Zonisamide Therapy Reduces Metabolic Consequences and Diminishes Nonalcoholic Fatty Liver Disease in Patients with Epilepsy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Study Design
2.2. Analysis of Biochemical Markers
2.3. Measurement of Hepatic Steatosis Index for Nonalcoholic Fatty Liver Disease
2.4. Statistical Analysis
3. Results
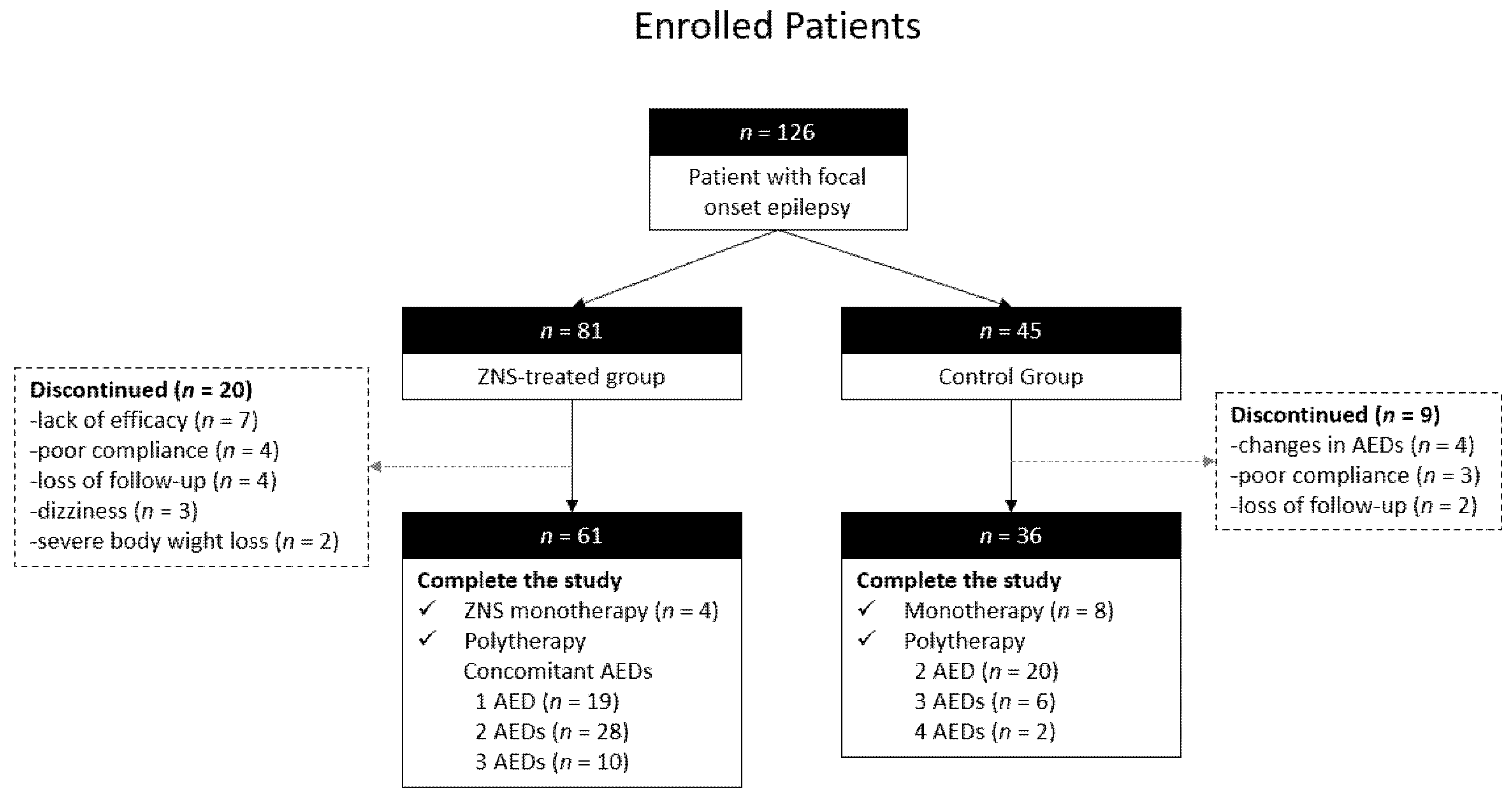
3.1. Clinical Characteristics of Patients
3.2. Changes in Body Weight, BMI, Circulating Biochemical Markers, and HSI after ZNS Therapy
3.3. GEE Analysis of Body Weight, BMI, Circulating Biochemical Markers, and HSI after ZNS Therapy
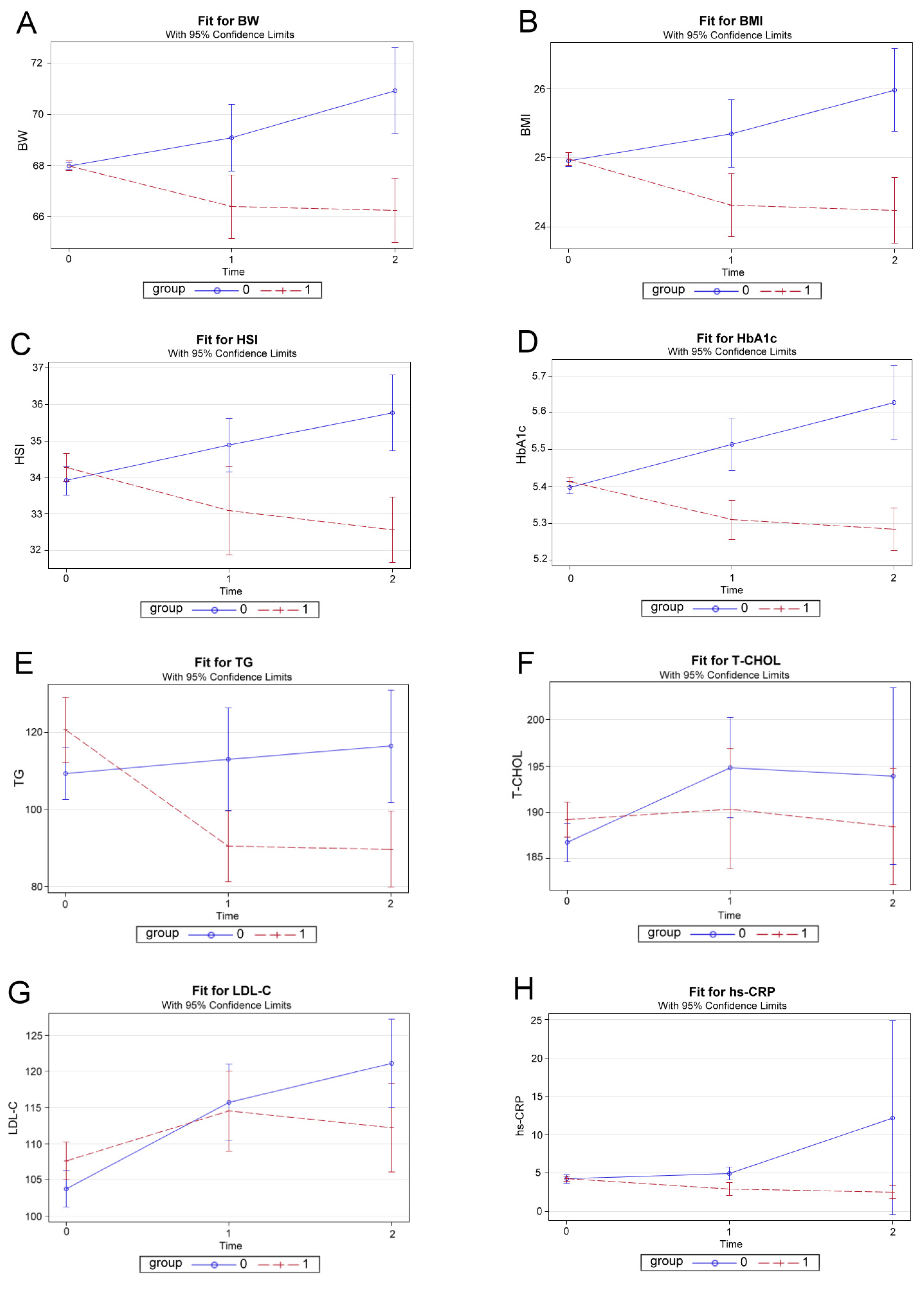
3.4. Effect Plots of ZNS Therapy by GEE Method with Adjustment for Sex, Age, and De-Pendent Variables at Baseline
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thijs, R.D.; Surges, R.; O’Brien, T.J.; Sander, J.W. Epilepsy in adults. Lancet 2019, 393, 689–701. [Google Scholar] [CrossRef]
- Chen, Z.; Brodie, M.J.; Liew, D.; Kwan, P. Treatment Outcomes in Patients With Newly Diagnosed Epilepsy Treated With Established and New Antiepileptic Drugs: A 30-Year Longitudinal Cohort Study. JAMA Neurol. 2018, 75, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Kwan, P.; Brodie, M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000, 342, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Brodie, M.J.; Kwan, P. Epilepsy in elderly people. BMJ 2005, 331, 1317–1322. [Google Scholar] [CrossRef] [Green Version]
- Brodie, M.J.; Mintzer, S.; Pack, A.M.; Gidal, B.E.; Vecht, C.J.; Schmidt, D. Enzyme induction with antiepileptic drugs: Cause for concern? Epilepsia 2013, 54, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, S. Metabolic consequences of antiepileptic drugs. Curr. Opin. Neurol. 2010, 23, 164–169. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Giménez, J.; Sánchez-Alvarez, J.C.; Cañadillas-Hidalgo, F.; Serrano-Castro, P.J.; Andalusian Epilepsy, S. Antiepileptic treatment in patients with epilepsy and other comorbidities. Seizure 2010, 19, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Tan, T.Y.; Lu, C.H.; Chuang, H.Y.; Lin, T.K.; Liou, C.W.; Chang, W.N.; Chuang, Y.C. Long-term antiepileptic drug therapy contributes to the acceleration of atherosclerosis. Epilepsia 2009, 50, 1579–1586. [Google Scholar] [CrossRef]
- Kwan, S.Y.; Chuang, Y.C.; Huang, C.W.; Chen, T.C.; Jou, S.B.; Dash, A. Zonisamide: Review of Recent Clinical Evidence for Treatment of Epilepsy. CNS Neurosci. Ther. 2015, 21, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Leppik, I.E. Zonisamide: Chemistry, mechanism of action, and pharmacokinetics. Seizure 2004, 13 (Suppl. 1), S5–S9, discussion S10. [Google Scholar] [CrossRef] [Green Version]
- Baulac, M.; Brodie, M.J.; Patten, A.; Segieth, J.; Giorgi, L. Efficacy and tolerability of zonisamide versus controlled-release carbamazepine for newly diagnosed partial epilepsy: A phase 3, randomised, double-blind, non-inferiority trial. Lancet Neurol. 2012, 11, 579–588. [Google Scholar] [CrossRef]
- Glauser, T.; Ben-Menachem, E.; Bourgeois, B.; Cnaan, A.; Guerreiro, C.; Kälviäinen, R.; Mattson, R.; French, J.A.; Perucca, E.; Tomson, T.; et al. Updated ILAE evidence review of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2013, 54, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Gupta, Y.K.; Gupta, M. Post traumatic epilepsy: A review of scientific evidence. Indian J. Physiol. Pharmacol. 2006, 50, 7–16. [Google Scholar]
- Kumar, B.; Medhi, B.; Modi, M.; Saikia, B.; Attri, S.V.; Patial, A. A mechanistic approach to explore the neuroprotective potential of zonisamide in seizures. Inflammopharmacology 2018, 26, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Ben-Menachem, E. Weight issues for people with epilepsy—A review. Epilepsia 2007, 48 (Suppl. 9), 42–45. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Drug interaction considerations in the therapeutic use of carbonic anhydrase inhibitors. Expert Opin. Drug Metab. Toxicol. 2016, 12, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Manco, R.; Agostinelli, S.; Coppola, G.; Chiarelli, F. The metabolic syndrome in overweight epileptic patients treated with valproic acid. Epilepsia 2010, 51, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Engin, A. The Definition and Prevalence of Obesity and Metabolic Syndrome. Adv. Exp. Med. Biol. 2017, 960, 1–17. [Google Scholar] [CrossRef]
- Daniels, Z.S.; Nick, T.G.; Liu, C.; Cassedy, A.; Glauser, T.A. Obesity is a common comorbidity for pediatric patients with untreated, newly diagnosed epilepsy. Neurology 2009, 73, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Janousek, J.; Barber, A.; Goldman, L.; Klein, P. Obesity in adults with epilepsy. Epilepsy Behav. 2013, 28, 391–394. [Google Scholar] [CrossRef]
- Vooturi, S.; Jayalakshmi, S. Metabolic syndrome in people with epilepsy. Epilepsy Behav. 2020, 106, 106992. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Chuang, H.Y.; Lin, T.K.; Chang, C.C.; Lu, C.H.; Chang, W.N.; Chen, S.D.; Tan, T.Y.; Huang, C.R.; Chan, S.H. Effects of long-term antiepileptic drug monotherapy on vascular risk factors and atherosclerosis. Epilepsia 2012, 53, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.A.; Hamed, E.A.; Hamdy, R.; Nabeshima, T. Vascular risk factors and oxidative stress as independent predictors of asymptomatic atherosclerosis in adult patients with epilepsy. Epilepsy Res. 2007, 74, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, S.; Skidmore, C.T.; Abidin, C.J.; Morales, M.C.; Chervoneva, I.; Capuzzi, D.M.; Sperling, M.R. Effects of antiepileptic drugs on lipids, homocysteine, and C-reactive protein. Ann. Neurol. 2009, 65, 448–456. [Google Scholar] [CrossRef]
- Mintzer, S.; Dimova, S.; Zhang, Y.; Steiniger-Brach, B.; De Backer, M.; Chellun, D.; Roebling, R. Effects of lacosamide and carbamazepine on lipids in a randomized trial. Epilepsia 2020, 61, 2696–2704. [Google Scholar] [CrossRef] [PubMed]
- Koo, D.L.; Nam, H. Effects of zonisamide monotherapy on bone health in drug-naive epileptic patients. Epilepsia 2020, 61, 2142–2149. [Google Scholar] [CrossRef]
- Wellmer, J.; Wellmer, S.; Bauer, J. The impact of zonisamide on weight. A clinical study in 103 patients with epilepsy. Acta Neurol. Scand. 2009, 119, 233–238. [Google Scholar] [CrossRef]
- Gadde, K.M.; Franciscy, D.M.; Wagner, H.R., 2nd; Krishnan, K.R. Zonisamide for weight loss in obese adults: A randomized controlled trial. JAMA 2003, 289, 1820–1825. [Google Scholar] [CrossRef]
- Gadde, K.M.; Kopping, M.F.; Wagner, H.R., 2nd; Yonish, G.M.; Allison, D.B.; Bray, G.A. Zonisamide for weight reduction in obese adults: A 1-year randomized controlled trial. Arch. Intern Med. 2012, 172, 1557–1564. [Google Scholar] [CrossRef] [Green Version]
- Shin, J.H.; Gadde, K.M.; Ostbye, T.; Bray, G.A. Weight changes in obese adults 6-months after discontinuation of double-blind zonisamide or placebo treatment. Diabetes Obes. Metab. 2014, 16, 766–768. [Google Scholar] [CrossRef] [Green Version]
- Johannessen, S.I.; Landmark, C.J. Antiepileptic drug interactions—Principles and clinical implications. Curr. Neuropharmacol. 2010, 8, 254–267. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kim, D.; Kim, H.J.; Lee, C.H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.H.; Cho, S.H.; Sung, M.W.; et al. Hepatic steatosis index: A simple screening tool reflecting nonalcoholic fatty liver disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Zeger, S.L.; Liang, K.Y. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986, 42, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Fang, J.; Chen, S.; Tong, N.; Chen, L.; An, D.; Mu, J.; Zhou, D. Metabolic syndrome among Chinese obese patients with epilepsy on sodium valproate. Seizure 2012, 21, 578–582. [Google Scholar] [CrossRef] [Green Version]
- Nair, S.S.; Harikrishnan, S.; Sarma, P.S.; Thomas, S.V. Metabolic syndrome in young adults with epilepsy. Seizure 2016, 37, 61–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.W.; Elmquist, J.K. Lighting up the hypothalamus: Coordinated control of feeding behavior. Nat. Neurosci. 2011, 14, 277–278. [Google Scholar] [CrossRef] [PubMed]
- Merlis, S. Diamox; a carbonic anhydrase inhibitor; its use in epilepsy. Neurology 1954, 4, 863–868. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrase inhibitors as emerging drugs for the treatment of obesity. Expert Opin. Emerg. Drugs 2012, 17, 11–15. [Google Scholar] [CrossRef] [PubMed]
- De Simone, G.; Di Fiore, A.; Menchise, V.; Pedone, C.; Antel, J.; Casini, A.; Scozzafava, A.; Wurl, M.; Supuran, C.T. Carbonic anhydrase inhibitors. Zonisamide is an effective inhibitor of the cytosolic isozyme II and mitochondrial isozyme V: Solution and X-ray crystallographic studies. Bioorg. Med. Chem. Lett. 2005, 15, 2315–2320. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Supuran, C.T.; Carta, F. Antiobesity carbonic anhydrase inhibitors: A literature and patent review. Expert Opin. Ther. Pat. 2013, 23, 725–735. [Google Scholar] [CrossRef]
- Klop, B.; Elte, J.W.; Cabezas, M.C. Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients 2013, 5, 1218–1240. [Google Scholar] [CrossRef] [Green Version]
- Look, A.R.G.; Wing, R.R. Long-term effects of a lifestyle intervention on weight and cardiovascular risk factors in individuals with type 2 diabetes mellitus: Four-year results of the Look AHEAD trial. Arch. Intern Med. 2010, 170, 1566–1575. [Google Scholar] [CrossRef] [Green Version]
- Kalliomaki, J.L.; Mollerstrom, J.; Sollberger, A. On the effects of a carbonic anhydrase inhibitor (diamox) in diabetes. Acta Med. Scand. 1956, 156, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Tzadok, M.; Nissenkorn, A.; Porper, K.; Matot, I.; Marcu, S.; Anikster, Y.; Menascu, S.; Bercovich, D.; Ben Zeev, B. The many faces of Glut1 deficiency syndrome. J. Child. Neurol. 2014, 29, 349–359. [Google Scholar] [CrossRef]
- Kawano, Y.; Cohen, D.E. Mechanisms of hepatic triglyceride accumulation in non-alcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 434–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinella, M.E. Nonalcoholic fatty liver disease: A systematic review. JAMA 2015, 313, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Masarone, M.; Rosato, V.; Dallio, M.; Gravina, A.G.; Aglitti, A.; Loguercio, C.; Federico, A.; Persico, M. Role of Oxidative Stress in Pathophysiology of Nonalcoholic Fatty Liver Disease. Oxid. Med. Cell. Longev. 2018, 2018, 9547613. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Noda, Y.; Packer, L. The anticonvulsant zonisamide scavenges free radicals. Epilepsy Res. 1998, 30, 153–158. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Heidari-Bakavoli, A.R.; Shoeibi, S.; Mirhafez, S.R.; Moohebati, M.; Esmaily, H.; Ghazavi, H.; Saberi Karimian, M.; Parizadeh, S.M.; Mohammadi, M.; et al. Association of Serum hs-CRP Levels With the Presence of Obesity, Diabetes Mellitus, and Other Cardiovascular Risk Factors. J. Clin. Lab. Anal. 2016, 30, 672–676. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.J.; Arnold, A.M.; Manolio, T.A.; Polak, J.F.; Psaty, B.M.; Hirsch, C.H.; Kuller, L.H.; Cushman, M. Association of carotid artery intima-media thickness, plaques, and C-reactive protein with future cardiovascular disease and all-cause mortality: The Cardiovascular Health Study. Circulation 2007, 116, 32–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| ZNS Group | Control Group | p | |
|---|---|---|---|
| Age (years) | 40.26 ± 10.90 | 40.47 ± 9.64 | 0.924 |
| Sex (female; male) | 27; 34 | 16; 20 | 0.999 a |
| Body weight (kg) | 67.92 ± 14.76 | 68.10 ± 13.92 | 0.952 |
| BMI (kg/m2) | 25.185 ± 4.468 | 24.620 ± 4.413 | 0.538 |
| hs-CRP (mg/L) | 4.551 ± 10.089 | 3.818 ± 5.734 | 0.691 |
| HbA1c (%) | 5.45 ± 0.38 | 5.33 ± 0.32 | 0.109 |
| Creatinine (mg/dL) | 0.796 ± 0.244 | 0.738 ± 0.159 | 0.163 |
| AST (U/L) | 20.97 ± 13.24 | 19.19 ± 6.43 | 0.454 |
| ALT (U/L) | 23.10 ± 15.52 | 19.944 ± 16.372 | 0.346 |
| HSI | 34.806 ± 6.969 | 32.996 ± 5.571 | 0.188 |
| tHcy (μmol/L) | 16.561 ± 19.443 | 15.813 ± 12.782 | 0.837 |
| Triglycerides (mg/dL) | 127.95 ± 94.48 | 97.08 ± 57.23 | 0.048 |
| Cholesterol (mg/dL) | |||
| Total | 194.02 ± 36.50 | 178.61 ± 27.50 | 0.031 |
| HDL-C | 58.48 ± 17.46 | 64.08 ± 17.89 | 0.133 |
| LDL-C | 112.18 ± 38.11 | 96.11 ± 26.74 | 0.028 |
| 12 Weeks | 24 Weeks | |||
|---|---|---|---|---|
| Difference Mean ± SD | p | Difference Mean ± SD | p | |
| Body weight (kg) | −1.61 ± 5.10 | 0.017 * | −1.75 ± 5.31 | 0.013 * |
| BMI (kg/m2) | −0.670 ± 1.909 | 0.008 * | −0.747 ± 2.031 | 0.006 * |
| hs-CRP (mg/L) | −1.340 ± 3.567 | 0.005 * | −1.753 ± 3.528 | <0.001 * |
| HbA1c (%) | −0.10 ± 0.22 | 0.001 * | −0.13 ± 0.24 | <0.001 * |
| Creatinine (mg/dL) | 0.036 ± 0.114 | 0.017 * | 0.048 ± 0.115 | 0.002 * |
| AST (U/L) | −2.31 ± 11.59 | 0.125 | −3.25 ± 12.11 | 0.041 * |
| ALT (U/L) | −3.46 ± 14.21 | 0.062 | −5.33 ± 13.36 | 0.003 * |
| HSI | −1.173 ± 5.646 | 0.110 | −1.701 ± 4.448 | 0.004 * |
| tHcy (μmol/L) | −2.065 ± 17.838 | 0.369 | −2.664 ± 18.669 | 0.269 |
| Triglycerides (mg/dL) | −30.34 ± 62.02 | <0.001 * | −31.07 ± 65.98 | 0.001 * |
| Cholesterol (mg/dL) | ||||
| Total | 1.15 ± 27.79 | 0.748 | −0.75 ± 26.89 | 0.827 |
| HDL-C | −1.80 ± 12.61 | 0.268 | −1.57 ± 12.61 | 0.333 |
| LDL-C | 6.89 ± 26.58 | 0.048 * | 4.57 ± 28.99 | 0.223 |
| Body Weight (kg) | BMI (kg/m2) | Hepatic Steatosis Index (HSI) | HbA1c (%) | |||||||||
| Effect | Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | Estimate | SE | p |
| Intercept | 0.220 | 1.648 | 0.894 | 0.746 | 0.774 | 0.336 | 7.522 | 1.377 | 0.009 | 0.814 | 0.170 | <0.0001 |
| ZNS group | −2.459 | 0.502 | <0.001 | −0.914 | 0.228 | <0.001 | −1.517 | 0.414 | <0.001 | −0.177 | 0.032 | <0.0001 |
| Control group | ||||||||||||
| 12 weeks | −0.603 | 0.594 | 0.311 | −0.276 | 0.185 | 0.137 | −0.380 | 0.485 | 0.434 | −0.022 | 0.025 | 0.380 |
| 24 weeks | −0.006 | 0.594 | 0.992 | −0.089 | 0.216 | 0.679 | −0.383 | 0.485 | 0.430 | 0.004 | 0.032 | 0.899 |
| Time = 0 week | ||||||||||||
| Sex (male vs. female) | 1.183 | 0.574 | 0.042 | 0.436 | 0.247 | 0.077 | 0.864 | 0.405 | 0.033 | 0.040 | 0.026 | 0.124 |
| Age (years) | 0.047 | 0.026 | 0.050 | 0.020 | 0.010 | 0.040 | 0.003 | 0.192 | 0.859 | 0.000 | 0.001 | 0.738 |
| Baseline b | 0.982 | 0.020 | <0.001 | 0.951 | 0.029 | <0.001 | 0.790 | 0.313 | <0.001 | 0.863 | 0.034 | <0.001 |
| Triglyceride (mg/dL) | Total Cholesterol (mg/dL) | LDL-C (mg/dL) | hs-CRP (mg/L) | |||||||||
| Effect | Estimate | SE | p | Estimate | SE | p | Estimate | SE | p | Estimate | SE | p |
| Intercept | 45.425 | 7.869 | <0.001 | 16.072 | 12.164 | 0.186 | 11.016 | 5.735 | 0.055 | 4.838 | 3.802 | 0.203 |
| ZNS group | −12.264 | 4.819 | 0.011 | −2.198 | 3.169 | 0.488 | −1.752 | 2.281 | 0.442 | −3.869 | 1.717 | 0.024 |
| Control group | ||||||||||||
| 12 weeks | −17.701 | 5.966 | 0.003 | 3.732 | 2.494 | 0.135 | 8.773 | 2.631 | 0.001 | −0.583 | 2.030 | 0.774 |
| 24 weeks | −16.907 | 6.447 | 0.009 | 2.196 | 2.888 | 0.447 | 9.299 | 2.631 | 0.000 | 1.847 | 2.030 | 0.363 |
| Time = 0 week | ||||||||||||
| Sex (male vs. female) | 17.142 | 4.775 | 0.000 | −1.393 | 2.960 | 0.638 | 1.218 | 2.163 | 0.574 | 2.168 | 1.682 | 0.198 |
| Age (years) | −0.063 | 0.163 | 0.701 | 0.465 | 0.174 | 0.008 | 0.410 | 0.104 | <0.001 | −0.080 | 0.081 | 0.321 |
| Baseline b | 0.616 | 0.035 | <0.001 | 0.827 | 0.054 | <0.001 | 0.745 | 0.032 | <0.001 | 0.910 | 0.097 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-R.; Chuang, H.-Y.; Chen, N.-C.; Chen, S.-F.; Hsu, C.-Y.; Chuang, Y.-C. Zonisamide Therapy Reduces Metabolic Consequences and Diminishes Nonalcoholic Fatty Liver Disease in Patients with Epilepsy. J. Clin. Med. 2021, 10, 3380. https://doi.org/10.3390/jcm10153380
Huang C-R, Chuang H-Y, Chen N-C, Chen S-F, Hsu C-Y, Chuang Y-C. Zonisamide Therapy Reduces Metabolic Consequences and Diminishes Nonalcoholic Fatty Liver Disease in Patients with Epilepsy. Journal of Clinical Medicine. 2021; 10(15):3380. https://doi.org/10.3390/jcm10153380
Chicago/Turabian StyleHuang, Chi-Ren, Hung-Yi Chuang, Nai-Ching Chen, Shu-Fang Chen, Chung-Yao Hsu, and Yao-Chung Chuang. 2021. "Zonisamide Therapy Reduces Metabolic Consequences and Diminishes Nonalcoholic Fatty Liver Disease in Patients with Epilepsy" Journal of Clinical Medicine 10, no. 15: 3380. https://doi.org/10.3390/jcm10153380
APA StyleHuang, C.-R., Chuang, H.-Y., Chen, N.-C., Chen, S.-F., Hsu, C.-Y., & Chuang, Y.-C. (2021). Zonisamide Therapy Reduces Metabolic Consequences and Diminishes Nonalcoholic Fatty Liver Disease in Patients with Epilepsy. Journal of Clinical Medicine, 10(15), 3380. https://doi.org/10.3390/jcm10153380