The APAC Score: A Novel and Highly Performant Serological Tool for Early Diagnosis of Hepatocellular Carcinoma in Patients with Liver Cirrhosis
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. Measurement of Serological Markers
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Performance of sPDGFRβ Levels for HCC Detection
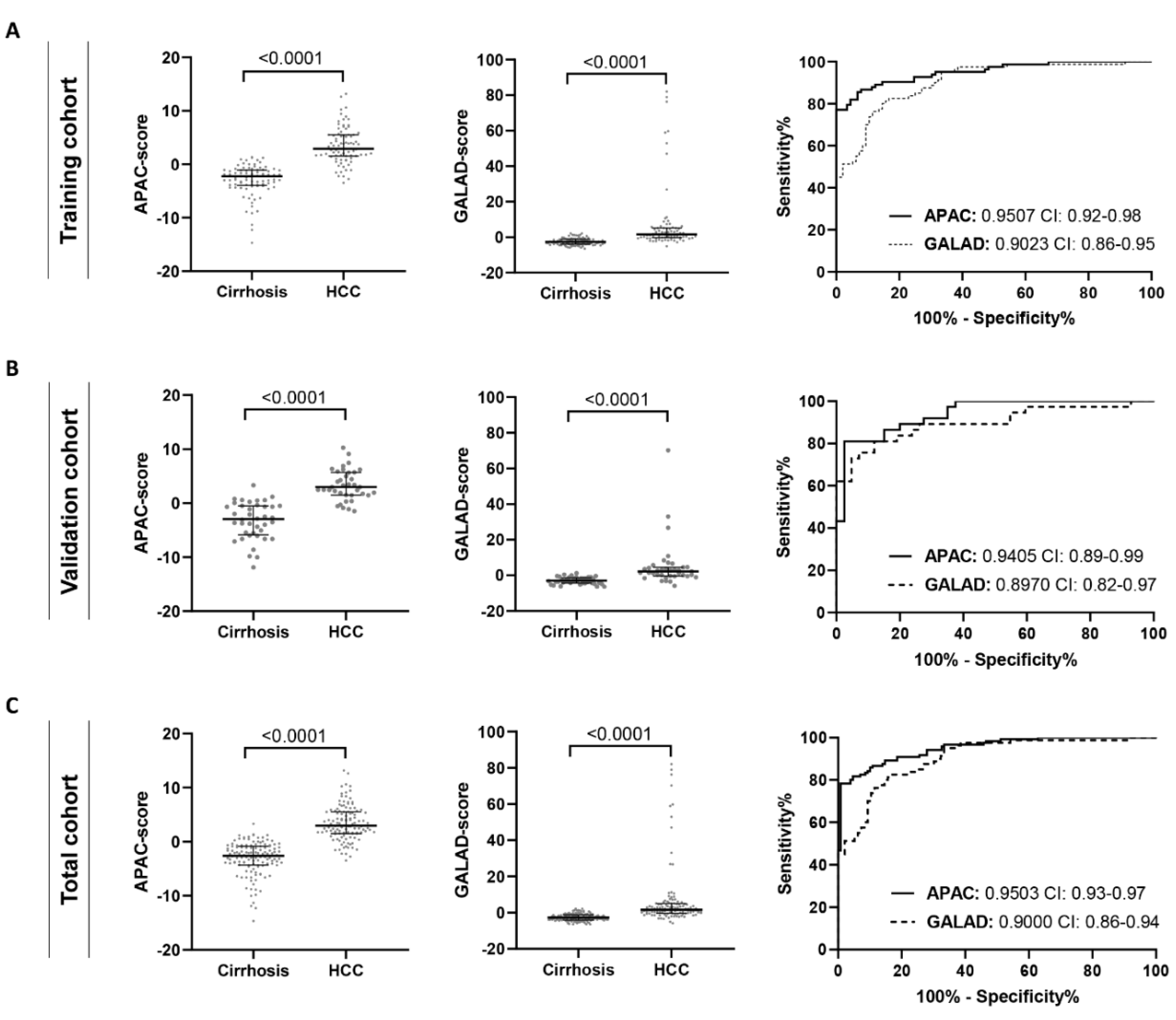
3.3. The APAC Score as a Superior Diagnostic HCC Test
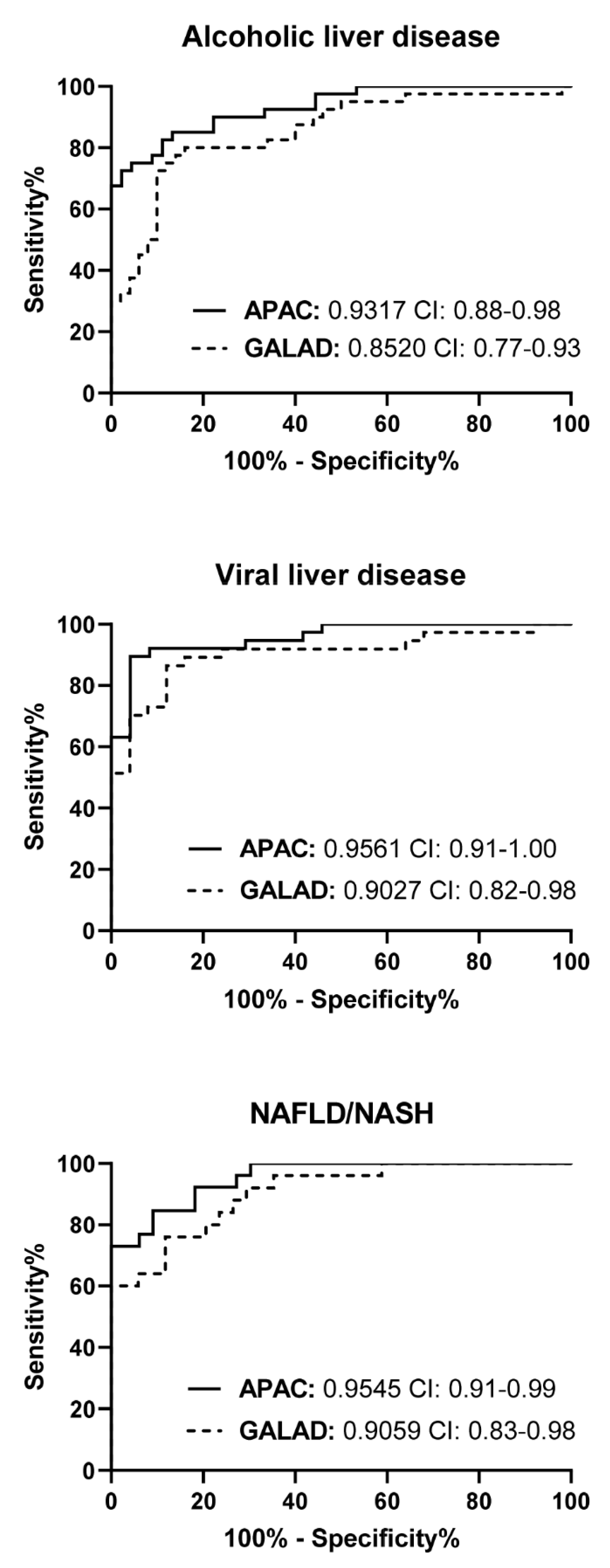
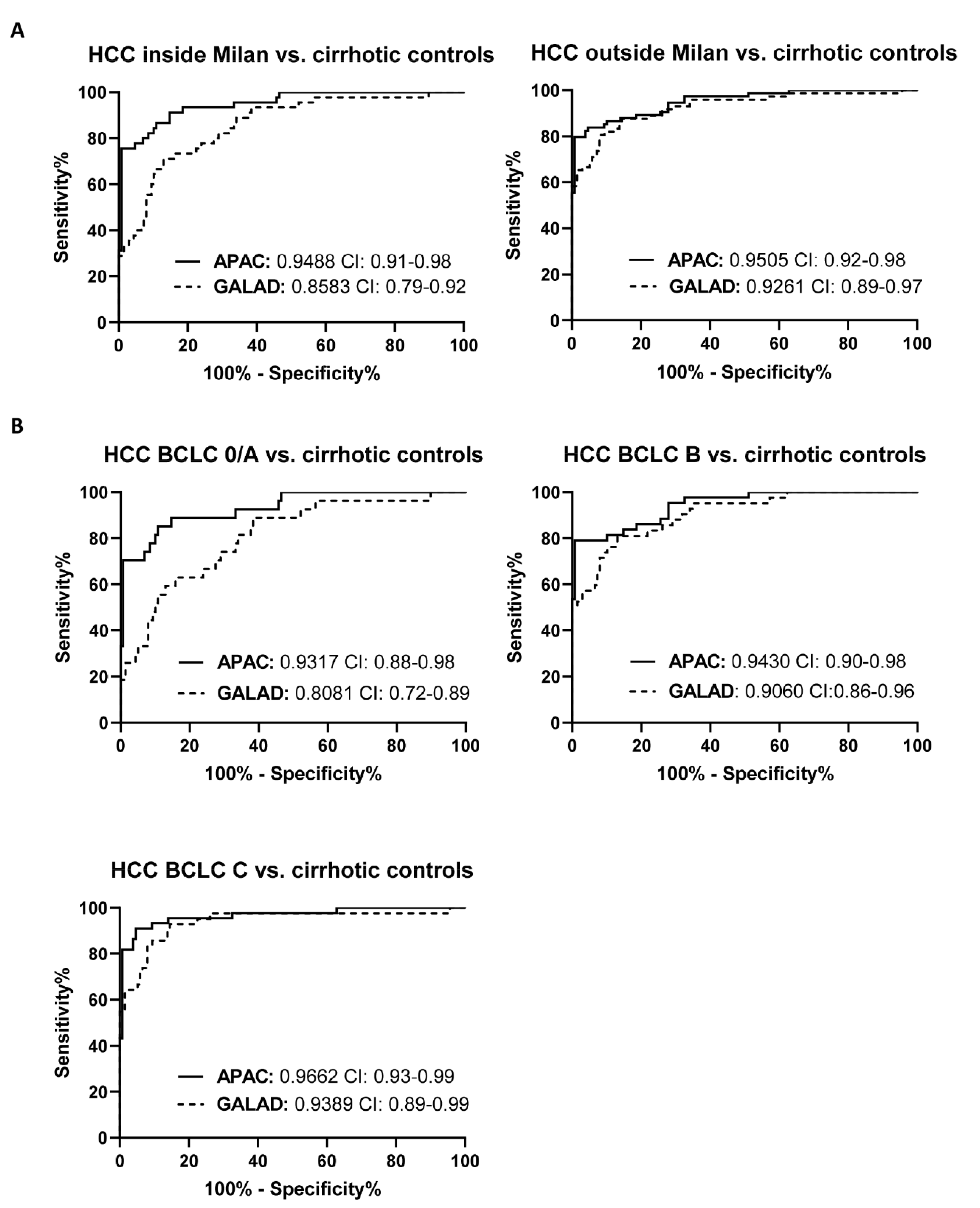
3.4. The Diagnostic Performance of the APAC Score Is Independent of the Stage of HCC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Shiels, M.S.; O’Brien, T.R. Recent Decline in Hepatocellular Carcinoma Rates in the United States. Gastroenterology 2020, 158, 1503–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, N.; Friedman, S.L.; Goossens, N.; Hoshida, Y. Risk factors and prevention of hepatocellular carcinoma in the era of precision medicine. J. Hepatol. 2018, 68, 526–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunasekaran, G.; Bekki, Y.; Lourdusamy, V.; Schwartz, M. Surgical Treatments of Hepatobiliary Cancers. Hepatology 2021, 73 (Suppl. 1), 128–136. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef]
- Fan, R.; Papatheodoridis, G.; Sun, J.; Innes, H.; Toyoda, H.; Xie, Q.; Mo, S.; Sypsa, V.; Guha, I.N.; Kumada, T.; et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J. Hepatol. 2020, 73, 1368–1378. [Google Scholar] [CrossRef]
- Singal, A.G.; Mittal, S.; Yerokun, O.A.; Ahn, C.; Marrero, J.A.; Yopp, A.C.; Parikh, N.D.; Scaglione, S.J. Hepatocellular Carcinoma Screening Associated with Early Tumor Detection and Improved Survival among Patients with Cirrhosis in the US. Am. J. Med. 2017, 130, 1099–1106.e1. [Google Scholar] [CrossRef] [Green Version]
- Heimbach, J.K.; Kulik, L.M.; Finn, R.S.; Sirlin, C.B.; Abecassis, M.M.; Roberts, L.R.; Zhu, A.X.; Murad, M.H.; Marrero, J.A. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology 2018, 67, 358–380. [Google Scholar] [CrossRef] [Green Version]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018, 69, 182–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simmons, O.; Fetzer, D.T.; Yokoo, T.; Marrero, J.A.; Yopp, A.; Kono, Y.; Parikh, N.D.; Browning, T.; Singal, A.G. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol. Ther. 2017, 45, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Wong, L.L.; Reyes, R.J.; Kwee, S.A.; Hernandez, B.Y.; Kalathil, S.C.; Tsai, N.C. Pitfalls in surveillance for hepatocellular carcinoma: How successful is it in the real world? Clin. Mol. Hepatol. 2017, 23, 239–248. [Google Scholar] [CrossRef]
- Johnson, P.J.; Pirrie, S.J.; Cox, T.F.; Berhane, S.; Teng, M.; Palmer, D.; Morse, J.; Hull, D.; Patman, G.; Kagebayashi, C.; et al. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol. Biomark. Prev. 2014, 23, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.D.; Addissie, B.D.; Mara, K.C.; Harmsen, W.S.; Dai, J.; Zhang, N.; Wongjarupong, N.; Ali, H.M.; Ali, H.A.; Hassan, F.A.; et al. GALAD Score for Hepatocellular Carcinoma Detection in Comparison with Liver Ultrasound and Proposal of GALADUS Score. Cancer Epidemiol. Biomark. Prev. 2019, 28, 531–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berhane, S.; Toyoda, H.; Tada, T.; Kumada, T.; Kagebayashi, C.; Satomura, S.; Schweitzer, N.; Vogel, A.; Manns, M.P.; Benckert, J.; et al. Role of the GALAD and BALAD-2 Serologic Models in Diagnosis of Hepatocellular Carcinoma and Prediction of Survival in Patients. Clin. Gastroenterol. Hepatol. 2016, 14, 875–886.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Best, J.; Bechmann, L.P.; Sowa, J.P.; Sydor, S.; Dechene, A.; Pflanz, K.; Bedreli, S.; Schotten, C.; Geier, A.; Berg, T.; et al. GALAD Score Detects Early Hepatocellular Carcinoma in an International Cohort of Patients with Nonalcoholic Steatohepatitis. Clin. Gastroenterol. Hepatol. 2020, 18, 728–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singal, A.G.; Tayob, N.; Mehta, A.; Marrero, J.A.; Jin, Q.; Lau, J.; Parikh, N.D. Doylestown Plus and GALAD Demonstrate High Sensitivity for HCC Detection in Patients with Cirrhosis. Clin. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Shiratori, Y.; Sato, S.; Obi, S.; Teratani, T.; Imamura, M.; Yoshida, H.; Shiina, S.; Omata, M. Des-gamma-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma: A prospective analysis of 227 patients. Cancer 2001, 91, 561–569. [Google Scholar] [CrossRef]
- Oka, H.; Saito, A.; Ito, K.; Kumada, T.; Satomura, S.; Kasugai, H.; Osaki, Y.; Seki, T.; Kudo, M.; Tanaka, M.; et al. Multicenter prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J. Gastroenterol. Hepatol. 2001, 16, 1378–1383. [Google Scholar] [CrossRef]
- Lambrecht, J.; Verhulst, S.; Mannaerts, I.; Sowa, J.P.; Best, J.; Canbay, A.; Reynaert, H.; van Grunsven, L.A. A PDGFRbeta-based score predicts significant liver fibrosis in patients with chronic alcohol abuse, NAFLD and viral liver disease. EBioMedicine 2019, 43, 501–512. [Google Scholar] [CrossRef] [Green Version]
- Kocabayoglu, P.; Lade, A.; Lee, Y.A.; Dragomir, A.C.; Sun, X.; Fiel, M.I.; Thung, S.; Aloman, C.; Soriano, P.; Hoshida, Y.; et al. beta-PDGF receptor expressed by hepatic stellate cells regulates fibrosis in murine liver injury, but not carcinogenesis. J. Hepatol. 2015, 63, 141–147. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.H.; Kneuertz, P.J.; Delgado, M.; Kooby, D.A.; Staley, C.A., 3rd; El-Rayes, B.F.; Kauh, J.S.; Sarmiento, J.M.; Hanish, S.; Cohen, C.; et al. Clinically relevant biomarkers to select patients for targeted inhibitor therapy after resection of hepatocellular carcinoma. Ann. Surg. Oncol. 2011, 18, 3384–3390. [Google Scholar] [CrossRef]
- Kaps, L.; Schuppan, D. Targeting Cancer Associated Fibroblasts in Liver Fibrosis and Liver Cancer Using Nanocarriers. Cells 2020, 9, 2027. [Google Scholar] [CrossRef]
- Gauthier, A.; Ho, M. Role of sorafenib in the treatment of advanced hepatocellular carcinoma: An update. Hepatol. Res. 2013, 43, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Tacke, F.; Boeker, K.H.W.; Klinker, H.; Heyne, R.; Buggisch, P.; Pathil, A.; Wiegand, J.; Cornberg, M.; Lange, C.; Berg, T.; et al. Baseline risk factors determine lack of biochemical response after SVR in chronic hepatitis C patients treated with DAAs. Liver Int. 2020, 40, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Singal, A.G.; Lampertico, P.; Nahon, P. Epidemiology and surveillance for hepatocellular carcinoma: New trends. J. Hepatol. 2020, 72, 250–261. [Google Scholar] [CrossRef] [Green Version]
- Llovet, J.M.; Bruix, J.; Fuster, J.; Castells, A.; Garcia-Valdecasas, J.C.; Grande, L.; Franca, A.; Bru, C.; Navasa, M.; Ayuso, M.C.; et al. Liver transplantation for small hepatocellular carcinoma: The tumor-node-metastasis classification does not have prognostic power. Hepatology 1998, 27, 1572–1577. [Google Scholar] [CrossRef]
- Caviglia, G.P.; Abate, M.L.; Petrini, E.; Gaia, S.; Rizzetto, M.; Smedile, A. Highly sensitive alpha-fetoprotein, Lens culinaris agglutinin-reactive fraction of alpha-fetoprotein and des-gamma-carboxyprothrombin for hepatocellular carcinoma detection. Hepatol. Res. 2016, 46, E130–E135. [Google Scholar] [CrossRef] [PubMed]
- Tarao, K.; Nozaki, A.; Ikeda, T.; Sato, A.; Komatsu, H.; Komatsu, T.; Taguri, M.; Tanaka, K. Real impact of liver cirrhosis on the development of hepatocellular carcinoma in various liver diseases-meta-analytic assessment. Cancer Med. 2019, 8, 1054–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of Hepatocellular Carcinoma. Hepatology 2021, 73 (Suppl. 1), 4–13. [Google Scholar] [CrossRef] [PubMed]

| Cirrhosis (n = 145) | HCC (n = 122) | p-Value | |
|---|---|---|---|
| Age, median (IQR), years | 54 (47 to 60) | 66 (60 to 72) | <0.0001 |
| Gender, n (%) | 0.07979 | ||
| Female | 46 (31.7%) | 27 (22.1%) | |
| Male | 99 (68.3%) | 95 (77.9%) | |
| Etiology, n (%) | 0.1917 | ||
| Alcohol | 51 (35.2%) | 41 (33.6%) | |
| HBV | 11 (7.6%) | 11 (9.0%) | |
| HCV | 18 (12.4%) | 27 (22.1%) | |
| NAFLD | 34 (23.4%) | 26 (21.4%) | |
| Other | 31 (21.4%) | 17 (13.9%) | |
| Child-Pugh class, n (%) | <0.0001 | ||
| A | 57 (39.3%) | 99 (81.1%) | |
| B | 58 (40.0%) | 22 (18.0%) | |
| C | 10 (6.9%) | 0 | |
| Laboratory results, median (IQR) | |||
| AST, IU/L | 57.0 (18.0 to 85.0) | 63.0 (25.0 to 95.5) | 0.1132 |
| ALT, IU/L | 35.0 (25.0 to 63.5) | 42.0 (29.5 to 73.0) | 0.0551 |
| ALP, IU/L | 135.0 (99.0 to 183.0) | 135.0 (98.0 to 200.5) | 0.6679 |
| GGT, IU/L | 94.0 (50.75 to 205.5) | 170.0 (87.00 to 278.5) | 0.0001 |
| Total bilirubin, mg/dL | 2.0 (1.3 to 4.2) | 1.1 (1.3 to 4.2) | <0.0001 |
| Albumin, g/L | 33.0 (29.0 to 39.0) | 37.1 (33.0 to 40.8) | 0.0003 |
| Thrombocytes, ×103/mm3 | 99.0 (70.0 to 149.0) | 125.0 (83.0 to 211.5) | 0.0059 |
| Creatinine, mg/dL | 0.92 (0.76 to 1.21) | 0.82 (0.70 to 0.98) | 0.0005 |
| INR | 1.40 (1.24 to 1.63) | 1.18 (1.07 to 1.29) | <0.0001 |
| CRP, mg/L | 1.28 (0.40 to 3.14) | 2.35 (0.62 to 14.9) | 0.0011 |
| AFP, ng/mL | 3.5 (2.1 to 6.0) | 24.0 (7.1 to 260.7) | <0.0001 |
| AFP-L3, % | 0.10 (0.10 to 8.10) | 14.70 (5.94 to 36.55) | <0.0001 |
| DCP, ng/mL | 0.69 (0.27 to 3.89) | 6.0 (1.42 to 57.46) | <0.0001 |
| Scoring parameters, median (IQR) | |||
| Fib-4 | 5.63 (2.95 to 8.59) | 5.27 (3.02 to 9.09) | 0.9377 |
| MELD | 14.10 (11.09 to 19.28) | 9.41 (7.75 to 11.58) | <0.0001 |
| ALBI | −1.72 (−2.42 to −1.22) | −2.35 (−2.73 to −1.85) | <0.0001 |
| GALAD | −2.79 (−4.03 to −1.230) | 1.56 (−0.21 to 4.99) | <0.0001 |
| Tumor size, n (%) | |||
| ≤2 cm | N.A. | 14 (11.5%) | N.A. |
| >2 to ≤3 cm | N.A. | 19 (15.6%) | N.A. |
| >3 to ≤5 cm | N.A. | 31 (25.4%) | N.A. |
| >5 cm | N.A. | 42 (34.4%) | N.A. |
| Tumor number, n (%) | |||
| 1 | N.A. | 45 (36.9%) | N.A. |
| 2 | N.A. | 18 (14.8%) | N.A. |
| ≥3 | N.A. | 56 (45.9%) | N.A. |
| BCLC stage, n (%) | |||
| Very early (0) | N.A. | 7 (5.7%) | N.A. |
| Early (A) | N.A. | 20 (16.4%) | N.A. |
| Intermediate (B) | N.A. | 44 (36.1%) | N.A. |
| Advanced (C) | N.A. | 44 (36.1%) | N.A. |
| Milan Criteria, n (%) | |||
| Inside | N.A. | 75 (61.5%) | N.A. |
| Outside | N.A. | 43 (35.2%) | N.A. |
| Cut-Off Value | AUC (95% CI) | p-Value AUC (vs. APAC) | Sensitivity, % | Specificity, % | PPV, % | NPV, % | |
|---|---|---|---|---|---|---|---|
| Training cohort | |||||||
| APAC | 0.4109 | 0.9507 (0.9202–0.9813) | - | 85.54 | 93.26 | 91.43 | 88.39 |
| sPDGFRβ, pg/mL | 7962 | 0.6214 (0.5414–0.7015) | <0.0001 | 68.24 | 54.46 | 55.76 | 67.09 |
| Creatinine, mg/dL | 1.025 | 0.6401 (0.5584–0.7218) | <0.0001 | 83.33 | 42.22 | 54.82 | 75.07 |
| AFP, ng/mL | 9.800 | 0.8478 (0.7920–0.9037) | 0.0001 | 65.06 | 90.00 | 84.55 | 75.38 |
| GALAD | −0.8141 | 0.9023 (0.8584–0.9463) | 0.0252 | 82.50 | 83.33 | 80.63 | 84.98 |
| Validation cohort | |||||||
| APAC | 0.6771 | 0.9405 (0.8920–0.9891) | - | 81.08 | 92.5 | 90.09 | 85.32 |
| sPDGFRβ, pg/mL | 10155 | 0.7039 (0.5892–0.8187) | 0.0006 | 83.78 | 56.82 | 62.01 | 80.64 |
| Creatinine, mg/dL | 0.9050 | 0.5923 (0.4670–0.7177) | <0.0001 | 64.86 | 54.76 | 54.76 | 64.94 |
| AFP, ng/mL | 12.95 | 0.8826 (0.8032–0.9619) | 0.0182 | 72.97 | 95.24 | 92.80 | 80.73 |
| GALAD | −0.5396 | 0.8970 (0.8231–0.9710) | 0.0137 | 81.08 | 88.1 | 85.15 | 84.69 |
| Total cohort | |||||||
| APAC | 0.7969 | 0.9503 (0.9258–0.9747) | - | 81.67 | 95.35 | 93.66 | 86.08 |
| sPDGFRβ, pg/mL | 9278 | 0.6470 (0.5813–0.7127) | <0.0001 | 83.61 | 43.45 | 55.43 | 75.91 |
| Creatinine, mg/dL | 0.9550 | 0.6266 (0.5583–0.6948) | <0.0001 | 72.73 | 49.24 | 54.66 | 68.22 |
| AFP, ng/mL | 6.350 | 0.8571 (0.8113–0.9028) | <0.0001 | 79.17 | 78.87 | 75.92 | 81.82 |
| GALAD | −0.6373 | 0.9000 (0.8620–0.9380) | 0.0031 | 81.20 | 85.51 | 82.50 | 84.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambrecht, J.; Porsch-Özçürümez, M.; Best, J.; Jost-Brinkmann, F.; Roderburg, C.; Demir, M.; Tacke, F.; Mohr, R. The APAC Score: A Novel and Highly Performant Serological Tool for Early Diagnosis of Hepatocellular Carcinoma in Patients with Liver Cirrhosis. J. Clin. Med. 2021, 10, 3392. https://doi.org/10.3390/jcm10153392
Lambrecht J, Porsch-Özçürümez M, Best J, Jost-Brinkmann F, Roderburg C, Demir M, Tacke F, Mohr R. The APAC Score: A Novel and Highly Performant Serological Tool for Early Diagnosis of Hepatocellular Carcinoma in Patients with Liver Cirrhosis. Journal of Clinical Medicine. 2021; 10(15):3392. https://doi.org/10.3390/jcm10153392
Chicago/Turabian StyleLambrecht, Joeri, Mustafa Porsch-Özçürümez, Jan Best, Fabian Jost-Brinkmann, Christoph Roderburg, Münevver Demir, Frank Tacke, and Raphael Mohr. 2021. "The APAC Score: A Novel and Highly Performant Serological Tool for Early Diagnosis of Hepatocellular Carcinoma in Patients with Liver Cirrhosis" Journal of Clinical Medicine 10, no. 15: 3392. https://doi.org/10.3390/jcm10153392
APA StyleLambrecht, J., Porsch-Özçürümez, M., Best, J., Jost-Brinkmann, F., Roderburg, C., Demir, M., Tacke, F., & Mohr, R. (2021). The APAC Score: A Novel and Highly Performant Serological Tool for Early Diagnosis of Hepatocellular Carcinoma in Patients with Liver Cirrhosis. Journal of Clinical Medicine, 10(15), 3392. https://doi.org/10.3390/jcm10153392








