.M. Prognosis of the upper limb following surgery and radiation for breast cancer. Breast Cancer Res. Treat. 2007, 110, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, P.; Rieger, R.; Shamiyeh, A.; Wayand, W. Morbidity following sentinel lymph node biopsy versus axillary lymph node dissection for patients with breast carcinoma. Cancer 2000, 88, 608–614. [Google Scholar] [CrossRef]
- Oosterwijk, A.; Nieuwenhuis, M.; Van Der Schans, C.; Mouton, L. Shoulder and elbow range of motion for the performance of activities of daily living: A systematic review. Physiother. Theory Pract. 2018, 34, 505–528. [Google Scholar] [CrossRef]
- Sugden, E.; Rezvani, M.; Harrison, J.; Hughes, L. Shoulder movement after the treatment of early stage breast cancer. Clin. Oncol. 1998, 10, 173–181. [Google Scholar] [CrossRef]
- Sagen, A.; Kaaresen, R.; Sandvik, L.; Thune, I.; Risberg, M.A. Upper Limb Physical Function and Adverse Effects After Breast Cancer Surgery: A Prospective 2.5-Year Follow-Up Study and Preoperative Measures. Arch. Phys. Med. Rehabil. 2014, 95, 875–881. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, R.R.; e Silva, M.P.P.; Gurgel, M.S.C.; Pastori-Filho, L.; Sarian, L. Immediate Breast Reconstruction With Transverse Latissimus Dorsi Flap Does Not Affect the Short-term Recovery of Shoulder Range of Motion After Mastectomy. Ann. Plast. Surg. 2010, 64, 402–408. [Google Scholar] [CrossRef]
- Kaya, T.; Karatepe, A.G.; Günaydin, R.; Yetiş, H.; Uslu, A. Disability and Health-Related Quality of Life after Breast Cancer Surgery: Relation to Impairments. South. Med. J. 2010, 103, 37–41. [Google Scholar] [CrossRef]
- Johnsson, A.; Fornander, T.; Rutqvist, L.-E.; Vaez, M.; Alexanderson, K.; Olsson, M. Predictors of return to work ten months after primary breast cancer surgery. Acta Oncol. 2009, 48, 93–98. [Google Scholar] [CrossRef]
- Norkin, C.C.; White, D.J. Measurement of Joint Motion: A Guide to Goniometry; FA Davis: Philadelphia, PA, USA, 2016. [Google Scholar]
- Ford-Smith, C.D.; Wyman, J.F.; Elswick, R., Jr.; Fernandez, T. Reliability of stationary dynamometer muscle strength testing in community-dwelling older adults. Arch. Phys. Med. Rehabil. 2001, 82, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, S.E. Pairwise multiple comparisons in repeated measures designs. J. Educ. Stat. 1980, 5, 269–287. [Google Scholar] [CrossRef]
- Springer, B.A.; Levy, E.; McGarvey, C.; Pfalzer, L.A.; Stout, N.; Gerber, L.; Soballe, P.W.; Danoff, J. Pre-operative assessment enables early diagnosis and recovery of shoulder function in patients with breast cancer. Breast Cancer Res. Treat. 2010, 120, 135–147. [Google Scholar] [CrossRef]
- Cinar, N.; Seckin, Ü.; Keskin, D.; Bodur, H.; Bozkurt, B.; Cengiz, Ö. The Effectiveness of Early Rehabilitation in Patients With Modified Radical Mastectomy. Cancer Nurs. 2008, 31, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Nesvold, I.-L.; Dahl, A.A.; Løkkevik, E.; Mengshoel, A.M.; Fosså, S.D. Arm and shoulder morbidity in breast cancer patients after breast-conserving therapy versus mastectomy. Acta Oncol. 2008, 47, 835–842. [Google Scholar] [CrossRef]
- Kootstra, J.J.; Dijkstra, P.U.; Rietman, H.; De Vries, J.; Baas, P.; Geertzen, J.H.B.; Hoekstra, H.J.; Hoekstra-Weebers, J.E.H.M. A longitudinal study of shoulder and arm morbidity in breast cancer survivors 7 years after sentinel lymph node biopsy or axillary lymph node dissection. Breast Cancer Res. Treat. 2013, 139, 125–134. [Google Scholar] [CrossRef]
- Belmonte, R.; Messaggi-Sartor, M.; Ferrer, M.; Pont, A.; Escalada, F. Prospective study of shoulder strength, shoulder range of motion, and lymphedema in breast cancer patients from pre-surgery to 5 years after ALND or SLNB. Support. Care Cancer 2018, 26, 3277–3287. [Google Scholar] [CrossRef]
- Klassen, O.; Schmidt, M.E.; Ulrich, C.M.; Schneeweiss, A.; Potthoff, K.; Steindorf, K.; Wiskemann, J. Muscle strength in breast cancer patients receiving different treatment regimes. J. Cachex. Sarcopenia Muscle 2016, 8, 305–316. [Google Scholar] [CrossRef]
- Baron, R.H.; Fey, J.V.; Borgen, P.I.; Stempel, M.M.; Hardick, K.R.; Van Zee, K.J. Eighteen Sensations After Breast Cancer Surgery: A 5-Year Comparison of Sentinel Lymph Node Biopsy and Axillary Lymph Node Dissection. Ann. Surg. Oncol. 2007, 14, 1653–1661. [Google Scholar] [CrossRef]
- Schulze, T.; Mucke, J.; Markwardt, J.; Schlag, P.M.; Bembenek, A. Long-term morbidity of patients with early breast cancer after sentinel lymph node biopsy compared to axillary lymph node dissection. J. Surg. Oncol. 2006, 93, 109–119. [Google Scholar] [CrossRef]
- Sclafani, L.M.; Baron, R.H. Sentinel Lymph Node Biopsy and Axillary Dissection: Added Morbidity of the Arm, Shoulder and Chest Wall After Mastectomy and Reconstruction. Cancer J. 2008, 14, 216–222. [Google Scholar] [CrossRef]
- Lee, C.H.; Chung, S.Y.; Kim, W.Y.; Yang, S.N. Effect of breast cancer surgery on chest tightness and upper limb dysfunction. Medicine 2019, 98, e15524. [Google Scholar] [CrossRef]
- Ducic, I.; Zakaria, H.M.; Felder, J.M., 3rd; Fantus, S. Nerve Injuries in Aesthetic Breast Surgery: Systematic Review and Treatment Options. Aesthet. Surg. J. 2014, 34, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Kilbreath, S.L.; Sullivan, G.; Refshauge, K.M.; Beith, J.M.; Harris, L.M. Factors That Affect Intention to Avoid Strenuous Arm Activity After Breast Cancer Surgery. Oncol. Nurs. Forum 2009, 36, 454–462. [Google Scholar] [CrossRef][Green Version]
- Shamley, D.R.; Srinanaganathan, R.; Weatherall, R.; Oskrochi, R.; Watson, M.; Ostlere, S.; Sugden, E. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res. Treat. 2007, 106, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Kwan, W.; Jackson, J.; Weir, L.M.; Dingee, C.; McGregor, G.; Olivotto, I.A. Chronic Arm Morbidity After Curative Breast Cancer Treatment: Prevalence and Impact on Quality of Life. J. Clin. Oncol. 2002, 20, 4242–4248. [Google Scholar] [CrossRef] [PubMed]
| Variable (N = 32) | TM&ALND (N = 7) | TM&SLNB (N = 8) | PM&ALND (N = 8) | PM&SLNB (N = 9) | P |
|---|---|---|---|---|---|
| Age (years) | 50.0 ± 11.1 | 53.6 ± 6.7 | 52.6 ± 7.9 | 52.8 ± 5.3 | 0.8 |
| Weight (kg) | 61.7 ± 8.3 | 66.5 ± 9.7 | 63.0 ± 7.9 | 58.4 ± 6.3 | 0.3 |
| BMI (kg/m2) | 23.3 ± 2.4 | 25.9 ± 3.7 | 25.5 ± 2.9 | 23.7 ± 1.9 | 0.2 |
| Muscle mass (kg) | 23.0 ± 2.3 | 23.6 ± 2.7 | 22.4 ± 2.3 | 20.8 ± 2.1 | 0.1 |
| Fat (%) | 30.1 ± 4.6 | 33.5 ± 5.5 | 33.0 ± 6.0 | 33.6 ± 6.6 | 0.6 |
| Stage (n, %) | |||||
| 0 | 0 | 3 (37.5%) | 2 (25%) | 4 (44.4%) | 0.6 |
| 1 | 1 (14.3%) | 3 (37.5%) | 2 (25%) | 5 (55.6%) | |
| 2 | 3 (42.9%) | 2 (25%) | 3 (37.5%) | 0 | |
| 3 | 3 (42.9%) | 0 | 1 (12.5%) | 0 | |
| Surgery site (n, %) | |||||
| Right | 5 (71.4%) | 4 (50%) | 4 (50%) | 6 (66.7%) | 0.8 |
| Dominant arm | 5 (71.4%) | 5 (62.5%) | 4 (50%) | 6 (66.7%) | 0.8 |
| Dissected LNs | 18 (9–25) | 7 (3–13) | 17.5 (10–26) | 9 (5–12) | <0.001 |
| Surgery duration (min) | |||||
| 108.9 ± 21.4 | 95 ± 16.1 | 122.3 ± 59.4 | 93.4 ± 40.0 | 0.5 | |
| Drainage removal day (n, %) | |||||
| 1st outpatient visit (POD 10) | 1 (14.3%) | 1 (12.5%) | 0 | 3 (33.3%) | 0.3 |
| 2nd outpatient visit (POD 17) | 6 (85.7%) | 4 (50%) | 4 (50%) | 4 (44.4%) | |
| 3rd outpatient visit (POD 24) | 0 | 3 (37.5%) | 3 (37.5%) | 1 (11.1%) | |
| 4th outpatient visit | 0 | 0 | 1 (12.5%) | 1 (11.1%) | |
| Neoadjuvant chemotherapy | |||||
| Yes | 6 (85.7%) | 1 (12.5%) | 7 (87.5%) | 1 (11.1%) | <0.001 |
| Baseline | POD 1 | POD 10 | POD 17 | POD 24 | P # | P baselinevs. POD1 | P POD1vs. POD10 | P POD10vs. POD17 | P POD17vs. POD24 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Shoulder range of motion | ||||||||||
| Flexion | ||||||||||
| Affected side (n = 29) | 174.0 ± 1.3 | 111.1 ± 8.6 **+ | 132.5 ± 4.7 **+ | 137.5 ± 4.2 **+ | 142.3 ± 4.6 **+ | <0.001 | <0.001 | 0.005 | 0.071 | 0.01 |
| Unaffected side (n = 31) | 176.4 ± 0.8 | 161.7 ± 3.6 ** | 174.4 ± 0.7 | 174.7 ± 0.9 | 174.9 ± 0.7 | 0.02 | <0.001 | 0.001 | 0.765 | 0.625 |
| Abduction | ||||||||||
| Affected side (n = 30) | 169.1 ± 2.8 | 90.2 ± 7.1 **+ | 84.1 ± 4.5 **+ | 87.9 ± 5.7 **+ | 91.8 ± 6.1 **+ | <0.001 | <0.001 | 0.386 | 0.437 | 0.164 |
| Unaffected side (n = 31) | 170.2 ± 1.9 | 153.7 ± 4.4 * | 166.0 ± 2.4 | 169.0 ± 2.1 | 171.2 ± 2.1 | 0.004 | 0.001 | 0.0.21 | 0.197 | 0.05 |
| Extension | ||||||||||
| Affected side (n = 30) | 47.4 ± 1.1 | 34.5 ± 2.1 **+ | 37.3 ± 1.6 **+ | 38.1 ± 1.8 **+ | 39.8 ± 1.6 **+ | <0.001 | <0.001 | 0.200 | 0.642 | 0.101 |
| Unaffected side (n = 31) | 46.0 ± 1.2 | 44.7 ± 1.8 | 44.1 ± 1.5 | 43.8 ± 1.6 | 45.8 ± 1.2 | 0.36 | 0.568 | 0.788 | 0.859 | 0.133 |
| Baseline | POD 1 | POD 10 | POD 17 | POD 24 | P # | P baselinevs. POD1 | P POD1vs. POD10 | P POD10vs. POD17 | P POD17vs. POD24 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Shoulder strength | ||||||||||
| Flexion | ||||||||||
| Affected side (n = 31) | 16.0 ± 0.9 | 7.9 ± 0.8 **+ | 7.8 ± 0.7 **+ | 8.8 ± 0.7 **+ | 8.9 ± 0.6 **+ | <0.001 | <0.001 | 0.890 | 0.017 | 0.850 |
| Unaffected side (n = 31) | 15.5 ± 0.8 | 12.7 ± 0.9 ** | 11.8 ± 0.8 ** | 11.7 ± 0.6 ** | 11.0 ± 0.5 ** | <0.001 | <0.001 | 0.173 | 0.866 | 0.071 |
| Abduction | ||||||||||
| Affected side (n = 31) | 15.5 ± 1.0 | 7.0 ± 0.6 **+ | 7.0 ± 0.6 **+ | 7.8 ± 0.7 **+ | 7.6 ± 0.5 **+ | <0.001 | <0.001 | 0.992 | 0.009 | 0.422 |
| Unaffected side (n = 31) | 15.0 ± 0.9 | 12.1 ± 0.9 ** | 11.2 ± 0.7 ** | 11.1 ± 0.6 ** | 11.0 ± 0.6 ** | <0.001 | <0.001 | 0.181 | 0.823 | 0.607 |
| Extension | ||||||||||
| Affected side (n = 31) | 22.3 ± 1.0 | 12.3 ± 1.0 **+ | 15.1 ± 1.0 ** | 15.5 ± 0.8 **+ | 16.1 ± 0.7 **+ | <0.001 | <0.001 | 0.001 | 0.426 | 0.521 |
| Unaffected side (n = 31) | 22.7 ± 1.2 | 17.6 ± 1.1 ** | 17.4 ± 1.0 ** | 19.0 ± 0.9 ** | 18.0 ± 0.8 ** | <0.001 | <0.001 | 0.783 | 0.047 | 0.147 |
| Horizontal adduction | ||||||||||
| Affected side (n = 31) | 19.6 ± 1.0 | 11.5 ± 1.1 **+ | 11.3 ± 1.0 **+ | 12.3 ± 0.9 **+ | 12.9 ± 1.0 **+ | <0.001 | <0.001 | 0.754 | 0.073 | 0.355 |
| Unaffected side (n = 31) | 19.5 ± 1.1 | 16.4 ± 1.0 ** | 16.7 ± 1.2 * | 16.7 ± 0.9 * | 15.3 ± 0.8 ** | <0.001 | <0.001 | 0.649 | 0.979 | 0.006 |
| Horizontal abduction | ||||||||||
| Affected side (n = 30) | 19.3 ± 1.2 | 12.2 ± 1.1 **+ | 11.5 ± 1.0 **+ | 12.7 ± 0.8 **+ | 12.0 ± 0.8 **+ | <0.001 | <0.001 | 0.312 | 0.066 | 0.263 |
| Unaffected side (n = 31) | 19.2 ± 1.0 | 17.4 ± 1.1 | 15.9 ± 1.2 * | 15.6 ± 0.8 ** | 14.9 ± 0.6 ** | <0.001 | 0.020 | 0.025 | 0.771 | 0.095 |
| Baseline | POD 1 | POD 10 | POD 17 | POD 24 | P # | P baselinevs. POD1 | P POD1vs. POD10 | P POD10vs. POD17 | P POD17vs. POD24 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Shoulder function score | ||||||||||
| ROM score | ||||||||||
| Affected side (n = 30) | 100.0 ± 0 | 63.3 ± 4.0 **+ | 67.8 ± 2.4 **+ | 70.5 ± 2.4 **+ | 73.5 ± 2.6 **+ | <0.001 | <0.001 | 0.208 | 0.146 | 0.011 |
| Unaffected side (n = 31) | 100.0 ± 0 | 93.6 ± 2.4 * | 97.9 ± 1.4 | 98.5 ± 1.5 | 100.4 ± 1.3 | 0.018 | 0.012 | 0.072 | 0.705 | 0.085 |
| Strength score | ||||||||||
| Affected side (n = 31) | 100.0 ± 0 | 52.9 ± 2.9 **+ | 55.3 ± 2.2 **+ | 61.8 ± 2.1 **+ | 62.5 ± 2.3 **+ | <0.001 | <0.001 | 0.369 | 0.001 | 0.964 |
| Unaffected side (n = 31) | 100.0 ± 0 | 83.1 ± 2.3 ** | 80.8 ± 2.8 ** | 82.5 ± 2.4 ** | 78.9 ± 2.9 ** | <0.001 | <0.001 | 0.335 | 0.461 | 0.035 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, J.; Kim, J.Y.; Yeon, S.; Ryu, J.; Min, J.J.; Park, S.; Kim, S.I.; Jeon, J.Y. Change in Shoulder Function in the Early Recovery Phase after Breast Cancer Surgery: A Prospective Observational Study. J. Clin. Med. 2021, 10, 3416. https://doi.org/10.3390/jcm10153416
Min J, Kim JY, Yeon S, Ryu J, Min JJ, Park S, Kim SI, Jeon JY. Change in Shoulder Function in the Early Recovery Phase after Breast Cancer Surgery: A Prospective Observational Study. Journal of Clinical Medicine. 2021; 10(15):3416. https://doi.org/10.3390/jcm10153416
Chicago/Turabian StyleMin, Jihee, Jee Ye Kim, Sujin Yeon, Jiin Ryu, Jin Joo Min, Seho Park, Seung Il Kim, and Justin Y. Jeon. 2021. "Change in Shoulder Function in the Early Recovery Phase after Breast Cancer Surgery: A Prospective Observational Study" Journal of Clinical Medicine 10, no. 15: 3416. https://doi.org/10.3390/jcm10153416
APA StyleMin, J., Kim, J. Y., Yeon, S., Ryu, J., Min, J. J., Park, S., Kim, S. I., & Jeon, J. Y. (2021). Change in Shoulder Function in the Early Recovery Phase after Breast Cancer Surgery: A Prospective Observational Study. Journal of Clinical Medicine, 10(15), 3416. https://doi.org/10.3390/jcm10153416







